ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Review Article - Biomedical Research (2022) Volume 33, Issue 7
The role of inflammation in cancer progression: Current understanding and future perspective.
Santhoshi Rani Nanchari1, Satti Vishnupriya2, Vijayalakshmi Venkatesan1*
1Department of Stem Cell Research and Molecular Biology, ICMR-National Institute of Nutrition, Hyderabad, India
2Department of Genetics, Osmania University, Hyderabad, India
- Corresponding Author:
- Vijayalakshmi Venkatesan
Department of Stem Cell Research and Molecular Biology
ICMR-National Institute of Nutrition
Hyderabad
India
Accepted date: 24 August, 2022
Inflammation is a primary response of the host to any infections and tissue damage, and it is characterized by a series of reactions. In general, it is accepted that inflammation is beneficial to the host, but it should be resolved in a timely manner. Persistent infection cumulatively causes successive inflammatory reactions, which dysregulates innate and/or adaptive immune functions and makes people susceptible to several diseases. Several shreds of evidence are suggesting that cancers originated due to failure in the host immunity and persistent inflammatory reactions. Amongst various signalling pathways, signalling mediated by NF- κB, COX-2, and JAK/STAT seems to play a major role in tumorigenesis. Several inflammatory markers are identified (Tumor Necrosis Factor-a (TNF-α, Interleukin-6 (IL-6), and C-Reactive Protein (CRP)), and regulated by several fold in response to infection and tissue damage vis a vis in proliferation, Epithelial- Mesenchymal Transition (EMT), invasion, and metastasis.
Chronic inflammation is known to elicit carcinogenesis and metastasis at distant sites either through direct interactions of inflammatory cells and cancer cells or indirect effects of inflammatory cells on other resident stromal cells. Chronic activation of the immune system by microbial infection is associated with tumors at numerous sites. In this review article, we are discussing how chronic inflammation is prone to developing disease and the role of inflammatory signalling markers in cancer development and progression and tumor microenvironment profile. We need to identify the novel inflammatory biomarkers which are significantly associated with chronic inflammation and cancer development; it might be helpful for the patients with a better therapeutic approach.
Keywords
Inflammation, EMT, Cancer development and progression, Cytokines, Chemokines.
Abbreviations
NF-κB: Nuclear Factor Kappa-light-chain-enhancer of activated B cells; COX-2: Cyclooxygenase 2; JAK-STAT: Janus Kinase (JAK)-Signal Transducer and Activator of Transcription (STAT) pathway; MMP: Matrix Metalloproteinase; TNF-a: Tumour Necrosis Factor-a; IL-6: Interleukin-6; CRP: C-Reactive Protein; EMT: Epithelial–Mesenchymal Transition; CAFs: Cancer Associated Fibroblasts; TLR4: Toll Like Receptor 4
Introduction
Inflammation is a primary response of the host against tissue damage caused by distinct factors like trauma, toxins, heat exposure, bacteria or fungal infections…etc. Deregulated inflammatory responses can cause excessive or long-lasting tissue damage, contributing to the development of acute or chronic inflammatory diseases. Studies by Rudoff Virchow first reported the link between inflammations with carcinogenesis and demonstrated that there is an increased infiltration of leukocytes in the malignant tissues, suggestive of a mechanistic association between inflammation and cancer [1]. Several other studies also concluded subsequently that the site of chronic inflammation could be the site of solid tumour origin [2]. Persistent inflammation co-precipitating with tissue damage and inhibition of host cell apoptosis contributes to carcinogenesis [3]. Understanding the role of inflammation in the origin of tumor initiation, tumor dissemination and tumor progression is difficult because several mediators and mechanisms contribute to various phases of inflammation at different stages of tumorigenesis and [4] affect immune surveillance and treatment response. One of the effector pathways by which the host fights with microbial infection is STING (Stimulator of Interferon Genes) dependent signalling pathway [5], and functions via the production of free radicals such as Reactive Nitrogen Intermediates (RNI), Nitric Oxide (NO), Peroxynitrite (ONOO-), Reactive Oxygen Intermediates (ROI), Hydroxyl Radical (HO•) and Superoxide (O2−) species. Excess free radicals are involved in oxidative DNA damage, nitration of DNA bases and cytotoxic, increasing the risk of cancer mutations [6-8]. However, note that not all chronic inflammatory diseases increase the risk of cancer and in fact, some of them such as psoriasis or rheumatoid arthritis may even reduce the cancer risk, or do not significantly promote tumorigenesis [9].
Interestingly, several inflammatory mediators like chemokines, cytokines, eicosanoids, Reactive Oxygen- Nitrogen Species (RONS), Cyclooxygenase (COX-2) and Nuclear Factor Kappa B (NFκB) etc participate in favor of cancer pathogenesis and tumor promotion by genomic alterations in the epithelium [10-14]. Cytokines, including Transforming Growth Factors (TGF-β), Interleukins, Tumor Necrosis Factor (TNF-α), and differentiation Macrophage Colony-Stimulating Factors (M-CSF), are secreted or membrane-bound molecules that pla a regulatory role in the growth, differentiation, and activation of immune cells [14,15].
Evidence from literature had shown that cytokine signalling could contribute to a large extent to tumour progression either by stimulation of cell growth/differentiation or by inhibition of apoptosis of altered cells at the inflammatory site [16]. Fibrosis-associated pleiotropic cytokine is also able to promote EMT, thereby enhancing the synthesis of fibroblast to mediate tissue repair and regulation of Matrix Metalloproteinase (MMP) secretion, increased tumour cell motility and invasion and metastasis [17,18]. Wendt et al., had shown that loss of carcinoma cells in responsiveness to TGF-β stimulation can also promote metastasis [11,12]. TGF- β in the presence of IL-6 promotes the differentiation of T helper cells 17 (Th17), whereas TGF-in the presence of IL-4 promotes the differentiation of interleukins (IL- 9 and IL-10) producing T cells, resulting in a lack of suppressive function as well as tissue inflammation [19]. Fascinatingly, the increased metastasis was found to be observed in carcinoma cells which are not showing any response to TGF-β stimulation and TGF-β is involved in increased chemokine production resulting in recruitment of pro-metastatic Myeloid-Derived Suppressor Cell (MDSC) at the tumour. Corroborating these, genetic and epigenetic alterations had also an impact on the TGF-β pathway in human cancer [11,12].
Chemokines (C-X-C family) targets all types of leukocytes, including hematopoietic cells, mature leucocytes of the innate immune system as well as naive, memory, and effector lymphocytes. Chemotactic migration of leucocytes largely depends on adhesive interaction within the substratum and recognition of a chemo attractant gradient [10,13,14]. Both aspects, cell adhesion and chemotaxis, are regulated by a family of chemotactic cytokines (chemokines). Loss of regulation in the control of leukocyte migration results in chronic inflammatory diseases [10,13,14]. Chemokine receptors are pleiotropic and redundant and belong to the large family of seven transmembrane domain receptors that couple to heterotrimeric GTP-binding proteins (G-proteins). The positive charge of these chemokines can bind to the proteoglycans of tissues, it may regulate the leukocyte migration that leads to inflammation and initiate induction of cancer [13,14]. Thus, interfering with chemokine function, form an interventional approach for the development of novel anti-inflammatory medication.
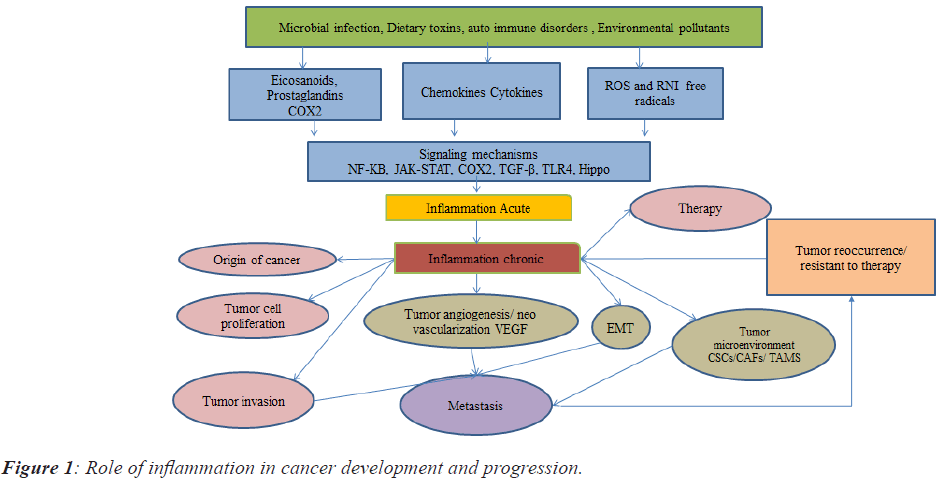
Derivatives from arachidonic acid and Polyunsaturated Fatty Acid (PUFAs) are like eicosanoids/prostaglandins/ leukotrienes/related bioactive lipid mediators that might contribute as major factors towards cell homeostasis and inflammatory processes. Deregulation of these lipid mediators might contribute to numerous diseases like cancer, arthritis, and heart diseases for example Cyclooxygenase2 (COX2) [20]. The bioactive lipid network of eicosanoids is a complex and challenging pathway to map in a physiological context and it is similar to the cytokine signalling pathway. However, advances in lipidomics pathways have helped to elucidate unique eicosanoids and related docosanoids with antiinflammatory and pro-resolution functions. Inhibiting the formation or receptor-mediated actions [21] of classical eicosanoids (that is prostaglandins and leukotrienes) by aspirin and other Non-Steroidal Anti-Inflammatory Drugs (NSAIDs), by the leukotriene inhibitor zileuton, and by leukotriene receptor antagonists during inflammation advocate as a strategy to alleviate pain, swelling, fever and asthmatic conditions [21]. However, pleiotropic effects are becoming increasingly appreciated for most eicosanoids and their related docosanoid compounds. The role of inflammation in cancer development and progression is represented in Figure 1.
The role of apoptosis in cancer inflammation
Programmed cell death or apoptosis is under the control of growth factors, hormones and cytokines, which are depending upon the receptor sites present on the target cells, which may trigger genetically controlled cell elimination [22-24]. Both extrinsic and intrinsic apoptotic pathways are linked to inflammation resulting in the activation of transcription factors such as NF-κB, STAT-3, and HIF-1 thereby in the accumulation of tumorigenic factors in the tumor microenvironment [25]. In the extrinsic pathway, infection or chronic inflammation is the driving force that causes the increase in cancer risk. Alternatively, in the intrinsic pathway, genetic alterations of oncogenes and/or tumor suppressor genes are the primary cause of cancer. Further, cells undergoing necrotic cell death due to starvation, and chemical or physical injury have also been shown to release factors into adjacent tissues, resulting in the transmigration of granulocytes to the damaged tissue. The gathering of neutrophils and the release of enzymes and free radicals exacerbate the inflammatory process and have an impact on tumorigenesis [26]. Macrophages engulf the necrotic bodies and reduce inflammation. Yang et al., defined necroptosis as cell death mediated through a pathway that depends on the Receptor-Interacting Protein kinases (RIP) 1-RIP3 complex and that can be inhibited by Necrostatin-1 (Nec-1) [27].
Inflammation with microbial infection leads to cancer development
Microbial infection is one of the primary inducers of inflammation; other inducers include autoimmune diseases. Epidemiologic studies have estimated that around 15% of the worldwide cancer incidence is associated with microbial infection [6]. Chronic infection with Chlamydia trachomatis results in Ovarian or cervical cancer, human papillomavirus (cervical cancer) or hepatitis B and C (hepatocellular carcinoma), Mycoplasma genitalium (STIS in males), Human Herpes Virus (HHV-8) (Kaposi’s sarcoma) Epistain bar virus (Nasopharyngeal and Helicobacter pylori (Gastric or colon cancer or inflammatory bowel disease) respectively [28-31]. Microbes infected tumours contain activated macrophages and fibroblasts; in addition to that, an inflammatory gene expression profile and lymphoreticular infiltrate [6] were observed. Inflammatory sensors include T and B lymphocytes, fibrocytes and endothelial cells, macrophages, dendritic cells, and mast cells [32]. Lipopolysaccharide of bacteria have been directly shown to affect lung fibroblast viability by activating TLR4 signalling, and TLR4-induced activation of the PI3KAkt signalling pathway, which acts by down regulation of Phosphatase and TENsin homolog (PTEN) served the function in the inflammatory signalling [33]. A recent study by Paris et al., 2017 suggested that miR-718 represses the proinflammatory cytokines by modulating PI3K-Akt signalling directly targeting the PTEN gene [3,34].
Inflammation and immunity with cancer development
Inflammation is mediated by immune cells as an immediate defense in response to infection or injury by noxious stimuli to eliminate or neutralize injurious signals (death of cell/apoptosis), and initiates healing and regenerative processes. The formation of inflammasomes has been viewed as primarily a pro-inflammatory component of the innate immune response. Innate immunity is a response to foreign microbial and viral structures, known as molecular structures of Pathogens/pathogen-Associated Molecular Structures (PAMS) or normal cellular constituents released upon injury and cell death, known as Damage-Associated Molecular structures (DAMs) [35]. These are recognized by Pattern-Recognition Receptors (PRRs), many of which belong to the TLR group (TLR4) [33,36]. DAMPs such as HMGB1, S100 proteins, and HSPs, activate inflammatory pathways and release IL-1, IL-6, LT-β, IFN-γ, TNF, and Transforming Growth Factor (TGF)-β promoting tumor progression in the early stage of cancer by inducing chronic inflammation [37]. Excess presence of adenosine, and uric acid also promote carcinogenesis by inflammation, immunosuppression, angiogenesis, and tumour cell proliferation [2,38,39]. Tumour derived cytokines like Fas ligand (FasL), Vascular Endothelial Growth Factor (VEGF), and TGF-h may also facilitate the suppression of immune response to tumors [40]. For example, IL-6, a key tumor-promoting inflammatory cytokine produced by innate immune cells, activates at least three regeneration promoting transcription factors like Yes-associated Protein 1 (YAP), Notch, and Signal Transducer and Activator of Transcription 3 (STAT3) which are also involved in inflammation, stem cell activation, survival advantage to tumour cells [25,35,40,41].
Association of cancer-associated fibroblasts and Cancer Stem Cells (CSCs) on inflammation and carcinogenesis
The tumour microenvironment is a heterogeneous population of cells composed of distinct cellular and structural factors, immune cells, tumour-associated macrophages/ neutrophils, lymphocytes, including the vasculature like endothelial cells, other stromal cells like cancer-associated fibroblasts and extracellular matrix. Cancer-Associated Fibroblasts (CAFs) are the critical component of the tumor microenvironment and exhibit a function in tumor-stromal crosstalk. Generally, fibroblasts are involved in tissue remodeling or repair, whereas cancer associated fibroblasts are involved in, a cascade of an inflammatory response by activating the NF-KB signalling mechanism thereby increasing the pro-inflammatory gene expression signatures leads to the acute and chronic inflammation results in tumorigenesis, metastasis, angiogenesis and increases the stem of cancer cells. CAFs are inflammatory immune regulators responsible for the recruitment of fibroblasts and induction of fibrosis [25]. CAFs can produce numerous chemokines, cytokines and including Osteopontin (OPN), CXCL1, CXCL2, TNF-α, IL-6, IL-1β, CCL-5, Stromal-Derived Factor-1α (SDF-1α), and CXCL13[25] and up-regulates the TLR4 signalling mechanisms [33,42]. During early tumorigenesis, fibroblasts sense changes in tissue architecture caused by increased proliferation of adjacent epithelial cells and respond to these changes by producing pro-inflammatory mediators [43]. Balachander et al. observed that CAFs were found to increase the infiltration of inflammatory cells to the tumor site and increase the invasiveness of the tumor cells in vivo when tumor cells were injected with CAFs grown on fibrous matrices [44]. In ovarian cancer, CAFs upregulated the expression of the pro-inflammatory factors IL-6, cyclooxygenase-2 and the chemokine (C-X-C motif) ligand [45]. In addition, CAFs may facilitate the invasiveness of originally noninvasive cancer cells, through protease-activated receptordependent Ca2+ signals and matrix metalloproteinase-1 upregulation [46,47]. The tumor microenvironment consists of another sort of cells known as Circulating Cells (CSCs) that also play a role in inflammation in solid tumor (ovarian, breast, colon, prostate) signalling mechanisms. CSCs were defined as cancer cells within the tumour that possess a high capacity of self-renewal, viability, metastatic ability, stem cellness of recurrence and they are not responded to conventional therapies (radiation and chemotherapy). CSCs were regulated by a number of signalling mechanisms and found to interact with inflammatory cytokines, including IL-1, IL-6 and IL- 8, activate the STAT3/NF-κB, an Interferon-Stimulated Regulatory Element (ISRE) [48] dependent signalling pathway in tumor and stromal cells, which stimulates cytokine production and self-renewal of CSCs [49]. Tumor Associated Macrophages (TAMs) also involved in inflammatory responses plays a role in cancer progression, angiogenesis and immune suppression [49].
The mechanism of inflammation on angiogenesis and carcinogenesis
Inflammation and hypoxia both are primary inducers of angiogenesis involved in many pathophysiological processes including tumor viability by Hypoxia-Inducible Factor 1 (HIF-1) activation. It is well-known as an adaptive strategy to hypoxia and consists of two subunits: HIF-1α and HIF-1β. HIF-1 activates transcription of genes involved in vascular/angiogenic reactivity, including VEGF, angiopoietin [4,50-52]. In (ANGPT1, ANGPT2) and Platelet-Derived Growth Factor (PDGF), which are secreted by hypoxic cells and stimulate epithelial cells [53]. Exosomes, extracted from high-grade ovarian cancer cells, induce angiogenesis, and activating transcription factor 2 and metastasis-associated 1 may serve a key function in the exosomal enhancement of tumor development [50,54-57]. Chronic inflammation induces the Vascular Endothelial Growth Factor (VEGF)/ Fibroblast Growth Factor (FGF) and eNOS, which have a function in tumor angiogenesis, viability and metastasis [58], and targeting VEGF to inhibit angiogenesis, may prevent cancer progression. Inflammation induced by NF-κB/ Nrf2 pathway, critical for pro-inflammatory gene expression, is considered to exhibit a function in tumor vascularization and inflamed tissues, and the NF-κB-inducing kinase and Nrf2 may be a therapeutic target in chronic inflammatory diseases and tumor neo angiogenesis [59,60]. Inflammation induced by Cyclooxygenase (COX2) and bradykines also plays a role in tumor cell proliferation and angiogenesis [61].
Inflammation-activated Epithelial-Mesenchymal Transition (EMT) and carcinogenesis
EMT is a biological process where epithelial cells lose their cell polarity and cell-cell interactions and gain migratory and invasive properties to become mesenchymal stem cells. EMT was first recognized as a feature of embryogenesis and it occurs in wound healing, organ fibrosis, cancer progression and cancer metastasis [62]. During the process of EMT, many transcription factors have been identified to repress E-cadherin such as Zinc finger protein SNAI1 (Snail), Zinc finger E-box-binding homeobox (ZEB), E2A immunoglobulin-enhancer binding factor (E47) and Krüppel-like factor 8 bound to and repressed the activity of the E-cadherin promoter; whereas Twist-related protein 1 (Twist) and FOXC2 were common factors that repressed E-cadherin transcription indirectly [63-67]. Furthermore, another study demonstrated that EMT was utilized by cancer cells to enhance aggressiveness by acquiring chemo resistance and stem-cell-like properties and escaping from host immunity [68]. The structural components of microbes including Helicobacter, Mycoplasma hyorhinis, Citrobacter rodentium, Epstein Bar Virus (EBV) and Hepatitis C (HCV) were found to be induce the EMT [17,65,69-71]. Li et al. identified that Lipopolysaccharide (LPS) promoted invasion and metastasis of liver hepatocellular carcinoma HepG2 cells and downregulated the expression of E-cadherin, suggesting that TLR4 may be involved in the process of EMT [72]. Furthermore, LPS was demonstrated to decrease E-cadherin expression in Human Intrahepatic Biliary Epithelial cells (HIBEpiCs) and increase the mesenchymal markers S100 calciumbinding protein A1 and sterile α motif [73]. In addition, it was hypothesized that LPS induced EMT of HIBEpiCs, through the TGF-β1/Smad2/3 signalling pathway [74,75]. Xie et al. has been suggested that Flagellin and muramyl dipeptides are two bacterial products that are also associated with EMT [76]. A previous study also revealed that flagellin induced EMT by activating NF-κB and Mitogen Activated Protein Kinase (MAPK), but failed to increase the level of Snail in A549 adenocarcinoma human alveolar basal epithelial cells and BEAS-2B human bronchial epithelial cells [72]. Another study revealed that EBV induced EMT of human corneal epithelial cells through activation of PI3K or Akt and ERK signalling pathways [29]. The EBV Latent Membrane Protein 1 (LMP1) and 2A (LMP2A) are involved in EMT; it was first identified that LMP1 induced EMT via Twist in nasopharyngeal carcinoma tissues [77]. Furthermore, LMP1 in lung epithelium predisposes cells to undergo EMT by enhancing signalling through the ERK signalling pathway and the interaction with TGF-β1 may also stimulate EMT [78]. In addition, HCV induces EMT: HCV core protein repressed E-cadherin expression by upregulating E12/E47 to induce EMT [79]. In cholangiocarcinoma, HCV has been shown to induce EMT, promoting carcinoma progression through a mechanism dependent on the lysyl oxidase-like 2 signalling pathways [80].
Conclusion
Considering these pathophysiological consequences of inflammation, inflammatory molecules/markers play a pivotal role in tumor initiation, tumor dissemination, formation of secondary tumors/tumor metastasis and angiogenesis. Targeting the procarcinogenic products of inflammation like free radicals, arachidonic acid metabolites, NFκB transcription factor, TNF-α, CXC chemokines and AKT can be an important approach to stopping cancer development and progression. Although inhibition of NFκB, and TNF-α impairs the normal function of immune cells, expression of inflammatory cytokines/ chemokines associated with immunosuppressive cells results in a lack of immunity might lead to the disease progression. Studies on inflammation-derived cancers with molecular approaches and knockout models might help address the problem of cancer origin. Several studies are showing that the consumption of natural compounds like omega 3 fatty acids, curcumin, S-adenosylmethionine, green tea, frankincense and capsaicin have antiinflammatory and antioxidant properties. Usually, the natural compounds do not have side effects and maintain normal cell homeostasis. Correlating the beneficial role of these compounds with chronic inflammation and subsequent development of tumour progression might be helpful for patients with better treatment.
Conflicts of Interest
All the Authors declared that, there are no conflicts of interest
Funding Statement
Grants Received from the Indian Council of Medical Research (3/1/3/ PDF (16)/2017 HRD)
Acknowledgment
Our sincere thanks to ICMR for providing the postdoctoral fellowship. We would like to thank the Director, the National Institute of Nutrition for the support of my post-doctoral research.