ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 10
TNF-α and IL-1β inhibitors, 3, 5-disubstituted-4, 5-dihydro-1H-pyrazoles
Musarat Amina1,2*, Satti NK1, Nawal M. Al Musayeib2, Bani S3 and Touseef Amna4
1Natural Products Chemistry Division, Indian Institute of Integrative Medicine (CSIR), Canal Road, Jammu Tawi, India
2Department of Pharmacognosy, College of Pharmacy, King Saud University, Riyadh, Saudi Arabia
3Cell Division, Indian Institute of Integrative Medicine (CSIR), Canal Road, Jammu Taw, India
4Department of Biology, Faculty of Science, Albaha University, Albaha, Kingdom of Saudi Arabia
- *Corresponding Author:
- Musarat Amina
Department of Pharmacognosy
College of Pharmacy, King Saud University, Saudi Arabia
Accepted date: February 8, 2017
A series of 3, 5-disubstituted-4, 5-dihydro-1H-pyrazoles have been synthesized under solvent free microwave irradiation method by the condensation of α, β-unsaturated ketones with hydrazine and its differently substituted derivatives. The chemical structures of the compounds were characterized by elemental analysis and spectroscopic data. All the synthetics were evaluated for their anti-inflammatory activity under in vivo conditions. The present study describes the potential of these pyrazole ring containing scaffolds to assess the TNF α (Tumor Necrosis Factor-alpha) and IL-1β (Interleukin-1 beta) inhibitory potential. TNF-α and IL-1β are inflammatory cytokines that are pro-inflammatory in nature and play a major role in inflammatory cascades of many pathologically dreadful diseases ranging from neurodegenerative disorders to autoimmune diseases such as rheumatoid arthritis.
Keywords
3, 5-disubstituted-4, 5-dihydro-1H-pyrazoles, Microwave irradiation, TNF-α, IL-1β, Anti-inflammatory
Introduction
Inflammation is the natural defense mechanism which keeps the body healthy and free of infections, but if go unchecked (chronic inflammation) can lead to various dreadful diseases ranging from neurodegenerative disorders to autoimmune diseases. The researchers during past decades have provided enough evidence in which cytokines have been implicated in diseases related to chronic inflammation and afterward several researches and scientists all over the world have targeted cytokines as a therapeutic target in autoimmune diseases such as rheumatoid arthritis [1]. Nowadays, the inhibition of cytokines production has also become a major focus of current drug development and therefore, an important method for evaluating bioactivity of drugs particularly in the field of inflammation [2,3].
On the other hand, during the last twenty years pyrazole ring has attracted continued attention, as it has become fairly accessible and has shown diverse biological attributes. Compounds with these ring systems have found to possess varied pharmacological activities such as anti-fungal [4], antibacterial [5,6], anti-inflammatory [7], anti-oxidant [8], anticonvulsant [9], anti-depressant [10], antiviral [11], anti-cancer ([12], anti-microbial [13], antitumor [14], anti-diabetic [15], anti-malarial [16], anesthetic, analgesic [17], anti-tuberculosis [18], pesticides and fungicide [19] activities. Apart from the aforementioned biological activities, pyrazoles and their reduced forms, pyrazolines are extensively useful synthons in organic synthesis [20] and serve as suitable building blocks to synthesize some other biologically active compounds such as natural products and constitutes a relevant synthetic target in the pharmaceutical industry.
A systematic investigation of this class of heterocyles revealed that many pyrazoline derivatives have found their clinical application as Non-Steroidal Anti-Inflammatory Drugs (NSAIDs). Antipyrine, 2, 3-dimethyl--phenyl-3-pyrazolin-5- one, was the first pyrazolone derivative used in the management of pain and inflammation. Phenyl butazone and its potent metabolite oxyphenbutazone, a prototype of pyrazolinedione NSAIDs, are potent anti-inflammatory agents [21]. Feprazone, the 4-(methyl butenyl)-analogue, is comparable to phenylbutazone in efficacy, but with fewer side effects on GI tract. Several related pyrazolidine-3, 5-diones, pyrazolin-3-ones and pyrazolin-5-ones are also available as NSAIDs; examples are felcobuzone, mefobutazone, morazone, famprofazone, and ramifenazone [22]. Besides these, pyrazoline derivatives such as N-acetyl-3, 5-diaryl-Δ2- pyrazolines [23], 1-acetyl-5-substituted aryl-3-(baminonaphthyl)- 2-pyrazolines [24], 3-(1-Acetyl-5-(phydroxyphenyl)- 2- pyrazolin-3-yl) indole, [25], bis (3-aryl-4, 5-dihydro-1H-pyrazol-1 carboxaldehydes) [26], 1- Thiocarbamoyl-3-substituted phenyl-5-(2-pyrrolyl)-4, 5- dihydro-(1H)-pyrazole [27], 5-(Substituted) aryl-3-(3- coumarinyl)-1-phenyl-2-pyrazolines [28], 5-(4 Fluorophenyl)-1-phenyl-3-(thiophen-2-yl)-4, 5-dihydro-1H pyrazole, 5-(4-Chlorophenyl)-1-phenyl-3-(thiophen-2-yl)-4, 5- dihydro-1H pyrazole, 3-(4-biphenyl)-5 substituted phenyl-2- pyrazolines, 1-benzoyl-3-(4-biphenyl)-5-substituted phenyl-2- pyrazolines [29], 1-((Benzoxazole/Benzimidazole-2-yl) thioacetyl) pyrazoline [30], 3, 2-(4, 5-dihydro-5-(4- morphilinophenyl)-1H-pyarazol-3-yl) phenols, Nphenylpyrazol- 1-carbothioamide [31], 1-(2’, 4’- Chloroacridine-9’-yl)-3-(5’-pyridine-4-yl)-(1, 3, 4-oxadiazol-2- ylthiomethyl)-pyrazole-5-one [32] and 1-substituted-5-aryl-3- (3-coumarinyl)-2-pyrazolines reported in the literature are found to possess good to excellent in vivo dose-dependent antiinflammatory activities [7]. Moreover, the recent success of pyrazole COX-2 inhibitor has further highlighted the importance of these heterocycles in medicinal chemistry.
Thus the enormous anti-inflammatory potential of pyrazolines and a plethora of biological activities as aforementioned prompted us to design an easy and convenient new synthetic route that will give high yield and rapid access to different pyrazolines to further explore in-depth anti-inflammatory activity associated with these heterocyles. Our present study reports specifically microwave-assisted synthesis of 3, 5- disubstituted-4, 5-dihydro-1H-pyrazoles via reaction of α, β- unsaturated ketones with hydrazine hydrate and its differently substituted methyl/phenyl derivatives under solvent free conditions, which have been found to possess an interesting profile of anti-inflammatory potential. All the synthetics were tested in vivo for their anti-inflammatory activity using carrageenan induced rat paw oedema model, the widely accepted acute model of inflammation [33]. Carrageenan induced oedema is a non-specific inflammation resulting from a complex of diverse mediators and since oedema of this type is highly sensitive to NSAIDs, carrageenan has been accepted as a useful agent for studying new anti-inflammatory drugs. Moreover our present findings also demonstrated the comparison between microwave and conventional method.
Materials and Methods
Chemistry
Melting points were measured on Buchi melting point B-545 and are uncorrected. Infrared spectrum was recorded on Hitachi 270-30 spectrophotometer in KBr pellets and values have been represented in cm-1. Mass spectra were recorded on JEOL JMS D-300 mass spectrometer at 70 ev. 1H and 13C NMR spectra were determined on Bruker DPX-200 spectrometer in CDCl3/CD3OD. Chemical shifts have shown in δ values (ppm) with Tetramethylsilane (TMS) as an internal reference. Elemental analytical data have been determined on Carlo Erba, Model 1106, elemental analyser. Column Chromatography (CC): silica gel (Merck, 60-120 mesh). Thin Layer Chromatography (TLC) cards from Merck (silica gel pre-coated aluminium cards with fluorescent indicator at 254 nm) were used for thin layer chromatography and TLC zones were visualized either by exposure to vanillin sulphuric acid, iodine vapour, or under UV light. Microwave irradiation was carried out in BPL BMC 900T commercial microwave oven operated at a frequency of 2450 MHz.
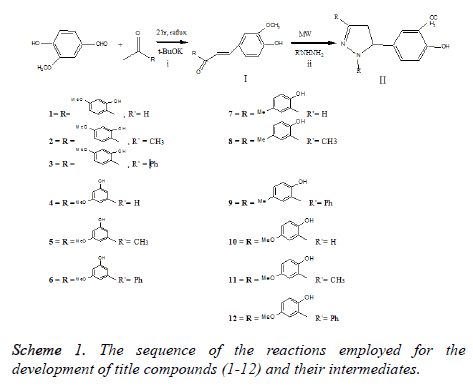
Synthesis and characterization of 3, 5-disubstituted-4, 5-dihydro-1H-pyrazoles (1-12)
Synthesis of α-β-unsaturated ketones (I): The α-β- unsaturated ketones were generated by Claisen-Schmidt condensation of vanillin with acetone or substituted acetophenones in presence of alkali following earlier established procedure [34] with suitable modifications.
Synthesis of 3, 5-disubstituted-4, 5-dihydro-1H-pyrazoles: The title compounds 3, 5-disubstituted-4, 5-dihydro-1Hpyrazoles II (1-12) were synthesized by cyclocondensation of α-β-unsaturated ketones I with hydrazine hydrate and its differently substituted methyl/phenyl derivatives. In a typical procedure, equimolar amounts of α-β-unsaturated ketones I and hydrazine hydrate substituted methyl/phenyl derivatives were taken in 100 ml of conical flask and mixed thoroughly. The reaction mixture was irradiated with microwaves (BPL BMC 900T commercial microwave) at 60% power (400 Watt) for 2-5 minutes, with short interruption of 10-20 sec to record the temperature and to monitor the reaction. Temperatures were recorded in the range of 50-68˚C. On completion of reaction (TLC), the reaction mixture was cooled to room temperature, acidified with dilute HCL. The product (3, 5-disubstituted-4, 5- dihydro-1H-pyrazoles) was separated, filtered, washed with cold water, dried and re-crystallized with ethanol. All the other compounds were synthesized (1-12) following the same procedure.
3-(2’-hydroxy-4’-methoxyphenyl)-5-(3’-methoxy-4- hydroxyphenyl)-4-5-dihydro-1H-pyrazole) (1): MS (m/z) [M +H]+: 315, calcd for C17H18N2O4; C, 65.20; H, 5.80; N, 8.85; Found: C, 64.96; H, 5.77; N, 8.91%.; 1HNMR (CDCl3): δ 3.12 (dd, 1H, J=4.42 Hz, Ha-4), 3,43 (dd, 1H, J=4.42 Hz, Hb-4), 3.93 (dd, 1H, J=4.45 Hz, H-5), 3.73 (S, 3H, -OCH3), 3.71 (S, 3H, -OCH3), 6.94 (m, 6H, Ar-H); 13CNMR: δ 146.43 (C-3), 40.69 (C-4), 74.71 (C-5), 114.53 (C-1’), 153.51 (C-2’), 108.47 (C-3’), 166.59 (C-4’), 113.92 (C-5’), 135.54 (C-6’), 128.56 (C-1”), 114.12 (C.2”), 148.31 (C-3”), 140.18 (C-4”), 116.32 (C-5”), 121.59 (C-6”), 55.96 (-OCH3), 55.35 (-OCH3).
3-(2’-hydroxy-4’-methoxyphenyl)-5-(3’-methoxy-4- hydroxyphenyl)-4-5-dihydro-1-methyl-pyrazole (2): MS (m/z) [M+H]+: 329, calcd for C18H20N2O4; C, 65.96; H, 6.14; N, 8.53; Found: C, 65.84; H, 6.14; N, 8.53; 1HNMR (CDCl3): δ 3.08 (dd, 1H, J=4.42 Hz and 4.42 Hz, Ha-4), 3,23 (dd, 1H, J=4.42 Hz and J=4.42 Hz, Hb-4), 3.54 (dd, 1H, J=4.45 Hz, H-5), 3.62 (S, 3H, -OCH3), 3.71 (S, 3H, -OCH3), 6.91 (m, 6H, Ar-H); 13CNMR: δ 146.51 (C-3), 40.52 (C-4), 72.82 (C-5), 116.12 (C-1’), 155.56 (C-2’), 109.12 (C-3’), 166.53 (C-4’), 113.64 (C-5’), 134.96 (C-6’), 128.51 (C-1”), 114.14 (C.2”), 148.43 (C-3”), 140.21 (C-4”), 116.30 (C-5”), 121.92 (C-6”), 55.98 (-OCH3), 55.39 (-OCH3), 43.58 (-NCH3).
3-(2’-hydroxy-4’-methoxyphenyl)-5-(3’-methoxy-4- hydroxyphenyl)-4-5-dihydro-1-phenyl-pyrazole (3): MS (m/z) [M+H]+: 391, calcd for C23H22N2O4; C, 70.86; H, 5.56; N, 7.09; Found: C, 70.75; H, 5.68; N, 7.17; 1HNMR (CDCl3): δ 3.10 (dd, 1H, J=4.42 Hz and J=4.42 Hz, Ha-4), 3, 41 (dd, 1H, J=4.42 Hz and J=4.42 Hz, Hb-4), 3.54 (dd, 1H, J=4.45 Hz, H-5), 3.91 (S, 3H, -OCH3), 3.73 (S, 3H, -OCH3), 6.12 (bs, 2H, H-3’, H-5’) 7.01 (m, 3H, H-6’, H-4”, H-5”), 6.12 (bs, 2H, H-3’, H-5), 6.92 (m, 2H, H-2”, H-6’”), 7.21 (m, 2H, H-3”, H-5”); 13CNMR: δ 146.32 (C-3), 40.39 (C-4), 76.71 (C-5), 115.01 (C-1’), 153.51 (C-2’), 108.52 (C-3’), 166.51 (C-4’), 113.19 (C-5’), 135.56 (C-6’), 128.53 (C-1”), 114.83 (C.2”), 148.90 (C-3”), 140.45 (C-4”), 116.31 (C-5”), 121.83 (C-6”), 143.74 (C-1’”), 113.17 (C-2’”), 129.93 (C-3’”), 116.62 (C-4’”), 129.93 (C-5’”), 113.17 (C-6’”), 55.9 (-OCH3), 55.39 (- OCH3).
3-(3’-hydroxy-5’-methoxyphenyl)-5-(3’-methoxy-4’- hydroxyphenyl)-4-5-dihydro-1H-pyrazole (4): MS (m/z) [M +H]+: 315, calcd for C17H18N2O4; C, 65.20; H, 5.80; N, 8.85; Found: C, 64.96; H, 5.77; N, 8.91; 1HNMR (CDCl3): δ 2.93 (dd, 1H, J=4.41 Hz and J=4.41 Hz, Ha-4), 3,48 (dd, 1H, J=4.41 Hz and J=4.41 Hz, Hb-4), 3.67 (dd, 1H, J=4.43 Hz, H-5), 3.80 (S, 3H, -OCH3), 3.93 (S, 3H, -OCH3), 6.84 (m, 6H, Ar-H); 13CNMR: δ 159.52 (C-3), 43.54 (C-4), 72.27 (C-5), 131.24 (C-1’), 101.23 (C-2’), 153.52 (C-3’), 114.30 (C-4’), 161.53 (C-5’), 120.95 (C-6’), 109.92 (C-1”), 128.16 (C.2”), 146.88 (C-3”), 145.51 (C-4”), 106.15 (C-5”), 109.17 (C-6”), 55.31 (- OCH3), 55.94 (-OCH3).
3-(3’-hydroxy-5’-methoxyphenyl)-5-(3’-methoxy-4’- hydroxyphenyl)-4-5-dihydro-1-methyl-pyrazole (5): MS (m/z) [M+H]+: 329, calcd for C18H20N2O4; C, 65.96; H, 6.14; N, 8.85; Found: C, 65.84; H, 6.14; N, 8.53; 1HNMR (CDCl3): δ 2.96 (dd, 1H, J=4.41 Hz and J=4.41 Hz, Ha-4), 3,48 (dd, 1H, J=4.41 Hz and J=4.41 Hz, Hb-4), 3.69 (dd, 1H, J=4.43 Hz, H-5), 3.84 (S, 3H, -OCH3), 3.93 (S, 3H, -OCH3), 2.78 (s, 3H, - NCH3), 5.64 (s, 1H, Ar-OH), 6.44 (dd, 1H, J=2.51 and 2.52 Hz H-4’), 6.71 (bs, 2H, H-2’, H-6’), 6.55 (d, 1H, H-2”), 7.03 (m, 2H, H-4”, H-5”) 13CNMR: δ 159.52 (C-3), 43.54 (C-4), 72.27 (C-5), 131.27 (C-1’), 101.28 (C-2’), 153.03 (C-3’), 114.27 (C-4’), 161.53 (C-5’), 120.96 (C-6’), 109.92 (C-1”), 128.18 (C. 2”), 146.87 (C-3”), 145.50 (C-4”), 106.16 (C-5”), 109.17 (C-6”), 55.33 (-OCH3), 55.95 (-OCH3), 41.89 (-NCH3).
3-(3’-hydroxy-5’-methoxyphenyl)-5-(3’-methoxy-4’- hydroxyphenyl)-4-5-dihydro-1-phenyl-pyrazole (6): MS (m/z) [M+H]+: 391, calcd for C23H22N2O4; C, 70.86; H, 5.56; N, 7.09; Found: C, 70.75; H, 5.56; N, 7.17; 1HNMR (CDCl3): δ 2.91 (dd, 1H, J=4.41 Hz and J=4.41 Hz, Ha-4), 3,48 (dd, 1H, J=4.41 Hz and J=4.41 Hz, Hb-4), 3.62 (dd, 1H, J=4.43 Hz, H-5), 3.82 (S, 3H, -OCH3), 3.93 (S, 3H, -OCH3), 5.64 (s, 1H, Ar-OH), 6.41 (dd, 1H, H-4’), 6.62 (bs, 2H, H-2’, H-6’), 6.43 (m, 1H, H-2”), 7.03 (m, 2H, H-4”, H-5”); 13CNMR: δ 159.32 (C-3), 43.52 (C-4), 72.17 (C-5), 131.48 (C-1’), 101.25 (C-2’), 153.58 (C-3’), 114.29 (C-4’), 161.50 (C-5’), 120.96 (C-6’), 109.91 (C-1”), 128.29 (C.2”), 146.74 (C-3”), 145.32 (C-4”), 106.15 (C-5”), 109.14 (C-6”), 144.01 (C-1’”), 112.23 (C-2’”), 129.13 (C-3’”), 116.90 (C-4’”), 129.13 (C-5’”), 112.23 (C-6’”), 55.31 (-OCH3), 55.94 (-OCH3), 41.89.
3-(2’-hydroxy-4’-methylyphenyl)-5-(3’-methoxy-4’- hydroxyphenyl)-4-5-dihydro-1H-pyrazole (7): MS (m/z) [M +H]+: 299, calcd for C17H18N2O3; C, 68.53; H, 6.12; N, 9.25; Found: C, 68.44; H, 6.08; N, 9.25; 1HNMR (CDCl3): δ 3.08 (dd, 1H, J=4.42 Hz and, J=4.42 Hz, Ha-4), 3, 51 (dd, 1H, J=4.42 Hz and, J=4.42 Hz, Hb-4), 3.95 (dd, 1H, J=4.45 Hz, H-5), 2.26 (S, 3H, -CH3), 3.86 (S, 3H, -OCH3), 6.95 (m, 6H, Ar-H); 13CNMR: δ 145.43 (C-3), 41.79 (C-4), 62.74 (C-5), 114.55 (C-1’), 155.53 (C-2’), 108.58 (C-3’), 119.47 (C-4’), 133.84 (C-5’), 127.82 (C-6’), 128.09 (C-1”), 131.12 (C.2”), 154.55 (C-3”), 146.88 (C-4”), 116.32 (C-5”), 119.47 (C-6”), 20.49 (-CH3), 55.97 (-OCH3).
3-(2’-hydroxy-5’-methylphenyl)-5-(3’-methoxy-4’- hydroxyphenyl)-4-5-dihydro-1-methyl-pyrazole (8): MS (m/z) [M+H]+: 313, calcd for C18H20N2O3; C, 69.34; H, 6.35; N, 8.87; Found: C, 69.21; H, 6.45; N, 8.97; 1HNMR (CDCl3): δ 3.00 (dd, 1H, J=4.42 Hz and, J=4.42 Hz, Ha-4), 3,51 (dd, 1H, J=4.42 Hz and, J=4.42 Hz, Hb-4), 3.71 (dd, 1H, J=4.45 Hz, H-5), 2.26 (S, 3H, -CH3), 2.79 (3, 3H, -CH3) 3.90 (S, 3H, - OCH3), 6.91 (m, 6H, Ar-H); 13CNMR: δ 145.52 (C-3), 41.66 (C-4), 72.28 (C-5), 116.10 (C-1’), 155.59 (C-2’), 109.10 (C-3’), 114.33 (C-4’), 131.27 (C-5’), 127.42 (C-6’), 128.03 (C-1”), 130.92 (C.2”), 152.77 (C-3”), 146.90 (C-4”), 116.30 (C-5”), 120.91 (C-6”), 20.50 (-CH3), 55.98 (-OCH3), 43.49 (- NCH3).
3-(2’-hydroxy-5’-methylphenyl)-5-(3’-methoxy-4’- hydroxyphenyl)-4-5-dihydro-1-phenyl-pyrazole (9): MS (m/z) [M+H]+: 375, calcd for C23H22N2O3; C, 73.78; H, 5.82; N, 7.48; Found: C, 73.78; H, 5.92; N, 7.48; 1HNMR (CDCl3): δ 3.06 (dd, 1H, J=4.42 Hz and, J=4.42 Hz, Ha-4), 3.45 (dd, 1H, J=4.42 Hz and, J=4.42 Hz, Hb-4), 3.90 (dd, 1H, J=4.45 Hz, H-5), 2.24 (S, 3H, -CH3), 3.84 (S, 3H, -OCH3), 6.66 (d, 1H, H-3’), 7.21 (m, 3H, H-4’, H-6’, H-5’’), 6.45 (m, 2H, H-2”, H-4”), 6.41 (m, 2H, H-2’”, H-6’”), 7.02 (m, 2H, H-3’”, H-5’”); 13CNMR: δ 145.00 (C-3), 40.39 (C-4), 61.74 (C-5), 117.03 (C-1’), 154.83 (C-2’), 110.71 (C-3’), 119.86 (C-4’), 132.67 (C-5’), 127.64 (C-6’), 128.04 (C-1”), 130.83 (C.2”), 154.32 (C-3”), 146.63 (C-4”), 116.31 (C-5”), 120.74 (C-6”), 143.71 (C-1’”), 112.41 (C-2’”), 129.62 (C-3’”), 117.01 (C-4’”), 129.62 (C-5’”), 112.41 (C-6’”), 20.52 (-CH3), 55.95 (-OCH3).
3-(2’-hydroxy-5’-methoxyphenyl)-5-(3’-methoxy-4’- hydroxyphenyl)-4-5-dihydro-1H-pyrazole (10): MS (m/z) [M+H]+: 315, calcd for C17H18N2O4; C, 64.83; H, 5.79; N, 8.82; Found: C, 64.96; H, 5.79; N, 8.91; 1HNMR (CDCl3): δ 3.07 (dd, 1H, J=4.41 Hz and, J=4.41 Hz, Ha-4), 3.50 (dd, 1H, J=4.42 Hz and J=4.42 Hz, Hb-4), 3.91 (dd, 1H, J=4.45 Hz, H-5), 3.76 (S, 3H, -OCH3), 3.87 (S, 3H, -OCH3), 4.84 (s, 1H, OH), 6.82 (m, 6H, Ar-H); 13CNMR: δ 145.56 (C-3), 41.86 (C-4), 62.93 (C-5), 116.24 (C-1’), 152.33 (C-2’), 114.59 (C-3’), 117.09 (C-4’), 154.06 (C-5’), 119.57 (C-6’), 112.66 (C-1”), 133.76 (C.2”), 151.97 (C-3”), 146.93 (C-4”), 116.60 (C-5”), 108.62 (C-6”), 55.97 (-OCH3), 56.03 (-OCH3).
3-(2’-hydroxy-5’-methoxyphenyl)-5-(3’-methoxy-4’- hydroxyphenyl)-4-5dihydro-1-methyl-pyrazole (11): MS (m/z) [M+H]+: 329, calcd for C18H20N2O4; C, 65.78; H, 6.14; N, 8.53; Found: C, 65.84; H, 6.14; N, 8.53; 1HNMR (CDCl3): δ 3.04 (dd, 1H, J=4.41 Hz and J=4.42 Hz, Ha-4), 3.21 (dd, 1H, 1H, J=4.42 Hz and J=4.42 Hz, Hb-4), 3.83 (dd, 1H, J=4.45 Hz, H-5), 3.80 (S, 3H, -OCH3), 3.87 (S, 3H, -OCH3), 2.74 (s, 3H, - NCH3), 4.95 (s, 1H, OH), 6.95 (m, 6H, Ar-H); 13CNMR: δ 145.51 (C-3), 41.73 (C-4), 65.74 (C-5), 116.52 (C-1’), 152.37 (C-2’), 114.62 (C-3’), 117.12 (C-4’), 153.93 (C-5’), 119.32 (C-6’), 112.24 (C-1”), 130.93 (C.2”), 151.94 (C-3”), 146.73 (C-4”), 116.59 (C-5”), 108.39 (C-6”), 55.98 (-OCH3), 56.03 (- OCH3), 43.43 (-NCH3).
3-(2’-hydroxy-5’-methoxyphenyl)-5-(3’-methoxy-4’- hydroxyphenyl)-4-5-dihydro-1-phenyl-pyrazole) (12): MS (m/z) [M+H]+: 391, calcd for C23H22N2O4; C, 70.63; H, 5.45; N, 8.84; Found: C, 70.75; H, 5.68; N, 7.17; 1HNMR (CDCl3): δ 2.97 (dd, 1H, J=4.41 Hz and J=4.40 Hz, Ha-4), 3.24 (dd, 1H, J=4.42 Hz and J=4.42 Hz, Hb-4), 3.57 (dd, 1H, J=4.44 Hz, H-5), 3.80 (S, 3H, -OCH3), 3.87 (S, 3H, -OCH3), 6.52 (d, 1H, H-3’), 6.47 (dd, 1H, H-4’), 7.02 (m, 3H, H-6’, H-4”, H-5”), 6.51 (m, 2H, H-2”, H-4”), 6.54 (m, 2H, H-2’”, H-6’”), 7.03 (m, 2H, H-3’”, H-5’”); 13CNMR: δ 145.27 (C-3), 41.64 (C-4), 62.52 (C-5), 116.72 (C-1’), 152.40 (C-2’), 115.65 (C-3’), 117.22 (C-4’), 154.09 (C-5’), 119.51 (C-6’), 112.62 (C-1”), 133.86 (C.2”), 151.61 (C-3”), 146.89 (C-4”), 116.58 (C-5”), 108.52 (C-6”), 143.31 (C-1’”), 112.35 (C-2’”), 129.42 (C-3’”), 116.83 (C-4’”), 129.42 (C-5’”), 112.35 (C-6’”), 55.97 (- OCH3), 56.03 (-OCH3).
Biological evaluation
Animals: The experiments were performed on male Wistar rats (weighing 130-150 g each) after obtaining the clearance from Institutional Animals Ethics Committee. All the animals were kept in standard cages and maintained under standard laboratory conditions (temperature 22 ± 2˚C with 12 h light/12 h dark cycle) with free access to pellet food (Lipton India Ltd) and water and libitum throughout the study. According to ethical regulations on animal research, all animals used in experimental work received humane care. Test materials have been prepared as a homogenized suspension in distilled water and orally administered to the experimental animals for the duration of experiment. Chemicals used; Carrageenan (Sigma Chemical Co. (St Louis, MO, USA), Mycobacterium Tuberculli (Difco, USA), TNF-α and IL-1β ELISA kits (R&D systems). All other reagents used were of analytical grade.
In vivo inhibition of the carrageenin-induced oedema
The acute anti-inflammatory effect was evaluated by carrageenan induced rat paw oedema according to the method of Winter et al. [35]. Oedema was induced by injection of 1% suspension of carrageenan in 0.9% sterile saline solution into the right plantar region of the rats. Compounds 1-12 (5 mg/kg) or Ibuprofen (100 mg/kg body weight), was administrated orally 1 h before injection of carrageenan. The paw volumes (up to the tibiotarsal articulation) were estimated by plethysmography after 4 h of carrageenan injection. Oedema is reported as the difference between the initial and final paw volumes. The inhibitory activity was calculated using the following formula.
Inhibition (%)=Control-Treated/Control × 100
In vivo adjuvant-induced arthritis
Adjuvant arthritis [36] was induced by the subplantar injection of 0.1 ml freshly prepared suspension (5.0 mg/ml) of steam killed Mycobacterium tuberculli in liquid paraffin. The volume of the injected paws was quantitated before and on day 13 after the adjuvant injection. Drug administration (compounds 1, 2 and 12 at multiple doses) was started 1 day before the adjuvant injection and continued till the termination of the experiment. After the termination of experimental period, blood was collected from the retro-orbital plexus of animals and allowed to clot for 1 h. After 1 h serum was separated and used for the estimation of TNF-α and IL-1β, using commercially available ELISA kits, according to the manufacturer’s instructions.
Ibuprofen (100 mg/kg body weight) was administered as a standard comparative drug.
Statistical analysis
All the data was analysed as mean ± S.E.M and the significance determined by applying the student’s-‘t’ test.
Results and Discussion
Chemistry
The sequence of the reactions employed for the development of title compounds (1-12) and their intermediates is depicted in Scheme 1.
α, β-unsaturated ketones I were synthesized according to reported method by Clasein-Schmidt condensation, i.e. reaction of vanillin with acetone or various substituted acetophenones in the presence of the alkali [34] produced above compounds in quantitative yields. The α, β-unsaturated ketones I thus prepared were further treated with hydrazine hydrate and its various substituted derivatives under microwave irradiation without the use of solvent, led to the formation of title compounds 3, 5-disubstituted-4, 5- dihydro-1H-pyrazoles II (Scheme 1) in excellent yields (80-90%). The structures of these compounds were confirmed by IR, NMR, and mass spectroscopy. The IR spectrum of α, β- unsaturated ketones I showed a strong band around 1665 cm-1 which indicates the presence of conjugated carbonyl group. In 1H NMR spectra the olefinic protons gave a singlet signal at 7.21 ppm and were in agreement with assigned structure and reported data [37]. For pyrazolines, 1H NMR spectra showed the presence of two doublet signals, at δ 2.91-3.12 (1H, Ha), 3.23-3.51 (1H, Hb) for prochiral CH2 protons, a double of doublet signal at δ 3.54-3.96 (1H, H5) for CH proton due to vicinal coupling with two non-equivalent geminal protons of adjacent carbon atom, for all the compounds. Further, disappearance of absorption band at 1665 cm-1 (C=O) of α, β- unsaturated ketones and appearance of absorption band at 1650 cm-1 (C=N) in IR spectra, confirms the presence of pyrozoline ring. Based on spectral studies, the structure of II was confirmed as 3, 5-disubstituted-4, 5-dihydro-1H-pyrazoles and is consistent with the reported data [37]. As a result of our studies related to the development of synthetic protocols using microwave irradiations, we report here a novel and easy access to 3, 5-disubstituted-4, 5-dihydro-1H-pyrazoles using a one-pot procedure. Moreover, synthetics prepared using microwave and classical methods on comparison (Table 1) gave us surprising results. The result shows that in microwave the formation of pyrazolines can be achieved in significant shorter time and in better yields. It was observed that the average reaction time between the two methods is 1:90 for the synthesis of 3, 5-disubstituted 4, 5-dihydro-1H-pyrazoles. Thus the present study is in favor of the microwave method and describes its superiority over previously reported classical heating method.
| Compound No. | Molecular formula | Time taken | Yield ˚A | ||
|---|---|---|---|---|---|
| Conventional | Microwave | Conventional | Microwave | ||
| 1 | C17H18N204 | 3 h | 2 min | 68.83 | 90.12 |
| 2 | C18H20N204 | 3 h | 2 min | 58.24 | 8 1 .24 |
| 3 | C23H22N204 | 3 h | 2 min | 65.58 | 90.41 |
| 4 | C17H18N204 | 3 h | 2 min | 89.82 | 90.12 |
| 5 | C18H20N204 | 3 h | 2 min | 45.78 | 86.34 |
| 6 | C23H22N204 | 3 h | 2 min | 63.54 | 86.27 |
| 7 | C17H18N203 | 3 h | 2 min | 66.87 | 89.34 |
| 8 | C18H20N203 | 3 h | 2 min | 66.86 | 80.34 |
| 9 | C23H22N203 | 3 h | 2 min | 65.58 | 90.41 |
| 10 | C17H18N204 | 3 h | 2 min | 47.34 | 80.67 |
| 11 | C18H20N204 | 3 h | 2 min | 54.67 | 8 1 .26 |
| 12 | C23H22N204 | 3 h | 2 min | 45.67 | 82.57 |
Table 1. Comparison of reaction time and yield of 3, 5-disubstiuted-4, 5-dihydro-1H-pyrazoles by microwave and conventional methods.
Biological evaluation
The compounds (1-12) were tested for their anti-inflammatory profile at the dose of 5 mg/kg p.o. and it was observed that compounds 1, 2, 3, 10, 11 and 12 showed maximum activity with the inhibition of 36.70%, 39.87%, 31.01%, 29.11%, 33.54% and 41.77% respectively which is indicative of their potent anti-inflammatory activity. Compound 12 showed most significant activity as compared to others. These results prompted us to further test the efficacy of these compounds at multiple doses in chronic model of inflammation. Since compounds 1, 2 and 12 showed highly significant activity they were selected for further studies. Adjuvant induced arthritis is the most widely used chronic model of inflammation for the screening of new anti-inflammatory drugs [38]. We first determined the paw swelling as reflection of rat paw inflammation and after treatment with compounds 1, 2 and 12 at the doses of 1, 2.5, 5 and 10 mg/kg p.o. for 13 days, highly significant inhibition of the paw swelling was obtained. The most significant inhibition of inflammation was exhibited by compound 12 at the dose of 10 mg/kg p.o. We also tested the effect of compounds 1, 2 and 12 on serum levels of TNF-α and IL-1β in arthritic control and treated groups, as blockade of these pro-inflammatory cytokines has been shown to be effective in both experimental and human arthritis [39]. Our results showed that compounds 1, 2 and 12 suppressed the TNF-α and IL-1β. Once again, the most significant suppression was shown by compound 12. These results clearly indicate that the as-synthesized compounds are potent natural antiinflammatory agents and therefore, could be suggested for possible further development for therapeutic usefulness.
Carrageenan induced oedema
The anti-inflammatory activity of compound 1-12 (5 mg/kg) was evaluated in the paw oedema model induced by carrageenan in male Wistar rats (n=6/group). As observed in (Table 2), the single oral administration of compounds 1-12 resulted in the inhibition of oedema, but the most significant anti-inflammatory activities were shown by compounds 1, 2, 3, 10, 11 and 12 (36.70%, 39.87%, 31.01%, 29.11%, 33.54% and 41.77% inhibition). However standard drug Ibuprofen was able to produce most significant inhibition of paw oedema at the dose of 100 mg/kg (50.63%).
| Groups | Concentration (mg/kg) | Mean ± S.E. | Activity (%) |
|---|---|---|---|
| Control | 1.58 ± 0.10 | ||
| Compound 1 | 5 | 1.00 ± 0.09 | 36.70%↓ |
| Compound 2 | 5 | 0.95 ± 0.12 | 39.87%↓** |
| Compound 3 | 5 | 1.09 ± 0.11 | 31.01%↓* |
| Compound 4 | 1.19 ± 0.16 | 24.68%↓ | |
| Compound 5 | 1.32 ± 0.10 | 16.45%↓ | |
| Compound 6 | 1.27 ± 0.19 | 19.62%↓ | |
| Compound 7 | 1.20 ± 0.09 | 24.05%↓ | |
| Compound 8 | 1.31 ± 0.12 | 17.08%↓ | |
| Compound 9 | 5 | 1.25 ± 0.13 | 20.88%↓ |
| Compound 10 | 5 | 1.12 ± 0.11 | 29.11%↓* |
| Compound 11 | 5 | 1.05 ± 0.09 | 33.54%↓* |
| Compound 12 | 5 | 0.92 ± 0.15 | 41.77%↓** |
| Ibuprofen | 100 | 0.78 ± 0.14 | 50.63%↓** |
Table 2. Effect of compound 1-12 (5 mg/kg/body weight) and Ibuprofen (100 mg/kg/body weight) on the oedema in the acute model of inflammation induced by carrageenan. Values represented the mean ± S.E.M, which were analysed by Student’s t-test, n=6, p-value: *<0.01,**<0.001.
Adjuvant-induced arthritis
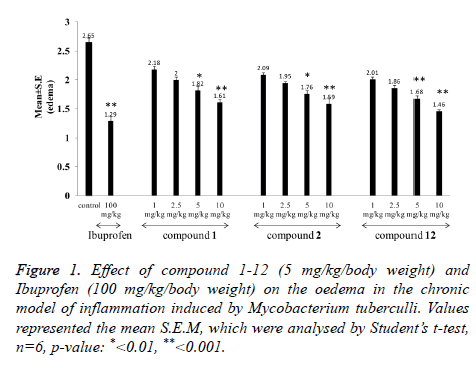
Effect on paw oedema: Figure 1 shows that groups of animals treated with compound 1, 2 and 12 showed suppression of oedema induced by adjuvant as compared to control in a dose dependent manner. Compound 1 decreased the paw volume from 2.65 ± 0.08 to 1.61 ± 0.06 (39.24% inhibition), compound 2 from 2.65 ± 0.08 to 1.59 ± 0.10 (40.00% inhibition) and compound 12 from 2.65 ± 0.08 to 1.46 ± 0.03 (44.90% inhibition) at the dose of 10 mg/kg p.o. which is suggestive of its anti-inflammatory activity.
Effect on TNF-α and IL-1 β
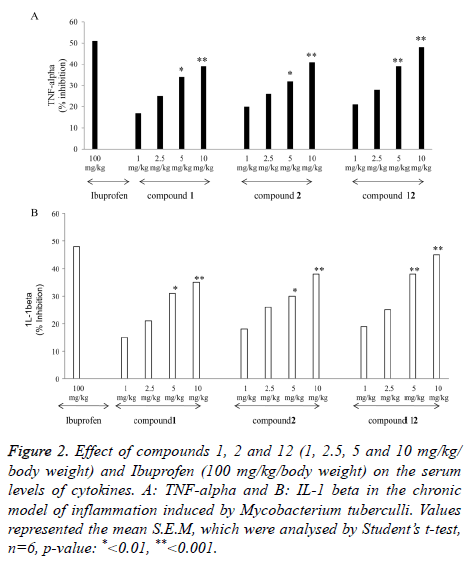
Figure 2 shows the effect of oral administration of compounds 1, 2 and 12 on the serum levels of cytokines. It was found that there was a significant suppression of TNF- αand IL-1β in the group of animals treated with compounds 1, 2 and 12 as compared to control. However highly significant suppression was obtained in the group of animals treated with compound 12. Compound 12 showed 48% suppression of TNF-α (Figure 2A) and 45% suppression of IL-1β (Figure 2A).
Figure 2. Effect of compounds 1, 2 and 12 (1, 2.5, 5 and 10 mg/kg/ body weight) and Ibuprofen (100 mg/kg/body weight) on the serum levels of cytokines. A: TNF-alpha and B: IL-1 beta in the chronic model of inflammation induced by Mycobacterium tuberculli. Values represented the mean S.E.M, which were analysed by Student’s t-test, n=6, p-value: *<0.01, **<0.001.
Conclusion
In conclusion, we have described an expedient, highly efficient and convenient procedure for the synthesis of 3, 5-disubstituted 4, 5-dihydro-1H-pyrazoles in excellent yields using α, β- unsaturated ketones with hydrazine and its differently substituted derivatives under solvent free conditions. This method offers significant advantages over reported conventional method [37]; featuring a simple reaction procedure and mild conditions, very short reaction time and high product yields. The screening results revealed that the compounds (1-12) exhibited moderate to considerable activity when compared with the standard Ibuprofen. The synthesized compounds showed anti-inflammatory activity in the range of 20-49% whereas standard drug showed 50% inhibition in paw oedema. The results of anti-inflammatory activity indicated that compounds 1, 2 and 12 possess maximum antiinflammatory potential as well as highly significant suppressor of TNF-α and IL-1 β. In the light of above results, it might be concluded that these compounds are potent anti-inflammatory in nature and thus further studies are required to evaluate and elucidate the mechanism of action in order to develop new natural anti-inflammatory agents.
Conflict of Interest
None
Acknowledgements
This research project was supported by a grant from the “Research Center of the Center for Female Scientific and Medical Colleges”, Deanship of Scientific Research, King Saud University (KSU). The authors are thankful to Cell Division, Indian Institute of Integrative Medicine (CSIR), Canal Road, Jammu Tawi, India, for providing laboratory facilities for biological activities.
References
- Tavares MCM, Junior SL, Coelho AVC, Marques TRNM, Jode A, Henrique TD, Heraclio SA, Amorim MMR, de Souza PRE, Crovella S. Tumor Necrosis Factor (TNF) alpha and Interleukin (IL) 18 genes polymorphisms are correlated with susceptibility to HPV infection in patients with and without cervical intraepithelial lesion. Ann Hum Biol 2015; 1-8.
- Keerthy HK, Mohan CD, Fuchs KJE, Rangappa S, Sundaram MS, Li F, Girish KS, Sethi G, Bender A, Rangappa KS. Novel synthetic biscoumarins target tumor necrosis factor-α in hepatocellular carcinoma in vitro and in vivo. J Bio Chem 2014; 3-5.
- Morais MC, Luqman S, Kondratyuk TP, Petronio MS, Regasini LO. Suppression of TNF-α induced NFκB activity by gallic acid and its semi-synthetic esters: possible role in cancer chemoprevention. Nat Prod Res 2010; 24: 1758-1765.
- Shailesh HS, Pankaj SP. Synthesis and biological activity of some novel phenylpyrazoline derivatives. Chem Sci Trans 2012; 632-637.
- El-Behairy MF, Mazeed TE, El-Azzouny AA, Aboul-Enein MN. Design, synthesis and antibacterial potential of 5-(benzo [d] [1, 3] dioxol-5-yl)-3-tert-butyl-1-substituted-4, 5-dihydropyrazoles. Saudi Pharm J 2015; 23: 202-209.
- Yusuf M, Solanki I. Synthesis and antimicrobial studies of furyl based new bispyrazolines linked via aliphatic chains. Saudi J Chem Soc 2015.
- Omneya KM. Synthesis and anti-inflammatory activity of 1- acetyl/propanoyl-5-aryl-3-(4-morpholinophenyl)-4, 5-dihydro-1H pyrazole derivatives. Med Chem Res 2012; 21: 3240-3245.
- Venkatesh P, Hari PK, Sharfudeen S, Soumya V, Spandana V, Priyanka J. Synthesis of coumarin fused pyrazoline-5-one derivatives and screening for their antimicrobial and antioxidant activity. J Pharm Res 2012; 5: 875-877.
- Singh V, Argal A, Mishra V, Raghuvanshi RA, Savita. Synthesis, structural analysis and biological evaluation of anticonvulsant activity of pyrazole derivatives containing thiourea. Inter J Res Phar Sci 2011; 1: 125-146.
- Rajendra Prasad Y, Lakshmana Rao A, Prasoona L, Murali K, Ravi Kumar P. Synthesis and antidepressant activity of some 1,3,5-triphenyl-2-pyrazolines and 3-(2-hydroxy naphthalen-1-yl)-1,5-diphenyl-2-pyrazolines. Bioorg Med Chem Lett 2005; 15: 5030-5034.
- Shahar Yar M, Bakht MA, Siddiqui AA, Abdullah MM, De Clercq E. Synthesis and evaluation of in vitro antiviral activity of novel phenoxy acetic acid derivatives. J Enzyme Inhib Med Chem 2009; 24: 876-882.
- Lv PC, Li HQ, Sun J, Zhou Y, Zhu HL. Synthesis and biological evaluation of pyrazole derivatives containing thiourea skeleton as anticancer agents. Bioorg Med Chem 2010; 18: 4606-4614.
- Karthikeyan MS, Holla BS, Kumari NS. Synthesis and antimicrobial studies on novel chloro-fluorine containing hydroxy pyrazolines. Eur J Med Chem 2007; 42: 30-36.
- Insuasty B, Tigreros A, Orozco F, Quiroga J, Abonía R. Synthesis of novel pyrazolic analogues of chalcones and their 3-aryl-4-(3-aryl-4,5-dihydro-1H-pyrazol-5-yl)-1-phenyl-1H-pyrazole derivatives as potential antitumor agents. Bioorg Med Chem 2010; 18: 4965-4974.
- Ahn JH, Kim HM, Jung SH, Kang SK, Kim KR. Synthesis and DP-IV inhibition of cyano-pyrazoline derivatives as potent anti-diabetic agents. Bioorg Med Chem Lett 2004; 14: 4461-4465.
- Domínguez JN, Charris JE, Caparelli M, Riggione F. Synthesis and antimalarial activity of substituted pyrazole derivatives. Arzneimittelforschung 2002; 52: 482-488.
- Jainey P, Bhat I. Antitumor, analgesic, and anti-inflammatory activities of synthesized pyrazolines. J Young Pharm 2012; 4: 82-87.
- Ali MA, Shaharyar M, Siddiqui AA. Synthesis, structural activity relationship and anti-tubercular activity of novel pyrazoline derivatives. Eur J Med Chem 2007; 42: 268-275.
- Kini S, Gandhi AM. Novel 2-pyrazoline derivatives as potential antibacterial and antifungal agents. Indian J Pharm Sci 2008; 70: 105-108.
- Behr LC, Fusco F, Jarboe CH. The chemistry of heterocyclic compounds. Wiley-Interscience New York 1967.
- William DA, Lemke TL. Foyes principle of medicinal chemistry. Williams Wilkins Lippincott Philadelphia (5th edn.) 2002; 751.
- Reynold JEFM. The extra pharmacopeia. Pharmaceutical Press London (30th edn.) 1993; 20: 594.
- Manna F, Chimentil F, Bolascol A,Cenicolaz ML, Amico M, Parrillo DC, Ross F, Marmo E. Anti-inflammatory, analgesic and antipyretic N-acetyl-2-pyrazolines and dihydrothieno coumarines. Eur J Med Chem 1992; 27: 633-639.
- Bansal E, Srivastava VK, Kumar A. Synthesis and anti-inflammatory activity of 1-acetyl-5-substituted aryl-3-(beta-aminonaphthyl)-2-pyrazolines and beta-(substituted aminoethyl) amidonaphthalenes. Eur J Med Chem 2001; 36: 81-92.
- Rani P, Srivastava VK, Kumar A. Synthesis and antiinflammatory activity of heterocyclic indole derivatives. Eur J Med Chem 2004; 39: 449-452.
- Barsoum FF, Hosni HM, Girgis AS. Novel bis (1-acyl-2-pyrazolines) of potential anti-inflammatory and molluscicidal properties. Bioorg Med Chem 2006; 14: 3929-3937.
- Kelekc NG, Yabanoglu S, Kupeli E, Salgın U, Ozgen O, Ucar G, Erdem Y, Kendi E, Yesiladaf A, Bilgin AA. A new therapeutic approach in Alzheimer disease: some novel pyrazole derivatives as dual MAO-B inhibitors and antiinflammatory analgesics. Bioorg Med Chem 2007; 15: 5775-5786.
- Khode S, Maddi V, Aragade P, Palkar M, Ronad PK, Mamledesai S, Thippeswamy AHM, Satyanarayana D. Synthesis and pharmacological evaluation of a novel series of 5-(substituted) aryl- 3-(3-coumarinyl)-1-phenyl-2 pyrazolines as novel anti-inflammatory and analgesic agents. Eur J Med Chem 2008; 21:1-7.
- Amir M, Kumar H, Khan SA. Synthesis and pharmacological evaluation of pyrazoline derivatives as new anti-inflammatory and analgesic agents. Bioorg Med Chem Lett 2008; 18: 918-922.
- Kaplancikli ZA, Turan-Zitouni G, Ozdemir A, Can Ov, Chevallet P. Synthesis and antinociceptive activities of some pyrazoline derivatives. Eur J Med Chem 2009; 44: 2606-2610.
- Joshi RS, Mandhane PG, Diwakar SD, Dabhade SK, Gill CH. Synthesis, analgesic and anti-inflammatory activities of some novel pyrazolines derivatives. Bioorg Med Chem Lett 2010; 20: 3721-3725.
- Chandra T, Garg N, Lata S, Saxena KK, Kumar A. Synthesis of substituted acridinyl pyrazoline derivatives and their evaluation for anti-inflammatory activity. Eur J Med Chem 2010; 45: 1772-1776.
- Luo P, Zhang Z, Yib T, Zhang H, Liu X, Mo Z. Anti-inflammatory activity of the extracts and fractions from Erigeron multi radiates through bioassay-guided procedures. J Ethnopharm 2008; 119: 232-237.
- Gillman H, Blatt A H, Dhar DN. Chemistry of chalcones and related compounds. Wiley-VCH New York USA Indian Chem Soc 1968; 45: 178.
- Winter CA, Risley EA, Nuss GW. Carrageenin-induced edema in hind paw of the rat as an assay for antiiflammatory drugs. Proc Soc Exp Biol Med 1962; 111: 544-547.
- Newbould BB. Chemotherapy of arthritis induced in rats by mycobacterial adjuvant. Br J Pharmacol Chemother 1963; 21: 127-136.
- Satti NK, Suri KA, Suri OP. Synthesis and biological activities of anti-inflammatory 4,5-Dihydro-1H-pyrazoles. Ind Drugs 1987; 24: 492-493.
- Mythilypriya R, Shanthi P, Sachdanandam P. Efficacy of Siddha formulation Kalpaamruthaa in ameliorating joint destruction in rheumatoid arthritis in rats. Chem-Bio Inter 2008; 176: 243-251.
- Mac Naul KL, Hutchinson NI, Parsons JN, Bayne EK, Tocci MJ. Analysis of IL-1 and TNF-alpha gene expression in human rheumatoid synoviocytes and normal monocytes by in situ hybridization. J Immunology 1990; 145: 4154-4166.