ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
- Biomedical Research (2010) Volume 21, Issue 2
Type 2 Diabetes and Vascular Complications: A pathophysiologic view
Khaled A. Ahmed1, 2, *, Sekaran Muniandy1, and Ikram S. Ismail3
1Department of Molecular Medicine, Faculty of Medicine, University of Malaya, 50603 Kuala Lumpur, Malaysia.
2Faculty of Dentistry, Ibb University, P.O.Box 70627, Ibb, Yemen.
3Department of Medicine, University of Malaya Medical Center, University of Malaya, 50603 Kuala Lumpur, Malaysia.
- *Corresponding Author:
- Khaled A. Ahmed
Department of Molecular Medicine
Faculty of Medicine, University of Malaya
50603 Kuala Lumpur
Malaysia
Tel: +603 7697 4717
Fax: +603 7967 4957
E-mail: khaaah@gmail.com
Accepted date: December 03 2009
Diabetes mellitus (DM) represents a range of metabolic disorders characterized by hypergly-cemia resulting from insulin deficiency or insulin resistance or both. Hyperglycemia, the pri-mary clinical manifestation of diabetes, is strongly associated with development of the diabetic complications. Complications caused by hyperglycaemia involve damage to the small vessels such as in neuropathy, nephropathy, and retinopathy, and large blood vessels as in cardiovas-cular diseases. It is well known established that in diabetes, long-term complications ensue from abnormal regulation of glucose metabolism. In fact, all manifestations of cardiovascular disease, coronary heart disease, stroke and peripheral vascular disease are substantially more common in patients with type 2 diabetes than in non-diabetic individuals. For example, pa-tients with type 2 diabetes (T2DM) have a two- to fourfold increased risk of fatal and non-fatal coronary events. Diabetes can lead to microvascular and macrovascular damage through a number of mechanisms, each of which may worsen or accelerate the others. The present re-view summarizes the information on the mechanisms of how vascular complications will de-velop in type 2 diabetes and this might be useful as a direction for further research to provide new strategies for prevention and treatment of these complications in their early stages.
Key Words
Diabetes mellitus, metabolic disorders, hyperglycemia, neuropathy, nephropathy
Introduction
The term diabetes mellitus describes a metabolic disorder of multiple etiologies characterized by chronic hyperglycemia with disturbances of carbohydrate, fat, and protein metabolism resulting from defects in insulin secretion and/or insulin action. Diabetes can be classified into two major classes: type 1 diabetes (T1DM) and type 2 diabetes (T2DM). T1DM is the classical form of diabetes and these subjects cannot survive without insulin treatment. T2DM is a group of genetically determined diseases which may be controlled by diet, hypoglycemic agents and/or exogenous insulin [1]. T2DM is mainly characterized by insulin resistance, but impairment in insulin secretion also occurs in type 2 diabetes [2,3].
Subjects with T2DM cannot compensate for insulin resistance at hyperglycemic levels by increasing insulin secretion [1]. Several human monogenic forms of diabetes have been identified including: maturityonset diabetes of the young (MODY) [4], which can be caused by muta-tions in the glucokinase gene. MODY is characterized by β-cells dysfunction and young age at diagnosis, usually less than 25 years, leading to early-onset T2DM. In addition, other minor classes include gestational diabetes, extreme insulin resistance caused by a defective insulin receptor gene, the diabetes-deafness and optic atrophy syndrome which is due to defects in mitochondrial genes [5], and latent autoimmune diabetes in adults (LADA) [6,7] which was introduced to define adult diabetic patients who initially present as type 2 diabetics but with immune markers of type 1 diabetes which, in a number of cases, progress to insulin dependency. However, LADA more closely resembles and shares common characteristics of T1DM including genetics, metabolic dysfunction, and autoimmune features, but LADA does not affect children and is classified distinctly as being separate from juvenile diabetes. T1DM and the minor classes of diabetes will not be discussed any further in this review.
Type 2 diabetes mellitus
Epidemiology
It is well established that the most common form of diabetes is type 2 diabetes. The global rise in diabetes [8, 9] occurs because of population growth and ageing, and because of increasing trends towards an unhealthy diet, obesity, and sedentary lifestyles [10]. Type 2 diabetes represents about 85% to 95% of the people with diabetes in developed countries and an even higher percentage in developing countries [11]. Amos et al. estimated that there were 124 million persons with diabetes in the world in 1997 and predicted this number would grow to 221 million in 2010 [12]. Another study group estimated that the number of persons with diabetes was 150 million in 2000 and this number is expected to double by 2025 [13]. In 2003, it was estimated that approximately 194 million people worldwide, or 5.1% in the age group 20-79, have diabetes.
The largest increase in the prevalence numbers is thought likely to appear in India, China and other developing countries. This estimate is expected to increase to 6.3% in the adult population, by 2025. In the United States, the National Health and Nutrition Examination Surveys (NHANES) I and II showed that the prevalence of DM between 1976 and 1994 among American adults increased from 6.6% to 7.8% [14]. Although the absolute increase is relatively small, when the U.S. population growth during this period is considered, the number of patients with DM almost doubled from an estimated 8 million to 15.6 million people. Similar pictures have been observed in Europe, in which DM affects about 8.5% of the adult population [15]. The European Region with 48 million and Western Pacific Region with 43 million currently have the highest number of people with diabetes. However, the prevalence rate of 3.1% for the Western Pacific Region is significantly lower than 7.9% in the North American Region and 7.8% in the European Region. By 2025, the region with the greatest number of persons with diabetes is expected to change to the South-East Asian Region with about 82 million. The region’s prevalence of 7.5% will however continue to be lower than that of North America, estimated at 9.7%, and Europe at 9.1%. [16].
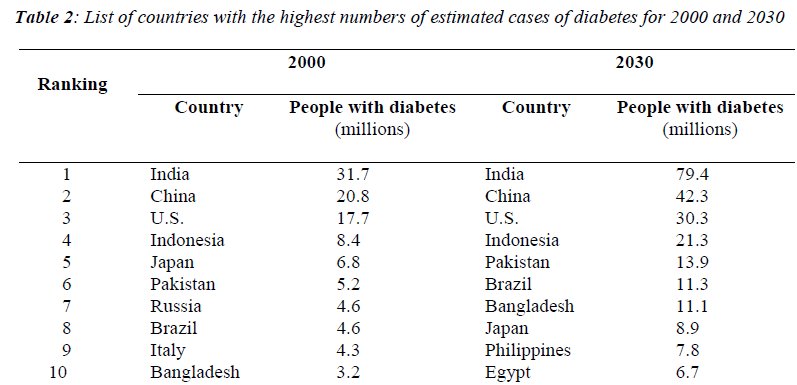
Gu et al. conducted a national study to investigate the prevalence of diabetes in 15,540 adults from 31 provinces in China. The authors reported that in China three out of four individuals with diabetes were undiagnosed and the prevalence of diabetes was 5.5% among the population aged 35 to 74 years in 2000. In addition, the age-standardized prevalence of diabetes of the population aged 35 to 74 living in urban area (7.8%) was higher than that of those living in rural area (5.1%) [17]. In Sweden, about 50,000 people are diagnosed with type 2 diabetes every year, with a prevalence of 4% or more [18]. Table 1 showed the 10 countries estimated to have the highest numbers of people with diabetes in 2000 and 2030 [19]. The 40-59 age group currently has the greatest number of persons with diabetes. By 2025, because of the aging of the world’s population, there will be 146 million aged 40-59 and 147 million aged 60 or older with diabetes.
Pathogenesis and major risk factors
Impaired insulin action and impaired pancreatic insulin secretion represent the principal pathophysiological abnormalities leading to increase blood glucose levels [20,21]. These are present to varying degrees in almost all patients with the common form of T2DM. Insulin resistance is a common pathologic state in which target cells fail to respond to the physiological effects of insulin occurring in peripheral organs and leading to abnormalities in glucose, lipid and protein metabolism [22]. When the target tissue does not respond to even high levels of insulin, glucose builds up in the blood resulting in high blood glucose or type 2 diabetes. In fact, insulin resistance is present in the majority of patients with impaired glucose tolerance or T2DM, and it is also found in up to 25 % of the general, apparently healthy population [23].
In response to elevated blood glucose concentration due to insulin resistance, pancreatic β-cells need to increase the insulin secretion to maintain homeostasis in glucose levels. Finally, β-cells become unresponsive to glucose due to pancreatic β-cells dysfunction and eventually type 2 diabetes develops. Although the etiology of the β-cell dysfunction of diabetes is incompletely understood, it is thought to result from both genetic and environmental factors [24,25]. Current study provided evidence to emphasis that both insulin resistance and β-cell dysfunction are associated processes and that β-cell dysfunction must be present for even minimal increases in blood glucose [26]. Family history, diet, and lack of physical activity are all major risk factors for developing T2DM. Dyslipidemia and high blood pressure are other risk factors that often appear before the clinical disease is evident [27]. Elevated levels of free fatty acids are also strong predictor of diabetes and correlate with hepatic glucose output, a major cause of diabetic hyperglycemia [28], high glucose levels, and obesity.
problems associated with CVD are severe in all parts of the world; however, the manifestations vary between different countries. In China, Japan and many Africans coun-tries for example, stroke is more common than coronary heart disease whereas, among Caucasian populations, coronary heart disease is more common. In some developed nations, such as the USA, Australia and Europe, where coronary heart disease rates were previously very high, mortality has fallen in recent decades [34]. However, in other areas such as Eastern Europe and the Middle East, the opposite is true. The “top ten” countries for both coronary and cerebrovascular disease mortality rates are now mainly from Eastern Europe and the former Soviet Union.
The clinical manifestations of CVD include coronary artery disease (CAD), cerebrovascular disease, and peripheral vascular disease. The underlying disease mechanism is accelerated atherosclerosis. The atherosclerotic process starts from fatty streaks, consisting of intimal deposits of lipids and macrophages with lipid droplets (foam cells), gradually developing into more advanced plaques. The process ends up in complicated atherosclerotic lesions, which through a plaque rupture and thrombosis can cause an acute myocardial infarction [35].
Type 2 diabetes and macrovascular complica-tions
Macrovascular complications of DM are due to accelerated atherosclerosis and have an important role in the increased morbidity and mortality suffered by these individuals [36]. The mechanism behind the relation between diabetes and atherosclerosis is not fully understood. Oxidative stress caused by production of reactive oxygen species (ROS) has been proposed as the main cause underlying insulin resistance, type 2 diabetes and vascular complications [37]. Additionally, both impaired glucose metabolism [38] and diabetic dyslipidemia [39] might contribute to the atherosclerotic process. Patients with T2DM are more at risk to develop macrovascular disease, both at an earlier age and in a higher frequency as compared to the general non-diabetic population.
The severity of cardiovascular complications in diabetes is demonstrated by the statistic that diabetics are 2 to 4 times more likely to have a stroke or die of heart disease than non-diabetics. Cardiovascular disease accounts for about 70% of all deaths in patients with diabetes [40]. It has been established in a more recent report that heart disease is the leading cause of diabetes-related deaths in the United States alone [41]. The presence of diabetes, in addition to any or all the other risk factors (such as smoking, hypertension, dyslipidemia, and genetic factors), approximately doubles the probability of developing macrovascular diseases and the available evidence suggests that strict diabetic control does not prevent or delay these complications [42]. Cardiovascular complications are often present already at the time of diagnosis of T2DM and subjects with impaired glucose tolerance (IGT) have an approximately twofold increase in the risk of macrovascular diseases [43]. Epidemiological studies have shown that the risk of cardiovascular mortality is two to three times higher in men and three to five times higher in women with diabetes than in non-diabetic subjects [44]. Ryden et al. showed that there is a steep rise in diabetes prevalence after the age of 50 in men and after the age of 60 in women [45].
Pathogenesis and major risk factors of diabetic complications
Hyperglycemia
A strong consistent relationship has been postulated between hyperglycemia and the incidence and progression of micro- and macrovascular complications in people with diabetes [46]. Studies on nondiabetic subjects have observed that even slightly elevated serum glucose concentrations increase risk for cardiovascular disease [47]. Epidemiological data have revealed hyperglycemia to be a major player in the development of the macrovascular complications such as CAD and stroke [41]. Prospective clinical studies in T2DM patients have shown an association between level of hyperglycemia and increased risk for mortality due to macrovascular disease [48,49]. The San Antonio Heart Study demonstrated that hyperglyce mia is a risk factor not only in Caucasians, but also in other ethnic groups [50]. The data of the UK prospective diabetes study (UKPDS) suggest that any improvement in glycemic control among patients with T2DM is likely to reduce the risk of diabetic complications [51].
Protein kinase C
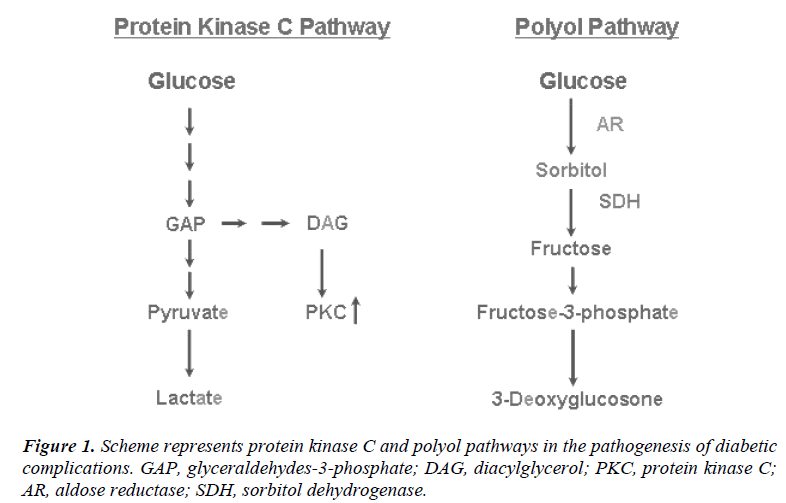
Protein kinase C (PKC) is a family of serine-threonine kinases that plays an important role in signal transduction mechanisms [52]. The PKC pathway is activated in diabetes as a result of hyperglycemia (Fig. 1). In this pathway, PKC is activated by the increased amounts of diacylglycerol (DAG), which are synthesized directly from glycolytic intermediates such as dihydroxyacetone phosphate and glyceraldehyde-3-phosphate [53]. It is possible that also advanced glycation end products and oxidants increase formation of DAG and activate PKC [54]. PKC appears to be activated in a range of diabetic tissues including the retina, kidney, heart, and aorta [55]. An activation of PKC has been implicated in many processes relevant to diabetic complications, including regulation of vascular permeability and flow, increased production of cytokines, and increased synthesis of basement membranes [56]. In diabetes microvascular complications, for example, PKC affects the activation of a number of growth factors and changes the function of vasoactive factors. These vasoactive factors include vasodilators such as nitric oxide (NO) as well as vasoconstrictors such as angiotensin II and endothelin-1 [57, 58].
Polyol pathway
Only a small proportion of glucose is metabolized to sorbitol during normoglycemia, while in hyperglycemia the enzyme aldose reductase is activated, leading to an accumulation of intracellular sorbitol and fructose that increases the flux through the polyol pathway [59]. Sorbitols and other polyols accumulate intracellularily, leading to osmotic damage and swelling. Aldose reductase (AR) is the first and ratelimiting enzyme of the polyol pathway, which converts monosaccharides (e.g. glucose) to their polyols or sugar alcohols (e.g. sorbitol) as seen in Fig. 1. This enzyme is widely distributed throughout the body, including those tissues that are susceptible to chronic diabetic complications (e.g. retina, lens, cornea, glomerulus, nervous system and the blood vessels) [60, 61]. In fact, alterations in sorbitol and fructose metabolism are implicated as factors contributing to vascular complications in diabetes mellitus [60].
Advanced glycation end products
Non-enzymatic glycation has recently attracted increasing interest as a crucial pathophysiologic event behind hyperglycemiarelated alterations and in the pathophysiology of the development of diabetic complications. Proteins and lipids exposed to aldose sugars go through reactions, which ultimately lead to the formation of advanced glycation end products (AGEs), a heterogeneous mixture of complex structures [62]. Although the series of reactions producing AGEs occurs at a very slow rate under normal circumstances, in diabetes their formation is accelerated to an extent related to the level and duration of hyperglycemia [63,64].
The potential pathophysiological significance of AGEs is associated with their accumulation in plasma, cells and tissues and their contribution to the formation of cross-links, generation of reactive oxygen intermediates, and interactions with particular receptors on cellular surfaces [65]. It has been reported by Cipollone and coworkers that advanced glycation end products might contribute to the atherosclerotic process seen in T2DM [66]. More details about AGEs and the well-characterized form of AGEs; Nε-(carboxymethyl)lysine were discussed by Ahmed et al. [67].
Genetic factors
Despite the crucial role of hyperglycemia on diabetic complications, other factors such as hypertension, obesity, and hyperlipidemia undoubtedly contribute. There are patients with longstanding hyperglycemia without complications and patients with short duration of disease who seem to be very prone to complications. Genetic factors are obviously important as prevalence is affected by the background population. Studies using several techniques including twin studies and diabetic animals have revealed definitive genetic predispositions to the development of diabetes [68,69] and these predispositions might be dependent on environmental stimulants.
An important variability in the incidence of diabetes-derived chronic complications exists, which may indicate the existence of a predetermined genetic susceptibility in its onset. The association between obesity and T2DM is well known and studies have started to focus on the biological basis for this link. In fact, about 60-90% of T2DM patients are overweight or obese thereby providing ample support for the term “diabesity” used to describe obesityrelated T2DM [70,71]. A strong support for the presence of an underlying genetic predisposition in those obese individuals who progress to T2DM has been provided by fact that while most T2DM patients are obese, most obese patients not necessary to have overt T2DM.
Type 2 diabetes and microvascular complica-tions
Diabetic retinopathy
Diabetes results in characteristic lesions in the retinal blood vessels. Diabetic retinopathy is still the most common cause of acquired blindness in the Western world [72]. Its prevalence increases most steeply between 5 to 15 years of diabetes duration, being about 60% after 20 years in the European population. This can result in for-mation of microaneurysms (minimal retinopathy), haemorrhages and increased leakage, causing retinal edema and lipid exudates (background retinopathy). When pathological development of new vessels in the retina or abnormal blood vessels and fibrous tissue (i.e. neovascularisation) occurs, the retinopathy is classified as proliferative retinopathy [73]. The formation of fibrous tissue may eventually cause retinal detachment and severe visual impairment [74]. Also, an excess of glucose activates the polyol pathway, which causes accumulation of sorbitol in the lens and is accompanied by cataracts [75]. The etiology of retinopathy includes hyperglycemiaassociated biochemical, anatomical, and functional changes.
Diabetic nephropathy
Diabetic nephropathy is estimated to develop in one third of both main types of diabetes [76]. Nephropathy is characterized by glomerular basement membrane thickening and arteriosclerosis of small arterioles. The hallmark of renal damage in diabetes is increased excretion of albumin in the urine. The natural history of diabetic nephropathy has been viewed as a descending path from normoalbuminuria to microalbuminuria, clinically overt diabetic nephropathy; i.e macroalbuminuria, and eventually to endstage renal disease. The term microalbu-miuria; i.e. incipient diabetic nephropathy, has been defined as urine albumin excretion rate 20-200 μg/min in a timed overnight or 30-300mg/24h urine collection [77] as determined by sensitive laboratory measurements. Urine albumin excretion rate exceeding these values is called macroalbuminuria and considered a sign of manifest diabetic nephropathy. It has been estimated that approximately half the patients with microalbuminuria will progress to overt nephropathy [78]. In fact, most of the hemodialysis patients and the patients receiving renal transplants have diabetes [79].
Diabetic neuropathy
The term diabetic neuropathy includes either a clinical or subclinical disorder without any additional causes of peripheral neuropathy other than diabetes. In fact, damage to the microvasculature in peripheral nerves is now becoming recognized as a major pathogenic factor in diabetic neuropathy [80]. It may affect both sensory and autonomic nerves, but distal symmetric polyneuropathy is probably the most common consequence which, together with peripheral vascular disease, is an important etiologic factor for foot ulcerations and lower limb amputations. Autonomic dysfunction is common in people with diabetes, but is only clinically apparent in a small percentage.
Diabetic neuropathy is encountered in about half of all people with diabetes either as a polyneuropathy or mononeuropathy [81] especially in patients over 60 years age with T2DM [82]. Although exact prevalence depends on the diagnostic criteria used to identify neuropathy, most studies suggest that 50% of patients with a 20-years history of either type 1 or type 2 diabetes have neuropathy [83,84]. Around 10% of these cases of neuropathy are associated with abnormal sensations and pain [85]. The incidence of neuropathy increases with duration of diabetes and is accelerated by poor control [86]. Additionally, the death rate is as high as 50% at three years after diagnosis of overt autonomic neuropathy [82].
How big is the problem?
Normally properly treated diabetes is symptomless, but continuing hyperglycemia seen in type 2 diabetes can give rise to chronic complications [87] including retinopathy, neuropathy and nephropathy [88], and macro-vascular complications [89]. A common denominator for all microvascular and macrovascular complications is extensive vascular damage. Both of these conditions are life threatening and may result in an altered mental state, loss of consciousness, and possibly death; therefore prompt medical attention is necessary to avoid adverse outcomes. Microvascular complications comprise changes in the small blood vessels of the eye that result in diabetic retinopathy, in the peripheral nerves, causing neuropathy, and finally in the kidney, causing diabetic nephropathy. As a result, diabetes is the most common cause of blindness, endstage renal disease [90], and limb amputation [91].
In macrovascular complications, accelerated atherosclerosis results in cardiovascular disease (CVD) such as coronary heart disease (CHD) and acute myocardial infarction (AMI). Through its effects on cardiovascular disease (70-80% of people with diabetes die of cardiovascular disease), diabetes is also now one of the leading causes of death. While the pathogenesis of these complications has been extensively studied for the past 50 years, no single etiology exists to explain all types of complications. Instead, multiple etiologies exist that are specific to each. The cost to care for patients with DM in the U.S. was approximately $132 billion. Of those costs, $40 billion was indirect medical expenses (disability, work loss, and premature deaths), and $92 billion dollars was direct medical expenses (those attributable to the disease itself, i.e. microvascular and macrovascular complications) [92]. In fact, approximately 25% of the total Medicare budget is used for the treatment of DM and its complications [93, 94].
In Africa, the burden of diabetes cost is huge and depending upon the individual and the family. It has been established that 50% of diabetes care is paid by the patients, 44% by the family, 2% by the employer, 2% charities and others, and only 2% by the government [95]. Therefore, recommendations founded on the results of Guerci et al. study showing benefits of intensive glycemic control [96]. Thus, development of tools and models for diabetes health care could potentially result in a substantial decrease in diabetes-associated vascular complications.
References
- Groop LC, Tuomi T. Non-insulin-dependent diabetes mellitus- a collision between thrifty genes and an affluent society. Ann Med 1997; 29: 37-53.
- Weyer C, Bogardus C, Mott DM, et al. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest 1999; 104: 787-794.
- Lebovitz HE. Diagnosis, classification, and pathogenesis of diabetes mellitus. J Clin Psychiatry 2001; 62 (Suppl 27): 5-9.
- Owen K, Hattersley AT. Maturityonset diabetes of the young: from clinical description to molecular genetic characterization. Best Pract Res Clin Endocrinol Metab 2001; 15: 309-323.
- Kahn CR, Vicent D, Doria A. Genetic of noninsulin-dependant (Type-II) diabetes mellitus. Ann Rev Med 1996; 47: 509-531.
- Tuomi T, Carlsson AL, Li H, et al. Clinical and genetic characteristics of type 2 diabetes with and without GAD antibodies. Diabetes 1999; 48: 150-157.
- Pozzilli P, Di Mario U. Autoimmune diabetes not requiring insulin at diagnosis (latent autoimmune diabetes of the adult): definition, characterization, and potential prevention. Diabetes Care 2001; 24: 1460-1467.
- Bagust A, Hopkinson PK, Maslove L, Currie CJ. The projected health care burden of Type 2 diabetes in the UK from 2000 to 2060. Diabet Med 2002; 19: 1-5.
- Motala AA, Pirie FJ, Gouws E, Amod A, Omar MK. High incidence of Type 2 diabetes mellitus in South African Indians: a 10-year follow-up study. Diabet Med 2003; 20: 23-30.
- Harris MI, Flegal KM, Cowie CC, et al. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults. The Third National Health and Nutrition Examination Survey, 1988-1994. Diabetes Care 1998; 21: 518-524.
- International Diabetes Federation [IDF]. Diabetes e-Atlas. Retrieved June 20, 2005, from http://www.-eatlas.idf.org.
- Amos AF, McCarty DJ, Zimmet P. The rising global burden of diabetes and its complications:estimates and projections to the year 2010. Diabet Med 1997; 14 (Suppl 5): S1-S85.
- King H, Aubert RE, Herman WH. Global burden of diabetes, 1995-2025: prevalence, numerical estimates, and projections. Diabetes Care 1998; 21: 1414-1431.
- Harris MI, Flegal KM, Cowie CC, et al. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults. The Third National Health and Nutrition Examination Survey, 1988-1994. Diabetes Care 1998; 21: 518-524.
- The Diabetes Epidemiology: Collaborative analysis Of Diagnostic criteria in Europe (DECODE) Study Group. Will new diagnostic criteria for diabetes mellitus change phenotype of patients with diabetes? Reanalysis of European epidemiological data. BMJ 1998; 317: 371-375.
- Sicree R, Shaw J, Zimmet P. The global burden of diabetes, Diabetes and impaired glucose tolerance: prevalence and projections. In: Gan D eds. Diabetes Atlas. 2nd ed. International Diabetes Federation, Brussels 2003; pp 15-71.
- Gu D, Reynolds K, Duan X, et al. Prevalence of diabetes and impaired fasting glucose in the Chinese adult population : International collaborative study of cardiovascular disease in Asia (InterASIA). D iabetologia 2003; 46: 1190-1198.18
- Farnkvist LM, Lundman BM. Outcomes of diabetes care: a populationbased study. Int J Qual Health Care 2003; 15: 301-317.
- Wild S, Roglic G, Green A, et al. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004; 27: 1047-1053.
- Taylor SI, Accili D, Imai Y. Insulin resistance or insulin deficiency. Which is the primary cause of NIDDM? Diabetes 1994; 43: 735-740.
- Kahn BB, Rossetti L. Type 2 diabetes who is conducting the orchestra? Nat Genet 1998; 20: 223-225.
- Kahn CR. Banting lecture. Insulin action, diabetogenes, and the cause of type II diabetes. Diabetes 1994; 43: 1066-1084.
- Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes 1988; 37: 1595-1607.
- Kahn SE, Porte D. ß-cell dysfunction in type 2 diabetes: pathophysiological and genetic bases. In: Scriver CR, Beaud et al., Sly WS, Valle D eds. The metabolic and molecular bases of inherited disease. McGrawHill, New York 2001; pp 1407-1431.
- Eriksson J, Lindstrom J, Tuomiletho J. Potential for the prevention of type 2 diabetes. Br Med Bul 2001; 60: 183-199.
- Leahy JL. Pathogenesis of type 2 diabetes mellitus. Arch Med Res 2005; 36: 197-209.
- Groop L, Forsblom C, Lehtovirta M. Characterization of the Prediabetic State. Am J Hypertension 1997; 10: 172S-180S.
- Bergman RN, Ader M. Free Fatty Acids and Pathogenesis of Type 2 Diabetes Mellitus. Trends Endocrinol Metab 2000; 11: 362-368.
- Hauner H. Insulin resistance and the metabolic syndromea challenge of the new millenium. Eur J Clin Nutr 2002; 56 (Suppl 1): S25-S29.
- Bonora E, Kiechl S, Willeit J, et al. Prevalence of insulin resistance in metabolic disorders. The Bruneck Study. Diabetes 1998; 47: 1643-1649.
- Rantala AO, Kauma H, Lilja M, et al. Prevalence of the metabolic syndrome in drugtreated hypertensive patients and control subjects. J Intern Med 1999; 45: 163-174.
- Alberti KGM, Zimmet PZ for the WHO Consultation. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus, provisional report of a WHO consultation. Diabet Med 1998; 15: 539-553.
- Thom T, Haase N, Rosamond W, et al. Heart Disease and Stroke Statistics-2006 Update: A Report From the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2006; 113: e85-e151.
- International Diabetes Federation. Diabetes and Cardiovascular Disease: Time to Act. Brussels: International Diabetes Federation 2001.
- Stary HC, Chandler AB, Dinsmore RE, et al. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis: A report from the Committee on Vascular Lesions of the Council on Atherosclerosis, American Heart Association. Circulation 1995; 92: 1355-1374.
- Wingard DL, Barrett-Connor E. Heart disease and diabetes. In: Harris M eds. Diabetes in America. 2nd ed. Bethesda, NIH NIDDK 1995; pp 429–456.
- Ceriello A, Motz E. Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease? The common soil hypothesis revisited. Arterioscler Thromb Vasc Biol. 2004; 24: 816-823.
- Bansilal S, Farkouh M, Fuster V. Role of insulin resistance and hyperglycemia in the development of atherosclerosis. Am J Cardiol 2007; 99(4A): 6B-14B.
- Haffner SM. Insulin resistance, inflammation, and the prediabetic state. Am J Cardiol 2003; 92(4A): 18J-26J.
- Laakso M. Hyperglycemia and cardiovascular disease in type 2 diabetes. Diabetes 1999; 48: 937-942.
- Centers for Disease Control and Prevention. National diabetes fact sheet: general information and national estimates on diabetes in the United States, 2005: Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2005.
- Davidson MB. Diabetes mellitus: diagnosis and treatment. W.B. Saunders Co., Los Angeles, California 1998.
- Pyorala K, Laakso M, Uusitupa M. Diabetes and atherosclerosis: an epidemiologic view. Diabetes Metab Rev 1987; 3: 463-524.
- Barrett-Connor EL, Cohn BA, Wingard DL, et al. Why is diabetes mellitus a stronger risk factor for fatal ischemic heart disease in women than in men? The Rancho Bernardo Study. JAMA 1991; 265: 627-631.
- Ryden L, Standl E, Bartnik M, et al. Guidelines on diabetes, prediabetes, and cardiovascular diseases: executive summary. The Task Force on Diabetes and Cardiovascular Diseases of the European Society of Cardiology (ESC) and of the European Association for the Study of Diabetes (EASD). Eur Heart J 2007; 28: 88-136.
- Hanssen KF. Blood glucose control and microvascular and macrovascular complications in diabetes. Diabetes 1997; 46 (Suppl.2): S101-S103.
- Laakso M. Cardiovascular disease and diabetes mellitus. Eur Heart J 2000; 2 (Suppl D): D26-D28.
- Standl E, Balletshofer B, Dahl B, et al. Predictors of 10-year macrovascular and overall mortality in patients with NIDDM: the Munich General Practitioner Project. Diabetologia 1996; 39: 1540-1545.
- Lehto S, Ronnemaa T, Haffner SM, et al. Dyslipidemia and hyperglycemia predict coronary heart disease events in middleaged patients with NIDDM. Diabetes 1997; 46: 1354-1359.
- Wei M, Gaskill SP, Haffner SM, et al. Effects of diabetes and level of glycemia on allcause and cardiovascular mortality. The San Antonio Heart Study. Diabetes Care 1998; 21: 1167-1172.
- Stratton IM, Adler AI, Neil HAW, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000; 321: 405-12.
- Ron D, Kazanietz MG. New insights into the regulation of protein kinase C and novel phorbol ester receptors. FASEB J 1999; 13: 1658-76.
- King GL, Ishii H, Koya D. Diabetic vascular dysfunctions: a model of excessive activation of protein kinase C. Kidney Int Suppl 1997; 60: S77-S85.
- Koya D, King GL. Protein kinase C activation and the development of diabetic complications. Diabetes 1998; 47: 859-866.
- Inoguchi T, Battan R, Handler E, et al. Preferential elevation of protein kinase C isoform beta II and diacylglycerol levels in the aorta and heart of diabetic rats: Differential reversibility to glycemic control by islet cell transplantation. Proc Natl Acad Sci USA 1992; 89: 11059-11063.
- Giardino I, Brownlee M. The biochemical basis of microvascular disease. In: Pickup J, Williams G eds. Textbook of diabetes. Blackwell Science, Oxford 1997; pp 42.1-42.16.
- Takagi C, King GL, Takagi H, et al. Endothelin-1 action via endothelin receptors is a primary mechanism modulating retinal circulatory response to hyperoxia. Invest Ophthalmol Vis Sci 1996; 37: 2099-2109.
- Candido R, Allen TJ. Haemodynamics in microvascular complications in type 1 diabetes. Diabetes Metab Res Rev 2002; 18: 286-304.
- Hawthorne GC, Bartlett K, Hetherington CS, et al. The effect of high glucose on polyol pathway activity and myoinositol metabolism in cultured human endothelial cells. Diabetologia 1989; 32: 163-66.
- Greene DA, Lattimer SA, Sima AF. Sorbitol, phosphoinositides, and sodium-potassium-ATPase in the pathogenesis of diabetic complications. N Engl J Med 1987; 316: 599-606.
- Harrison HE, Stribling D, Armstrong FM, et al. Aldose reductase in the etiology of diabetic complications: I. Introduction. J Diabetes Complicat 1989; 3: 6-11.
- Harding JJ. Nonenzymatic covalent posttranslational modification of proteins in vivo. Adv Protein Chem 1985; 37: 247-334.
- Vlassara H, Bucala R, Striker L. Biology of disease. Pathogenic effects of advanced glycosylation: bio-chemical, biologic, and clinical implications for diabetes and aging. Lab Invest 1994; 70: 138-151.
- Vlassara H. Recent progress in advanced glycation end products and diabetic complications. Diabetes 1997; 46 (Suppl 2): S19-S25.
- Schmidt AM, Hori O, Cao R, et al. RAGE. A novel cellular receptor for advanced glycation end products. Diabetes 1996; 45 (Suppl 3): S77–S80.
- Cipollone F, Iezzi A, Fazia M, et al. The receptor RAGE as a progression factor amplifying arachidonate-dependent inflammatory and proteolytic response in human atherosclerotic plaques: role of glycemic control. Circulation 2003; 108: 1070-1077.
- Ahmed KA, Muniandy S, Ismail IS. Ne-(Carboxymethyl)lysine and Coronary Atherosclerosis-Associated Low Density Lipoprotein Abnormalities in Type 2 Diabetes: Current Status. J Clin Biochem Nutr 2009; 44: 14-27.
- Beck-Nielsen H, Vaag A, Poulsen P, Gaster M. Metabolic and genetic influence on glucose metabolism in type 2 diabetic subjects-experiences from relatives and twin studies. Best Pract Res Clin Endocrinol Metab 2003; 17: 445-467.
- Chen D, Wang MW. Development and application of rodent models for type 2 diabetes. Diabetes Obes Metab 2005; 7: 307-317.
- Astrup A, Finer N. Redefining type 2 diabetes: 'diabesity' or 'obesity dependent diabetes mellitus'? Obes Rev 2000; 1: 57-59.
- Felber JP, Golay A. Pathways from obesity to diabetes. Int J Obes Relat Metab Disord 2002; 26 (Suppl 2): S39-S45.
- Klein R, Klein BE, Moss SE. Epidemiology of proliferative diabetic retinopathy. Diabetes Care 1992; 15: 1875-91.
- Aiello LP, Gardner TW, King GL, et al. Diabetic retinopathy. Diabetes Care 1998; 21: 143-56.
- Forrester JV, Knott RM. Pathogenesis of diabetic retinopathy and cataract. In: Pickup J, Williams G eds. Textbook of Diabetes. Blackwell Science, Oxford 1997; pp 45.1 -45.19.
- American Diabetes Association. Diabetic retinopathy. Position Statement. Diabetes Care 1998; 21 (Suppl 1): S47-S49.
- O'Bryan GT, Hostetter TH. The renal hemodynamic basis of diabetic nephropathy. Semin Nephrol 1997; 17: 93-100.
- Mogensen CE, Chachati A, Christensen CK, et al. Microalbuminuria: an early marker of renal involvement in diabetes. Uremia Invest 1986; 9: 85-95.
- Krolewski AS, Warram JH, Freire MB. Epidemiology of late diabetic complications. A basis for the development and evaluation of preventive programs. Endocrinol Metab Clin North Am 1996; 25: 217-242.
- American Diabetes Association. Diabetic Nephropathy. Position Statement. Diabetes Care 1998; 21 (Suppl 1): S50-S53.
- Vinik AI, Holland MT, Le Beau JM, et al. Diabetic neuropathies. Diabetes Care 1992; 15: 1926 -75.
- Sheetz MJ, King GL. Molecular understanding of hyperglycemia.s adverse effects for diabetic complications. JAMA 2002; 288: 2579-2588.
- King’s Fund Policy Institute Report, Commissioned by the British Diabetic Association. Counting the cost: the real impact of non insulin dependent diabetes. London, King’s Fund 1996.
- Feldman EL, Stevens MJ, Russell JW, et al. Diabetic neuropathy. In: Becker KL ed. Principles and practice of endocrinology and metabolism. Baltimore, Lippincott Williams & Wilkins 2001; pp 1391-1399.
- Apfel SC. Nerve regeneration in diabetic neuropathy. Diabetes Obes Metab 1999; 1: 3-11.
- Calcutt NA. Potential mechanisms of neuropathic pain in diabetes. Int Rev Neurobiol 2002; 50: 205-228.
- Feldman EL, Stevens MJ, Russell JW. Diabetic peripheral and autonomic neuropathy. In: Sperling MA eds. Contemporary endocrinology. Totowa, N.J, Humana Press 2002; pp 437-461.
- United Kingdom Prospective Diabetes Study Group. Intensive bloodglucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352: 837-853.
- Vinik AI, Maser RE, Mitchell BD, et al. Diabetic autonomic neuropathy. Diabetes Care 2003; 26: 1553-1579.
- Pyorala K, Laakso M, Uusitupa M. Diabetes and atherosclerosis: an epidemiologic view. Diabetes Metab Rev 1987; 3: 463-524.
- Creager MA, Lüscher TF, Cosentino F, et al. Diabetes and vascular disease: Pathophysiology, clinical consequences, and medical therapy: Part I. Circulation 2003; 108:1527-1532.
- Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA 2002; 287: 2570-2581.
- Centers for Disease Control and Prevention. National diabetes fact sheet: general information and national estimates on diabetes in the United States, 2002. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention 2003.
- Finkelstein EA, Fiebelkorn IC, Wang G. National medical spending attributable to overweight and obesity: How much, and who's paying? Health Affairs 2003; W3: 219-226.
- Finkelstein EA, Fiebelkorn IC, Wang G. State-level estimates of annual medical expenditures attributable to obesity. Obesity Research 2004; 12: 18-24.
- Mbanya JC, Mbanya D. Diabetes cost in sub-Saharan Africa. J Cardiovasc Risk. 2003; 10: 191-193.
- Guerci B, Drouin P, Grange V, et al. Self-monitoring of blood glucose significantly improves metabolic control in patients with type 2 diabetes mellitus: the Auto-Surveillance Intervention Active (ASIA) study. Diabetes Metab 2003; 29: 587-594.