ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2021) Red Cell Immunology and Genotyping
Tibetan medicine RenQingMangJue capsule for COVID-19 Treatment
Yu Wang, Guozhen Ma, Xudong Tian, Yibing Zhang, Xia Song, Zhiming Zhang*
Department of Intensive Medicine, Affiliated Hospital of Gansu University of Traditional Chinese Medicine, Lanzhou, Gansu, China
- Corresponding Author:
- Dr. Zhiming Zhang
Department of Intensive Medicine
Affiliated Hospital of Gansu University of Traditional Chinese Medicine
Lanzhou Gansu
China
E-mail: zhangzhjmingys@163.com
Accepted date: 04 August, 2021
The severe coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has spread globally. By now no specific drug has been proven effective for the treatment of patients with COVID-19. RenQingMangJue (RQMJ), as a traditional Tibetan medicine (TTM) for detoxification, was shown beneficial for digestive diseases and biological toxicity. In this study, RQMJ capsule (RQMJ-C) was used to treat COVID-19 positive patients combined with conventional treatments. Patients with RQMJ-C taken orally once in a day for 10 days showed improvement in main symptoms like fever, weakness and cough, compared to the control patients. Moreover, RQMJ-C associated with a difference in time to clinical improvement in fever, weakness, cough, pharyngodynia, chest distress and digestive symptoms for example poor appetite. RQMJ-C was proven safe and no side-effect within 10 days’ treatment. Therefore, RQMJ-C could be beneficial for the treatment of COVID-19 symptoms but that needs further investigation in a larger population and a longer term.
Introduction
Well known, The COVID-19 pandemic caused by SARS- CoV-2 has spread globally. Although most infections are self- limited, about 15% of infected adults progress to severe pneumonia, which requires treatment with supplemental oxygen, and an additional 5% patients become critical illness with hypoxaemic respiratory failure, acute respiratory distress syndrome, and even die in the end.1-4 The ongoing pandemic of SARS-CoV-2 infections has led to more than 6177454 cases and 369212 deaths globally as of June 25, 2020.With the continued increase in cases and affected regions in the whole world, protection and treatment options should be made to protect high-risk populations, especially for older patients and those with existing conditions. However, there are still not enough effective drugs to treat with it.
Tibetan medicine is based on the practice and cognition of life and disease. It provides a quick and effective way to solve viral infectious diseases which has unknown etiology, unclear diagnosis, complicated mechanism or no medicine available. RQMJ, one of the classical prescriptions of TTM, has been widely used in treating digestive diseases, peptic ulcer, herpes zoster, food poisoning and ulcerative colitis for many years. RQMJ capsule (RQMJ-C) was not only recorded in a Tibetan medical classic, but also approved by the Ministry of Health as one of the national protected traditional Chinese medicine (TCM) in 1997.5,6 The composition of RQMJ-C is complex, which contains TERMINALIAE BELLIRICAE FRUCTUS, FRUCTUS SYZYGII CUMINI, CROCI STIGMA, BOVIS CALCULUS ARTIFACTUS, MUSK ARTIFACTUS, CINNABARIS and more.7 RQMJ-C has the effect of clearing away heat and detoxifying, benefiting liver and nourishing stomach, clearing sores and rejuvenating eyes. In many conditions, RQMJ-C is used as a multi-ingredient formula.1 Up to now; there haven’t been reports about RQMJ-C related heavy metal poisoning.
RQMJ-C has been used in treating infectious bronchitis (IB) and symptomatic treatment of viral infectious diseases such as influenza and upper respiratory tract infection, so we infer that RQMJ-C may be effective for COVID-19. In this work, we used RQMJ-C to treat patients with COVID-19 in the Houhu District of Wuhan Central Hospital. After being screened, 20 patients were treated and hospitalized, and they were discharged from the hospital in the later period. RQMJ-Cs were permitted to take back home, but the data of patients who took RQMJ-C home were not collected. Clinical observations have shown that RQMJ-C has a certain effect in improving patients' shortness of fever, cough and weakness as well as other symptoms. In the feedback of the patients' medication, the effect is particularly prominent. Because continuous observation is not possible, detailed statistics are not made.
Methods and Materials
Study design
This study was an investigator-initiated, randomized individually trial to evaluate the effectiveness and safety of RQMJ-C in adults (aged ≥18 years) admitted into hospital with COVID-19. The trial was done at Wuhan Central Hospital Houhu District in Wuhan, Hubei, China. Ethical approval was obtained from the institutional review boards of Wuhan Central Hospital Houhu District. Written informed consent or legal representative was obtained from all involved patients.
Patients
Eligible patients were men and non-pregnant women with COVID-19 who were aged at least 18 years and were RT-PCR positive for COVID-19. Exclusion criteria included pregnancy or breast feeding; severe primary cardiovascular disease, liver disease, kidney disease, hematology disease, lung disease; mental illness; possibility of transfer to a non-study hospital within 72h; and enrolment into an investigational treatment study for COVID-19 with other drugs therapy in a week before screening. Eligible patients were randomly assigned to RQMJ- C group. Randomization was achieved according to the level of respiratory support. Eligible patients were assigned to receive medication in individually numbered packs, according to the sequential order of the randomization center (Wuhan Central Hospital Houhu District central pharmacy). The use of other conventional treatments, including ribavirin and methylprednisolone, was permitted.
Procedures and outcomes
Patients received 4 RQMJ-C orally in the morning once a day for a total of 10 days. Patients were assessed once a day by well-trained nurses using diary cards or related forms. The safety assessment comprised daily monitoring for adverse events, clinical laboratory testing (days 1, 3, 7, and 10), electrocardiogram (days 1 and 10), and daily vital signs measurements. The clinical endpoint was time to clinical improvement within 10 days after randomization. Clinical improvement was defined as the main symptoms and signs improved significantly and efficacy index>30% according to TCM syndrome score of new coronary pneumonia in 2020.
Statistics analysis
Spss20.0 statistical software was adopted for statistical processing, and bilateral tests were used for all statistical tests. The attribute data was described by the number of cases and the composition ratio. The variables data was expressed by mean ± standard deviation. Chi-square test, continuously modified chi-square test and Fisher accurate probability method was used to count the data, P ≤ 0.05 indicated that the difference was statistically significant [1-3].
Results
Between Feb, 2020, and April, 2020, 20 patients were screened, all of whom were eligible. 20 patients confirmed COVID-19 positive by RT-PCR were assigned to receive RQMJ-C combined with conventional treatments, and 38 patients obtained from other group’s statistics were adopted as the control. The median age of patients in this study was 65 years; sex distribution was 10 (50.0%) men versus 10 (50.0%) women in the RQMJ-C group and 18 (47.4%) versus 20 (52.6%) in the control group (table 1). Most patients were in grade 2 of the three ordinal scale based on the severity of clinical status. Some imbalances like comorbidities existed at enrolment between the two groups, but no other major differences in symptoms, signs, laboratory results, disease severity, or treatments were observed between groups at baseline. Median time from symptom onset to data analysis was 5 days. No important differences were apparent between the two groups in other treatments received.
| RQMJ-C group (n=20) | Control group (n=38) | |
| Age, years | 66.38±11.32 | 60.2±17.01 |
| Sex | ||
| Men | 10 (50.0%) | 18 (47.4%) |
| Women | 10(50.0%) | 20 (52.6%) |
| Severity Grading | ||
| Light | 0 (0%) | 0 (0%) |
| Ordinary | 15 (75.0%) | 28 (73.7%) |
| Severe | 5 (25.0%) | 20 (16.3%) |
Table 1. Patient characteristic.
The final follow-up was on April 15, 2020. Intention-to-treat (ITT) analysis and safety analysis were done in the population enrolled.
Compared with the control group, RQMJ-C group showed higher curative ratios of fever (100% vs 67.6%), cough (65.0% vs 30.6%) and weakness (80% vs 58.6%) within 10 days (Table 2).
| RQMJ-C group (n=20) | Control group (n=38) | P | ||
|---|---|---|---|---|
| Fever | 12/12, 100.0% | 23/34, 67.6% | - | 0.044 |
| Cough | 13/20, 65.0% | 11/36, 30.6% | 6.229 | 0.013 |
| Weakness | 16/20, 80.0% | 17/29, 58.6% | 1.584 | 0.208 |
Table 2. Outcomes in two groups (cases with improvement/ total, %).
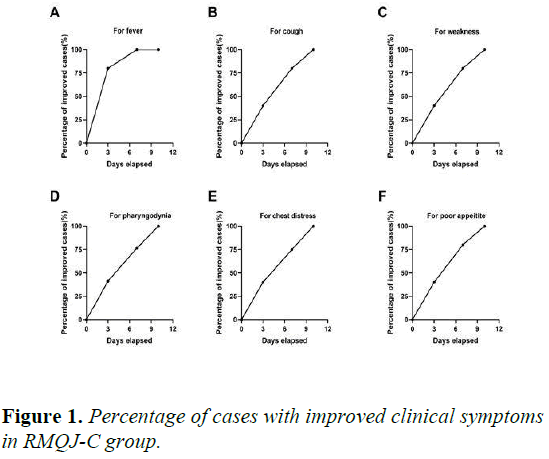
Moreover, with RQMJ-C treatment for 3, 7 and 10 days, compared with the baseline, 80.0%, 100.0% and 100.0% of the total showed improvement in fever, and 40.0%, 80.0% and 100.0% of the total showed improvement in cough and weakness (Figures 1A-1C).
More than these three main clinical symptoms, RQMJ-C treatment had obvious effect in the treatment of pharyngodynia (41.2%, 76.5% and 100%), chest distress (40.0%, 75.0% and 100%) and digestive symptoms for example poor appetite (40.0%, 70.0% and 100%) for 3,7 and 10 days (Figures 1D-1F).
In addition, RQMJ-C also had high security. No cases with RQMJ-C treatment showed aggravated clinical status compared to 6/38 (15.8%) in the control group. Adverse events were reported in 8 (21.1%) of 38 patients in the control group and 0 (0%) of 20 in the RQMJ-C group [4,5].
The most common adverse events in the control group were anorexia (5/8, 67.5%) and vomiting (3/8, 37.5%). No significant abnormalities were found in liver function, renal function and electrocardiogram between the two groups.
Discussion
In December, 2019, the COVID-19 pandemic, which was caused by SARS-CoV-2, has emerged in China. The main clinical manifestations of COVID-19 are fever, cough and weakness. So far there has been no specific drug for the treatment of COVID-19. Traditional Chinese medicine and integrated traditional Chinese and western medicine have played an active role in the prevention and control of this epidemic. Especially, China's ethnomedicine has recognized infectious diseases since ancient times, and formed a medical system including theory, therapies, formula and herbal medicines for such diseases. TTM has always been an essential part of China's ethnomedicine, and RQMJ-C is one of the most important drugs of TTM and has been commonly used for the treatment of digestive diseases. Sometimes, RQMJ-C can be used to treat viral infectious diseases such as influenza. In this study, we want to explore the effect of RQMJ-C on COVID-19.
The cases in this study are all from patients with mild new COVID-19 diagnosed clinically in Hubei. These patients did not take other related medicines within 1 week and took part in this study voluntarily, agreeing to follow up. We classified patients based on symptoms, for patients with shortness of breath and poor appetite during the recovery period. Patients received RQMJ-C orally for 10 days. We objectively evaluated the improvement of TCM syndrome scores of patients before and after treatment. At the same time, we monitored and recorded the safety indicators such as blood, urine, feces routine, liver and kidney function of patients and the adverse reactions during the test period to evaluated the safety and effectiveness of RQMJ-C. Finally, we analyzed the recorded data, and we found that RQMJ-C has a certain effect in improving the patients' shortness of fever, weakness, and cough as well as poor appetite, etc; the effect is particularly prominent in the feedback of medications for discharged patients. According to the above statistical data during hospitalization, RQMJ-C has a significant effect on the treatment of COVID-19 in the late period of TCM syndrome, with an effective rate of 100%, and a recovery rate of 89% for diarrhea; 80% for weakness. In addition, RQMJ-C had no adverse events during the treatment of COVID-19 and had good safety.
The recovery period is the fourth stage of COVID-19, the first 3 stages are early stage, advanced stage and critical stage. It is a stage where traditional medical treatment has unique advantages. At this stage, patients still have weakness, anorexia, abnormal mood et al. Xue Honghao11 et al. conducted a clinical analysis of traditional Chinese medicine for 66 patients with common type of COVID-19 during the recovery period. Studies have shown that among 66 patients with new coronavirus pneumonia in the recovery period, patients with loss of appetite (anorexia, belching nausea, and full stomach) accounted for up to 43.9%. The good effect of RQMJ-C on digestive system symptoms such as anorexia and diarrhea in patients with COVID-19 indicates the great value of the drug in the treatment of COVID-19.
On March 20, Nanshan Zhong, an academician of the Chinese Academy of Engineering, claimed that TTM has great potential in reducing symptoms, shortening fever time, and even reducing viral load of COVID-19. It is worth digging and developing research. However, TTM has always existed such problems: 1. unclear mechanism of TTM for “epidemic prevention” ; 2. weak basic research on epidemic prevention, unclear anti-viral mechanism of medicine and insufficient pharmacodynamics research; 3. insufficient number of mature Tibetan prescriptions for prevention, treatment and rehabilitation; 4. high-level evidence-based research is lacking. Through case observations, this study confirms that RQMJ-C has very good application prospects for diarrhea, anorexia, cough, chest tightness, and general fatigue and other symptoms during COVID-19. It provides support for the use of TTM in the prevention and treatment of COVID-19.
Conclusion
During the epidemic in Wuhan, doctors adopted a standard treatment plan for the treatment of patients. The patients enrolled in this trial had different combinations of drugs. Basically, western medicine and traditional Chinese medicine were differently used for the treatment in the early stage. The main combined drugs included Tawei, ribavirin, methylprednisolone, levofloxacin and so on, the traditional Chinese medicine is mainly composed of prescription in Gansu traditional Chinese Medicine. In 20 cases, antiviral drugs were basically combined. A small number of patients with bacterial infections had used antibiotics, and patients who used Tibetan medicines used fewer Chinese medicines. The above situation interfered greatly with our research. In addition, the number of cases in this test was insufficient. That would make a great impact on the accuracy of our results.
RQMJ-C’s effect on COVID-19 needs further testing and verification. We will also improve the corresponding entry criteria and formulate a more detailed experimental plan in order to obtain more accurate experimental results.
References
- Sallon S, Dory Y, Barghouthy Y, et al. Internet Is mercury in Tibetan Medicine toxic? Clinical, neurocognitive and biochemical results of an initial cross-sectional study[J]. Exp Biol Med. 2017;242: 316-332.
- Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72314 Cases From the Chinese Center for Disease Control and Prevention[J]. JAMA. 2020;13:1239-1242.
- Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study[J]. Lancet. 2020; 395: 507-513.
- Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study[J]. Lancet. 2020; 395: 1054-1062.
- Hao YF, Jiang JG. Origin and evolution of China Pharmacopoeia and its implication for traditional medicines[J]. Mini Rev Med Chem. 2015; 15(7): 595-603