ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
- Biomedical Research (2016) Volume 27, Issue 3
The expression of TIPE2 in peripheral blood mononuclear cells in patients with acute coronary syndrome.
Department of Cardiology, Weifang People’s Hospital, Weifang, Shandong, P.R.China
Accepted date: February 22, 2016
Objective: We aimed to examine the expression and significance of tumor necrosis factor-a-induced protein-8 like-2 (TIPE2) in peripheral blood mononuclear cells (PBMCs) in patients with acute coronary syndrome (ACS).
Methods: TIPE2 mRNA and protein levels in PBMCs from ACS patients were detected by RT-PCR, qRT-PCR and western blot. The interleukin-10 (IL-10) and interferon-γ (IFN-γ) concentrations of blood serum were detected by ELISA. We also assessed the association between TIPE2 mRNA expression and IL-10 and IFN-γ concentrations.
Results: Our research found that TIPE2 mRNA and protein expression were decreased in ACS patients compared with stable angina pectoris (SAP) and healthy person. The level of IL-10 in ACS patients was obviously lower than that in SAP individuals and normal controls, while the level of IFN-γ in ACS patients was significantly higher than that in control subjects. Furthermore, the expression level of TIPE2 mRNA was positively correlated with IL-10 concentration.
Conclusion: Our data indicate that reduced TIPE2 expression may contribute to the pathogenesis of ACS.
Keywords
TIPE2, Acute coronary syndromes, IL-10, IFN-γ.
Introduction
Acute coronary syndromes (ACS) including unstable angina pectoris (UAP) and acute myocardial infarction (AMI) induced by atherosclerosis (AS) is a chronic inflammation of arterial vessel wall characterized by abnormal metabolism of lipid [1,2]. Many researchers believe that the injury of the vessel wall endothelium, infiltration of monocytes, activation of macrophages by oxidized low-density lipoprotein (ox-LDL) and excessive release of cytokines are the key factors for formation of the atherosclerotic plaque. Atherosclerotic plaque instability often leads to plaque rupture and thrombosis, which is the main cause of acute cardiocerebrovascular events [3-5]. The numbers of elderly people with ACS is rising in our country and ACS severely affects the health of older men; however, the mechanisms of ACS still remain unclear [6]. Accumulating evidence demonstrates that many immune cells and cytokines including pro-inflammatory as well as anti-inflammatory cytokines play an essential role in the development of ACS [7,8].
Tumor necrosis factor-α-induced protein-8 like-2 (TIPE2) is a newly identified immune negative regulator which plays an important role in maintaining immune homeostasis [9]. It belongs to tumor necrosis factor-α-induced protein-8 (TNFAIP8) family. TIPE2 predominantly expresses in immune cells and immune tissues, such as lymphocytes and monocytes and its deficiency in mice leads to multiorgan inflammation, enlarged spleen and premature death [10,11]. Interestingly, it is found that TIPE2 highly expresses in macrophages and serves as a negative regulator of innate immunity through negatively regulating both T cell receptor, inhibiting Toll-like receptor(TLR) and NF-κB signaling pathway [12,13]. Studies suggest that TIPE2 is associated with systemic autoimmunity, diabetic nephropathy, and hepatitis B in human [14,15]. Recent studies reveal that TIPE2 plays a crucial atheroprotective role by regulating macrophage responses to oxidized low-density lipoprotein [16]. However, expression and significance of TIPE2 in patients with acute coronary syndromes still remains enigmatic. In the present study, we explored the role of TIPE2 in ACS by detecting the mRNA and protein levels of TIPE2 in PBMCs from ACS patients and healthy controls, and measured the plasma levels of IL-10 and IFN-γ in patients with ACS.
Patients and Method
Patients
The study group comprised of 35 persons with SAP (22 men and 13 women, mean age: 55.8 ± 6.7 years), 28 patients with UAP (19 men and 9 women, mean age 59.5 ± 7.9 years), 27 patients with AMI (18 men and 9 women, mean age 60.1 ± 6.9 years) and 33 healthy controls (20 men and 13 women, mean age 56.9 ± 8.1 years) that were recruited from Weifang People’s Hospital from 2011 to 2014. Inclusion criteria: stable angina pectoris is judged by typical exertional chest discomfort that is associated with down sloping or horizontal ST-segment depression >1 mm in an exercise test. Acute myocardial infarction confirmed by significant rise of troponin I and creatine kinase-MB levels, and unstable angina confirmed by chest pain at rest with definite ischemic electrocardiographic changes, including ST-segment changes and/or T-wave inversion and angiographic evidence of coronary artery stenosis (>70%). Patients with peripheral vascular disease, autoimmune diseases, heart failure, renal failure, malignant tumor, diabetes or treatment with anti-inflammatory drugs were excluded. The healthy subjects were persons who visited the hospital for medical check-ups and they were all without any clinical signs or atherosclerosis history and were not undergoing any treatments. Laboratory assessments including serum creatinine levels, LDL, highdensity lipoprotein (HDL), total cholesterol (TC), C-reactive protein (CRP) and blood glucose levels were determined. History of hypertension and current tobacco use were also evaluated. Thirty seven percent of recruited participants were current cigarette smokers, 41% had a history of hypertension. All the subjects provided informed consent. Subject characteristics are presented in table 1.
| Characteristics | Control(n=33) | SAP(n=35) | UAP(n=28) | AMI(n=27) |
|---|---|---|---|---|
| Female, n (% ) | 13 (39%) | 13(37%) | 9(32%) | 9(33%) |
| Male, n (% ) | 20 (61%) | 22(63%) | 19(68%) | 18(67%) |
| Age(years) | 56.9 ± 8.1 | 55.8 ± 6.7 | 59.5 ± 7.9 | 60.1 ± 6.9 |
| TC(mmol/L) | 3.92 ± 1.03 | 4.24 ± 0.67 | 5.15 ± 0.98 | 4.93 ± 0.82 |
| LDL-C(mmol/L) | 2.15 ± 0.57 | 2.33 ± 0.59 | 2.58 ± 0.60 | 2.62 ± 0.71 |
| HDL-C(mmol/L) | 1.21 ± 0.22 | 1.13 ± 0.26 | 1.09 ± 0.32 | 1.08 ± 0.35 |
| GLU(mmol/L) | 5.14 ± 0.98 | 5.49 ± 1.03 | 5.62 ± 1.21 | 5.67 ± 1.32 |
| CRP (mg/L) | 2.01 ± 1.78 | 2.57 ± 1.13 | 4.88 ± 2.12* | 5.31 ± 1.14* |
| Creatinine(µmol/L) | 72.13 ± 15.22 | 80.34 ±16.41* | 84.53 ±20.98* | 83.25 ± 21.89* |
| Smoking, n(%) | 10(30) | 14(40) | 10(36) | 12(44) |
| Hypertension, n(%) | 11(33) | 12(34) | 14(50) | 13(48) |
| Data shown as mean ± SEM. TC: total cholesterol; LDL-C: low-density lipoprotein cholesterol; HDL-C: high-density lipoprotein cholesterol; GLU: fasting glucose; *P < 0.05, versus control | ||||
Table 1: Characteristics of the studied subjects.
RNA isolation and Quantitative real-time PCR
Venous blood samples were collected at the time of patient enrollment. PBMC were separated by density gradient centrifugation from peripheral blood. RNA was isolated by using Trizol reagent (Invitrogen, California, USA), followed by reverse transcription using the Reverse-Transcribe Kit (TIANGEN, Beijing, China). We first performed RT-PCR with TIPE2 specific primers (sense 5’- CCCTCGAGGCCGCCACCACCATGG-3’, and anti-sense 5’- CG GGATCCGAGCTTCCCTTCG-3’) for 28 cycles (95°C for 30sec, 58°C for 30sec and 72°C for 30sec). Then quantitative real-time PCR was performed using the SYBR Green I RealTime PCR kit (Takara, Dalian, China). Primer sequences for TIPE2 used were: 5'- GGAACATCCAAGGCAAGACTG-3'(sense) and 5'- AGCACCTCACTGCTTGTCTCATC-3'(antisense). The thermal cycle profile was as follows: 95˚C for 10 sec, followed by 40 cycles of amplification 95˚C for 5 sec, 60˚C for 41 sec. GAPDH (sense 5'-AACGGATTTGGTCGTATTGGG3' and antisense 5'-CCTGGAAGATGGTGATGGGAT-3') was applied as control. Each sample was conducted in triplicate. Quantitative real time PCR was analyzed using the 2−ΔΔCt method.
Western blot
Proteins were extracted from PBMC using Trizol reagent according to the manufacture's protocol and protein concentration was determined using BCA protein assay reagent (Pierce, Rockford, IL, USA). Total proteins (30 μg) were loaded in SDS-PAGE and electro-blotted onto polyvinylidene difluoride membrane (Millipore, Billerica, MA, USA). After blockage with 5% non-fat milk, the membranes were incubated with anti-TIPE2 antibody (1:500, Boster Biological Technology Inc, China) and anti-β-actin (1:1000, Santa Cruz, CA) overnight at 4°C, followed by washing three times and incubation with HRP-conjugated secondary antibodies (goat anti-rabbit IgG or goat anti-mouse IgG, ZSGB-Bio, Beijing, China,) for 1 h at room temperature. After washing, the bounds were visualized with ECL reagent (Pierce, Rockford, IL, USA). Densitometry analysis was performed to quantify protein bands.
Cytokine measurement by ELISA
Plasma IL-10 and IFN-γ levels were detected by enzyme-linked immunosorbent assay (ELISA) following the manufacture's protocol (eBioscience, USA). All assays were run in triplicate and the mean value was used for statistical analysis.
Statistical analysis
All data were expressed as mean ± SEM. One-way ANOVA was used to compare difference among groups. Correlations analysis involved Pearson’s correlation test. A P value <0.05 was considered significant.
Results
Baseline clinical characteristics of the studied subjects
The age, sex, hypertension or smoking did not differ significantly among the four groups. However, CRP (C-reactive protein) level was significantly higher in patients with ACS and SAP.
The creatinine level in ACS was also significantly higher than that of control group. The other clinical characteristics including lipid, lipoprotein fractions and fasting glucose are listed in table 1.
The expression of TIPE2 mRNA in PBMC from patients with ACS and healthy controls
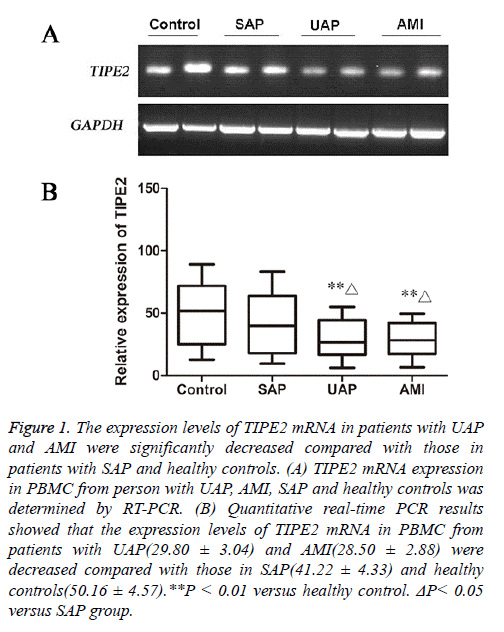
Both RT-PCR and Quantitative real-time PCR results showed that TIPE2 mRNA levels in PBMC from patients with AMI and UAP were significantly decreased than that in healthy controls(P<0.01).
Further analysis showed that TIPE2 mRNA was reduced in patients with AMI and UAP compared with that in patients with SAP (P<0.05, Figure 1).
Figure 1: The expression levels of TIPE2 mRNA in patients with UAP and AMI were significantly decreased compared with those in patients with SAP and healthy controls. (A) TIPE2 mRNA expression in PBMC from person with UAP, AMI, SAP and healthy controls was determined by RT-PCR. (B) Quantitative real-time PCR results showed that the expression levels of TIPE2 mRNA in PBMC from patients with UAP(29.80 ± 3.04) and AMI(28.50 ± 2.88) were decreased compared with those in SAP(41.22 ± 4.33) and healthy controls(50.16 ± 4.57).**P < 0.01 versus healthy control. ΔP< 0.05 versus SAP group.
Analysis of TIPE2 protein levels in PBMC from patients with ACS and healthy controls
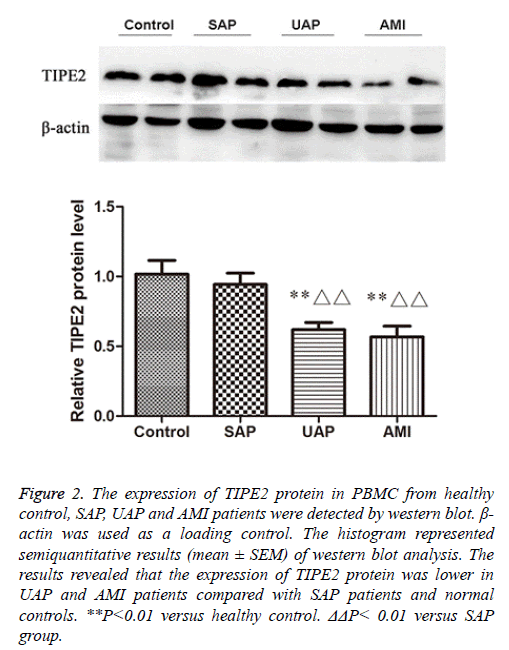
We further confirmed the expression levels of TIPE2 protein in PBMC from the four groups by western blot.
We observed that the expression of TIPE2 protein was lower in AMI and UAP patients compared with normal controls and SAP patients (Figure 2), which suggests the down-regulation of TIPE2 expression in ACS patients.
Figure 2: The expression of TIPE2 protein in PBMC from healthy control, SAP, UAP and AMI patients were detected by western blot. β- actin was used as a loading control. The histogram represented semiquantitative results (mean ± SEM) of western blot analysis. The results revealed that the expression of TIPE2 protein was lower in UAP and AMI patients compared with SAP patients and normal controls. **P<0.01 versus healthy control. ΔΔP< 0.01 versus SAP group.
Serum IL-10 and IFN-γ levels in patients with ACS and healthy controls
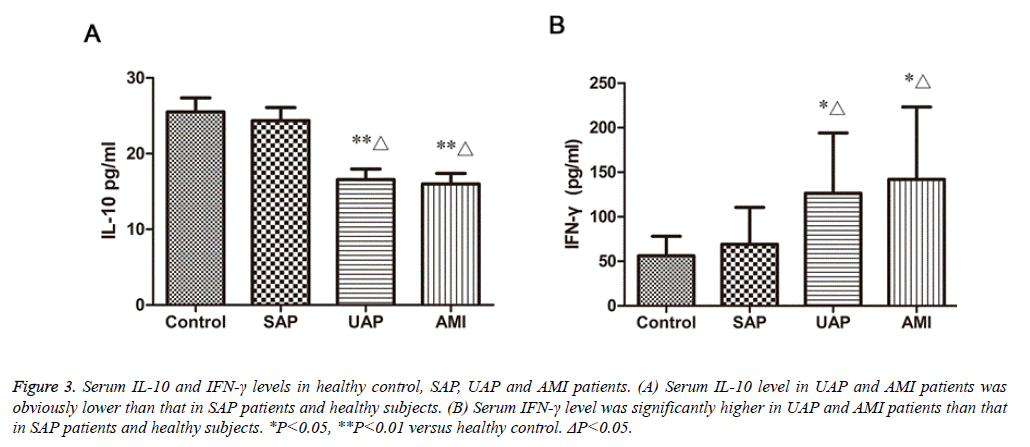
Plasma IL-10 and IFN-γ levels of the four studied groups are shown in fig.3. The IL-10 concentrations in patients with AMI, UAP (15.98 ± 1.39 pg/ml, 16.57 ± 1.38 pg/ml, respectively) were significantly decreased compared with those in SAP and normal controls (23.37 ± 1.71 pg/ml, 25.52 ± 1.83 pg/ml, respectively) (P<0.01, Figure 3A). The IFN-γ concentrations in patients with AMI and UAP (142.1 ± 19.68 pg/ml, 126.3 ± 16.46 pg/ml, respectively) were significantly higher than those in patients with SAP and healthy people (69.12 ± 9.25 pg/ml, 56.08 ± 4.77 pg/ml, respectively) (P<0.05, Figure 3B). The difference in IL-10 and IFN-γ between SAP patients and healthy people was not significant.
Figure 3: Serum IL-10 and IFN-γ levels in healthy control, SAP, UAP and AMI patients. (A) Serum IL-10 level in UAP and AMI patients was obviously lower than that in SAP patients and healthy subjects. (B) Serum IFN-γ level was significantly higher in UAP and AMI patients than that in SAP patients and healthy subjects. *P<0.05, **P<0.01 versus healthy control. ΔP<0.05.
Correlation of TIPE2 expression with plasma cytokines and other parameters
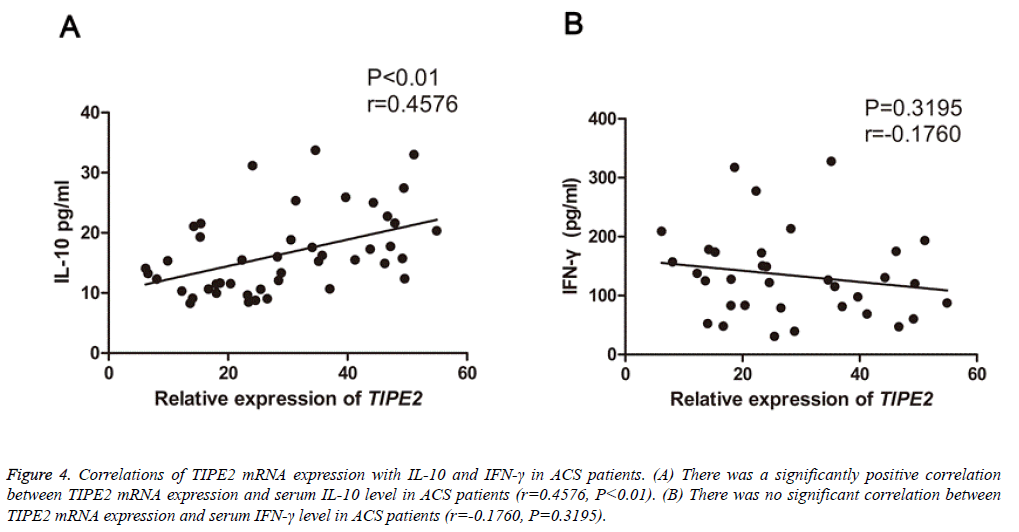
To evaluate the clinical significance of TIPE2 in ASC, we subsequently analyzed the correlations of TIPE2 mRNA expression with IL-10, IFN-γ, lipid and lipoprotein fractions (triglycerides, high density lipoprotein cholesterol and low-density lipoprotein cholesterol), fasting glucose and CRP in patients with ASC. Interestingly, a positively correlation was found between TIPE2 mRNA levels and serum IL-10 (P<0.05, r=0.4576, Figure 4A) in all ACS patients. However, the TIPE2 mRNA levels failed to correlate with IFN-γ concentrations (P=0.3195, r=-0.1760, Figure 4B) and other parameters mentioned above (data not shown).
Figure 4: Correlations of TIPE2 mRNA expression with IL-10 and IFN-γ in ACS patients. (A) There was a significantly positive correlation between TIPE2 mRNA expression and serum IL-10 level in ACS patients (r=0.4576, P<0.01). (B) There was no significant correlation between TIPE2 mRNA expression and serum IFN-γ level in ACS patients (r=-0.1760, P=0.3195).
Discussion
TIPE2 has been recognized as a negative regulator of innate and adaptive immunity, which involves in many inflammation-related diseases. ACS is a non-resolving inflammatory disease in which immune mechanisms interact with metabolic risk factors initiate and accelerate its progress [17,18]. Previous studies revealed that TIPE2 deficiency accelerated atherosclerosis development in mice [19], however, the exact expression patterns and significance of TIPE2 in ACS patients has never been explored. In this study, we provided the first evidence that both TIPE2 mRNA and protein in ACS patients were down-regulated compared with healthy person and SAP patients, which suggests TIPE2 may play a potential role in ACS development.
In recent years, a number of studies have found that TIPE2 plays an important role in several human inflammatory diseases. However, TIPE2 may participate in the pathogenesis of some chronic inflammatory diseases by different mechanism. For example, TIPE2 mRNA expression was significantly decreased in systemic lupus erythematosus (SLE) patients, and the TIPE2 mRNA levels negatively correlated with the SLE disease activity index (SLEDAI) and the myxoma resistance protein (MX1) in the SLE patients [20]. In addition, decreased expression of TIPE2 was found in asthmatic children, and TIPE2 mRNA was negatively correlated with serum total immunoglobulin E, eosinophil, and interleukin-4 (IL-4) [21]. However, increased expression of TIPE2 was found in several inflammation diseases, such as diabetic patients and chronic rejection patients [22]. Given the different role of TIPE2 played in different inflammation diseases, our next question is how TIPE2 affected the ACS.
It has been widely acknowledged that CD4+ T lymphocytes including Th1 and Th2 cells play a critical role in the progression of ACS [23,24]. Th1 cytokines, such as IFN-γ and IL-2, contribute to inflammatory effects by activating macrophages, endothelial and smooth muscle cells [25,26]. Th2 cells may play a protective role in the progression of ACS by secretion of anti-inflammatory cytokines such as IL-10. Increased evidence indicates that Th1/Th2 imbalance involved in the occurrence and development of ACS [27]. In this study, we measured the levels of IFN-γ and IL-10 in ACS patients and SAP patients and healthy controls. Our results showed a significantly lower level of serum IL-10 but higher level of serum IFN-γ in ACS patients. These data are in agreement with the previous reports that elevated IFN-γ and decreased IL-10 secretion in atherosclerosis diseases [28,29].
We further analyzed the correlations of TIPE2 mRNA expression with IL-10 and IFN-γ levels in ACS patients. The results showed obviously positive correlations of TIPE2 expression with IL-10. Unfortunately, the correlation between TIPE2 and IFN-γ was not significant. Considering the close relationship between decreased IL-10 level and the development of coronary atherosclerotic plaque inflammatory, TIPE2 may also to some extent affect the development of ACS by regulate IL-10 secretion. It is found that TIPE2 highly expresses in macrophages and serves as a negative regulator through inhibiting TLR signaling, we think that reduced TIPE2 may mainly affect Th2 response, thus regulate IL-10 secretion.
In conclusion, this study is the first to demonstrate the reduced expression of TIPE2 in PBMC in patients with ACS, and the expression of TIPE2 mRNA is positively relate to serum IL-10, which suggests that TIPE2 may play an important role in the pathogenesis of ACS. However, further studies are needed to explore the exact mechanism of TIPE2 in ACS.
References
- Greaves DR, Channon KM. Inflammation and immune responses in atherosclerosis. Trends Immunol. 2002; 23: 535-541.
- Salisbury D, Bronas U. Inflammation and immune system contribution to the etiology of atherosclerosis: mechanisms and methods of assessment. Nurs Res 2014; 63: 375-385.
- Zhu JJ, Wang X. Chronic inflammation, autoimmunity and atherosclerosis. Sheng Li Ke Xue Jin Zhan 2002; 33: 327-331.
- Scott J. The pathogenesis of atherosclerosis and new opportunities for treatment and prevention. J Neural Transm Suppl 2002; 1-17.
- Dzau VJ, Braun-Dullaeus RC, Sedding DG. Vascular proliferation and atherosclerosis: new perspectives and therapeutic strategies. Nat Med 2002; 8: 1249-1256.
- Liang Y, Tan HQ, Zhu J, Zhang Y, Liu LS. Chinese Coordinating Center for Organization to Assess Strategies for Ischemic S. Risk factors of sudden death and death from arrhythmia in patients with non-ST elevation acute coronary syndromes in China. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2005; 17: 142-145.
- Hajishengallis G, Sharma A, Russell MW, Genco RJ. Interactions of oral pathogens with toll-like receptors: possible role in atherosclerosis. Ann Periodontol 2002; 7: 72-78.
- Alber HF, Frick M, Suessenbacher A, Doerler J, Schirmer M, Stocker EM, Dichtl W, Pachinger O, Weidinger F. Effect of atorvastatin on circulating proinflammatory T-lymphocyte subsets and soluble CD40 ligand in patients with stable coronary artery disease--a randomized, placebo-controlled study. Am Heart J 2006; 151: 139.
- Sun H, Gong S, Carmody RJ, Hilliard A, Li L, Sun J, Kong L, Xu L, Hilliard B, Hu S, Shen H, Yang X, Chen YH. TIPE2, a negative regulator of innate and adaptive immunity that maintains immune homeostasis. Cell 2008; 133: 415-426.
- Freundt EC, Bidere N, Lenardo MJ. A different TIPE of immune homeostasis. Cell 2008; 133: 401-402.
- Zhang G, Hao C, Lou Y, Xi W, Wang X, Wang Y, Qu Z, Guo C, Chen Y, Zhang Y, Liu S. Tissue-specific expression of TIPE2 provides insights into its function. Mol Immunol. 2010; 47: 2435-2442.
- Zhang X, Wang J, Fan C, Li H, Sun H, Gong S, Chen YH, Shi Y. Crystal structure of TIPE2 provides insights into immune homeostasis. Nat Struct Mol Biol 2009; 16: 89-90.
- Zhang L, Shi Y, Wang Y, Zhu F, Wang Q, Ma C, Chen YH, Zhang L. The unique expression profile of human TIPE2 suggests new functions beyond its role in immune regulation. Mol Immunol 2011; 48: 1209-1215.
- Xi W, Hu Y, Liu Y, Zhang J, Wang L, Lou Y, Qu Z, Cui J, Zhang G, Liang X, Ma C, Gao C, Chen Y, Liu S. Roles of TIPE2 in hepatitis B virus-induced hepatic inflammation in humans and mice. Mol Immunol 2011; 48: 1203-1208.
- Zhang S, Zhang Y, Wei X, Zhen J, Wang Z, Li M, Miao W, Ding H, Du P, Zhang W, He M, Yi F. Expression and regulation of a novel identified TNFAIP8 family is associated with diabetic nephropathy. Biochim Biophys Acta 2010; 1802: 1078-1086.
- Lou Y, Liu S, Zhang C, Zhang G, Li J, Ni M, An G, Dong M, Liu X, Zhu F, Zhang W, Gao F, Chen YH, Zhang Y. Enhanced atherosclerosis in TIPE2-deficient mice is associated with increased macrophage responses to oxidized low-density lipoprotein. J Immunol 2013; 191: 4849-4857.
- Choi YH, Lee WH, Lee Y, Kim JK, Lee SY, Park JE. Correlation between monocyte and T-lymphocyte activation markers in patients with acute coronary syndrome. Jpn Heart J 2000; 41: 605-615.
- Anogeianaki A, Angelucci D, Cianchetti E, D'Alessandro M, Maccauro G, Saggini A, Salini V, Caraffa A, Tete S, Conti F, Tripodi D, Shaik-Dasthagirisaheb YB. Atherosclerosis: a classic inflammatory disease Int J Immunopathol Pharmacol. 2011; 24: 817-825.
- Zhang G, Zhang W, Lou Y, Xi W, Cui J, Geng M, Zhu F, Chen YH, Liu S. TIPE2 deficiency accelerates neointima formation by downregulating smooth muscle cell differentiation. Cell Cycle 2013; 12: 501-510.
- Li D, Song L, Fan Y, Li X, Li Y, Chen J, Zhu F, Guo C, Shi Y, Zhang L. Down-regulation of TIPE2 mRNA expression in peripheral blood mononuclear cells from patients with systemic lupus erythematosus Clin Immunol 2009; 133: 422-427.
- Ma Y, Liu X, Wei Z, Wang X, Wang Z, Zhong W, Li Y, Zhu F, Guo C, Zhang L, Wang X. The expression and significance of TIPE2 in peripheral blood mononuclear cells from asthmatic children Scand J Immunol 2013; 78: 523-528.
- Jia L, Gui B, Tian P, Yao G, Fu R, Wang L, Ge H, Ou Y. TIPE2, a novel biomarker for clinical chronic kidney allograft rejection. Artif Organs 2013; 37: 221-225.
- Keaney JF. Jr. Immune modulation of atherosclerosis. Circulation 2011; 124: 559-560.
- Zhu ZF, Meng K, Zhong YC, Qi L, Mao XB, Yu KW, Zhang W, Zhu PF, Ren ZP, Wu BW, Ji QW, Wang X, Zeng QT. Impaired circulating CD4+ LAP+ regulatory T cells in patients with acute coronary syndrome and its mechanistic study. PLoS One 2014; 9: e88775.
- Hu Z, Li D, Hu Y, Yang K. Changes of CD4+CD25+ regulatory T cells in patients with acute coronary syndrome and the effects of atorvastatin. J Huazhong Univ Sci Technolog Med Sci 2007; 27: 524-527.
- Sardella G, De Luca L, Francavilla V, Accapezzato D, Di Roma A, Gianoglio O, Colantonio R, Mancone M, Fedele F, Paroli M. Effect of coronary percutaneous revascularization on interferon-gamma and interleukin-10 producing CD4+ T cells during acute myocardial infarction. Int J Immunopathol Pharmacol 2007; 20: 791-799.
- Methe H, Brunner S, Wiegand D, Nabauer M, Koglin J, Edelman ER. Enhanced T-helper-1 lymphocyte activation patterns in acute coronary syndromes. J Am Coll Cardiol 2005; 45: 1939-1945.
- Ben-Hadj-Khalifa-Kechiche S, Cornillet-Lefebvre P, Gillot L, Abboud N, Ben-Khalfallah A, Addad F, Almawi WY, Mahjoub T. Interleukin-10 microsatellite variants and the risk of acute coronary syndrome among Tunisians. Int J Immunogenet 2011; 38: 37-43.
- Dopheide JF, Knopf P, Zeller GC, Vosseler M, Abegunewardene N, Munzel T, Espinola-Klein C. Low IL-10/TNFalpha Ratio in Patients with Coronary Artery Disease and Reduced Left Ventricular Ejection Fraction with a Poor Prognosis After 10 Years. Inflammation 2015; 38: 911-922.