ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 1
The effects of polysaccharides from Maca (Lepidium meyenii Walp.) on exhaustive exercise-induced oxidative damage in rats
1Department of Physical Education, Yunnan University of Finance and Economics, Kunming 650221, PR China
2Department of Physical Education, Hebei Normal University of Science and Technology, Qinhuangdao, PR China
- *Corresponding Author:
- Rong-Wei Li
Department of Physical Education, Hebei Normal University of Science and Technology
West Hebei Street, Qinhuangdao, PR China
Accepted date: May 04, 2016
The present study aimed to investigate the effects of polysaccharides from Maca (MCP) on oxidative damage induced by exhaustive swimming exercise using rat models. The rats were divided into five groups: sedentary control group (SC), exercise control group (EC), exercise+50 mg/kg MCP treated group (EM-50), exercise+100 mg/kg MCP treated group (EM-100), exercise+200 mg/kg MCP treated group (EM-200). SC and EC groups received distilled water while three MCP treated groups received different doses of MCP (50, 100 and 200 mg/kg bw) by oral gavage once daily. After 28 days, the rats were forced to swim in a plastic tank until being exhausted, and the following biochemical parameters of oxidative damage were measured: aspartate aminotransferase (AST), creatine kinase (CK), lactate dehydrogenase (LDH), superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), glutathione reductase (GR), reduced glutathione (GSH), oxidized glutathione (GSSG), malondialdehyde (MDA), and 8-hydroxy-2'-deoxyguanosine (8-OHdG). In all MCP treated groups, serum LDH and CK levels were significantly lower than those in the EC group, whereas in the E-100 and M-200 groups, a decrease was also seen for serum AST levels. Furthermore, the GSSG and 8-OHdG levels in the E-100 and M-200 groups, as well as the MDA levels in all MCP treated groups were significantly lower. In contrast, all MCP treated groups exhibited significantly higher levels of SOD, CAT, GPx, GSH and GSH/GSSG ratio in muscle. The GR levels in muscle were also significantly increased in the E-100 and M-200 groups. These results demonstrate that MCP might have protective effects on exhaustive exerciseinduced oxidative damage.
Keywords
Polysaccharides, Maca, Exhaustive exercise, Oxidative damage, Protective effects, Biochemical parameters.
Introduction
It has been reported that strenuous physical exercise is associated with an increased free radicals and reactive oxygen species (ROS) production attributed to high oxygen consumption [1], which may reach 10-20 times the systemic levels and as much as 100-200 times the skeletal muscle levels, resulting in substantially increased mitochondrial electron flux [2]. Under normal circumstances, ROS are neutralized by an elaborate antioxidant defence system consisting of enzymes such as superoxide dismutase (SOD), catalase, glutathione peroxidase (GPx), glutathione reductase (GR), and nonenzymatic substances such as reduced glutathione (GSH) and vitamins A, C, E, and selenium [3]. When the ROS production overwhelms the capacity of the antioxidant defence system, oxidative stress is initiated [4], consequently oxidative damage of cellular macromolecules such as lipids, proteins and nucleic acids may occur. Skeletal muscle is the tissue with the largest mass in the body consisting of postmitotic cells, which are more prone to accumulate oxidative damage [5]. Recently, a number of studies have demonstrated that many antioxidant compounds, naturally occurring from plant sources, can be a useful strategy to reduce exercise-induced oxidative damage in humans and animal models [6]. Maca (Lepidium meyenii Walp.) from the Brassicaceae family native to the Americas grows in the suni and puna ecosystems of Peru (altitude>3500 m above sea level) [7]. Maca roots has been commonly used as traditional medicine and herbal foods for health promotion in the Andean region for thousands years. Traditionally it has been used to enhance both female and male fertility, improve sexual dysfunction, prevent osteoporosis, and relieve menopausal syndrome and increase physical endurance [8]. In the past few years, many studies have demonstrated that Maca roots extracts have a wide range of biological activities including antioxidant, antidepressant, antiviral, anti-fatigue, antihyperlipidemic, hypoglycemic, improving sexual performance, enhancing fertility, spermatogensis, and preventing estrogen-deficient bone loss [9-14]. To date, its biological activities are mainly attributed to phytosterols, alkaloids, isothiocyanates, glucosinolates, macaenes, and macamides [15]. Besides, recent studies have suggested that the polysaccharides from Maca (MCP) also exhibit strong antioxidant activities [16-18], possibly protecting against exercise-induced oxidative damage. Based on the aforementioned considerations, the present study was undertaken to investigate the protective effects of MCP on oxidative damage induced by exhaustive swimming exercise using rat models.
Materials and Methods
Reagents and chemicals
The diagnostic kit for the determination of aspartate aminotransferase (AST) was purchased from MingFeng Biological Technology Co. Ltd. (Shanghai, China). The diagnostic kits for the determination of reduced glutathione (GSH) and oxidized glutathione (GSSG) were purchased from Amyjet Scientific Inc. (Wuhan, China). The diagnostic kits for the determination of creatine kinase (CK), lactate dehydrogenase (LDH), superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), glutathione reductase (GR) and malondialdehyde (MDA) were purchased from JianCheng Biologic Project Co. (Nanjing, China). The enzymelinked immunosorbent assay kit for the determination of 8- hydroxy-2'-deoxyguanosine (8-OHdG) was purchased from the Control of Aging, Nikken SEIL Co. Ltd. (Shizuoka, Japan). Water was prepared in ultra-pure water system (Purkinje, Beijing, China). All other chemicals were of analytical grade and purchased from local suppliers (Kunming, China).
Plant material and extraction
The dried roots of Maca were purchased from the Yunnan Chinese Herbal Medicine Company (Kunming, China). The polysaccharides from Maca (MCP) were extracted according to a published method with minor modification [12]. Briefly, the plant material were grind to fine powder and extracted with distilled water in a ultrasonic cleaner bath set at a selected extraction conditions (solid/liquid ratio of 1:25, extraction time of 20 min, ultrasonic power of 200 W and extraction temperature of 50°C). After filtration to remove debris fragments, the filtrate was concentrated by rotary vacuum evaporator at 60°C, and subsequently was dried with a spray dryer. Then, 20 g Maca extracts was dissolved in 200 ml water, and 2 ml 10% (w/v) amylase and 1.0 ml 0.2 M phosphate buffer (pH 6.5) was added then. After enzymatic hydrolysis in water bath at 50°C for 2 h, 1 ml glucoamylase was added and incubated for 1 h. Enzymatic solution was heated to 100°C rapidly to inactivate enzyme reaction, and then cooled and diluted to 250 ml. The diluents extract solution was centrifuged (2,000 ×g, 30 min), then the supernatant was precipitated with addition of 4 times volume 95% (v/v) ethanol, and the precipitate was collected after centrifugation, redissolved in distilled water and treated with Sevag’s reagent several times to remove protein and then dialyzed against distilled water for 48 h at 4°C. MCP was again precipitated with ethanol and the precipitate thus obtained was lyophilized. The polysaccharides content was determined by the phenol-sulphuric acid method using glucose as a standard. Extraction yield of MCP was calculated as the polysaccharides content of extraction divided by dried roots of Maca weight, and the calculated extraction yield of MCP was 2.37% (w/w).
Animal care and maintenance
Male Sprague Dawley (SD) rats, weighing 220 ± 20 g, were provided by the Experimental Animal Center of Kunming Medical University (Kunming, China). The animals were maintained under constant conditions (temperature of 21-23°C and relative humidity of 50 ± 5%) with free access to standard rodent diet and tap water under a 12-h light/dark cycle. All procedures involving the use of laboratory animals were in accordance with the National Institutes of Health guidelines and were approved by the Institutional Animal Ethics Committee (IAEC) of Yunnan University of Finance and Economics (Kunming, China). Every effort was made to minimize the number of used animals and their suffering.
Grouping and MCP treatment
After acclimatization to laboratory conditions for 1 week, the animals were randomly divided into five groups of 10 rats each. (1) Sedentary control group (SC), (2) exercise control group (EC), (3) exercise + 50 mg/kg MCP treated group (EM-50), (4) exercise+100 mg/kg MCP treated group (EM-100), (5) exercise+200 mg/kg MCP treated group (EM-200). The rats of control group (SC and EC) were orally given vehicle (distilled water) in a volume of 1.0 ml once daily for 28 days. Three MCP treated group (EM-50, EM-100, and EM-200) were orally given MCP the same volume of MCP (50, 100 and 200 mg/kg bw) once daily for 28 days.
Exhaustive exercise protocol
In this study, swimming was chosen as the model of exerciseinduced oxidative damage since it is a natural behaviour of rodents, less stressful and it can prevent foot injury, which may generate ROS unrelated to strenuous exercise [19]. The protocol was adapted from a previous study with some modifications [20]. Briefly, 30 min after the last treatment with MCP or distilled water, the rats were allowed to swim with constant loads (tagged to the tail base) corresponding to 5% of their body weight. The swimming exercise was carried out in an acrylic plastic tank (90 cm × 45 cm × 45 cm) with 40 cm deep with water maintained at 28 ± 2°C. The rats were considered to be exhausted when they could not rise to the surface to breathe after 10 s [21]. The mean time to exhaustion was 106.81 min.
Biochemical analysis
After the completion of exhaustive swimming exercise, the rats were anesthetized with sodium pentobarbital (40 mg/kg bw, ip). Blood samples from the eyeballs were collected in the test tube without any anticoagulant for measurement of serum LDH, CK and AST. Serum was prepared by centrifugation (2,000 ×g, 10 min) at 4°C, decanted immediately, and was stored at -80°C until assays were performed. Next, the gastrocnemius muscles were carefully removed and rinsed in ice cold physiological saline solution, blotted dry and stored at -80°C until required for the SOD, CAT, GPx, GR, GSH, GSSG, MDA and 8-OHdG analyses. All the biochemical parameters were measured using diagnostic kits under the manufactures' instructions.
Statistical analysis
The statistical analysis was performed using the statistical package for the Social Sciences (version18.0; SPSS, Chicago, IL, USA). Data were expressed as mean ± standard deviation (SD) and assessed with one-way analysis of variance (ANOVA) followed by Fisher’s least-significant difference (LSD). A value of p<0.05 was considered to be significant.
Results and Discussion
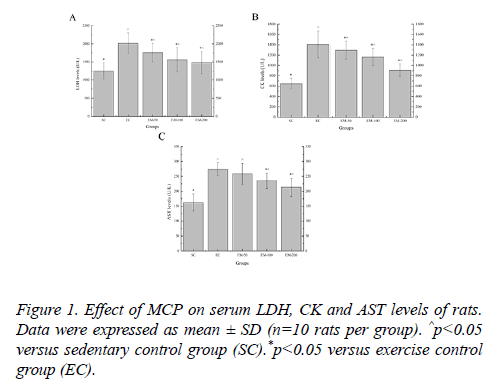
Effect of MCP on serum LDH, CK and AST levels of rats
Effect of MCP on serum LDH, CK and AST levels are shown in Figure 1. Compared with the SC group, the LDH levels in the EC, EM-50, E-100 and M-200 groups were significantly increased (p<0.05) by 60.59%, 40.06%, 24.67% and 18.22%, respectively; the CK levels in the EC, EM-50, E-100 and M-200 groups were significantly increased (p<0.05) by 117.52%, 99.92%, 79.93% and 40.01%, respectively; the AST levels in the EC, EM-50, E-100 and M-200 groups were significantly increased (p<0.05) by 69.03%, 58.91%,44.87% and 31.29%, respectively. Compared with the EC group, the LDH levels in the EM-50, E-100 and M-200 groups were significantly decreased (p<0.05) by 14.65%, 28.81% and 35.84%, respectively; the CK levels in the EM-50, E-100 and M-200 groups were significantly decreased (p<0.05) by 9.21%, 20.89% and 55.37%, respectively; the AST levels in the E-100 and M-200 group were significantly decreased (p<0.05) by 16.67% and 28.74%, respectively.
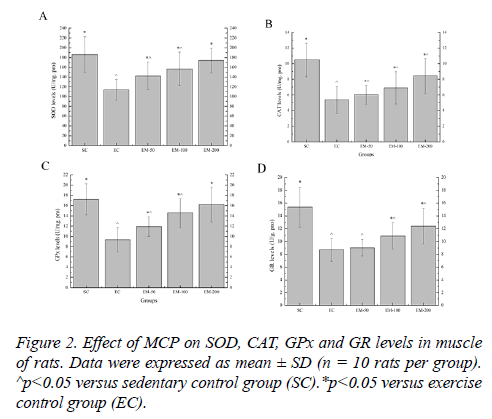
Effect of MCP on SOD, CAT, GPx and GR levels in muscle of rats
Effect of MCP on the SOD, CAT, GPx and GR levels in muscle are shown in Figure 2. Compared with the SC group, the SOD levels in the EC, EM-50, and EM-100 groups were significantly decreased (p<0.05) by 63.82%, 31.04% and 18.89%, respectively; the CAT levels in the EC, EM-50, E-100 and M-200 groups were significantly decreased (p<0.05) by 95.34%, 73.68%, 51.81% and 24.14%, respectively; the GPx levels in the EC, EM-50, and EM-100 groups were significantly decreased (p<0.05) by 84.08%, 44.67% and 18.26%, respectively; the GR levels in the EC, EM-50, E-100 and M-200 groups were significantly decreased (p<0.05) by 76.87%, 70.21%, 41.40% and 24.05%, respectively. Compared with the EC group, the SOD levels in the EM-50, E-100 and M-200 groups were significantly increased (p<0.05) by 25.02%, 37.79% and 52.93%, respectively; the CAT levels in the EM-50, E-100 and M-200 groups were significantly increased (p<0.05) by 12.48%, 28.68% and 57.36%, respectively; the GPx levels in the EM-50, E-100 and M-200 groups were significantly increased (p<0.05) by 27.24%, 55.66% and 73.18%, respectively; the GR levels in the E-100 and M-200 groups were significantly increased (p<0.05) by 25.09% and 42.58%, respectively.
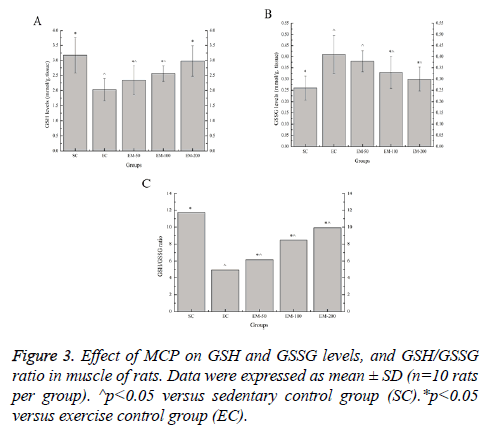
Effect of MCP on GSH and GSSG Levels, and GSH/ GSSG ratio in muscle of rats
Effect of MCP on the GSH and GSSG levels, and GSH/GSSG ratio in muscle are shown in Figure 3. Compared with the SC group, the GSH levels in the EC, EM-50, and EM-100 groups were significantly decreased (p<0.05) by 56.16%, 35.47% and 23.83%, respectively; the GSSG levels in the EC, EM-50, E-100 and M-200 groups were significantly increased (p<0.05) by 57.69%, 46.15%, 26.92% and 15.38%, respectively; the GSH/GSSG ratio in the EC, EM-50, E-100 and M-200 groups were significantly decreased (p<0.05) by 137.17%, 90.58%, 38.61% and 18.23%, respectively. Compared with the EC group, the GSH levels in the EM-50, E-100 and M-200 groups were significantly increased (p<0.05) by 15.27%, 26.11% and 46.80%, respectively; the GSSG levels in the E-100 and M-200 groups were significantly decreased (p<0.05) by 24.24% and 36.67%, respectively; the GSH/GSSG ratio in the EM-50, E-100 and M-200 groups were significantly increased (p<0.05) by 24.44%, 71.11% and 100.61%, respectively.
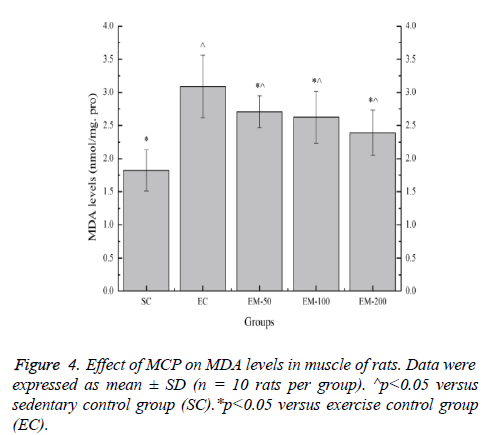
Effect of MCP on MDA levels in muscle of rats
Effect of MCP on MDA levels in muscle of rats is shown in Figure 4. Compared with the SC group, the MDA levels in the EC, EM-50, EM-100 and M-200 groups were significantly increased (p<0.05) by 69.78%, 48.90%, 44.51% and 31.32%, respectively. Compared with the EC group, the MDA levels in the EM-50, E-100 and M-200 groups were significantly decreased (p<0.05) by 14.02%, 17.49% and 29.29%, respectively.
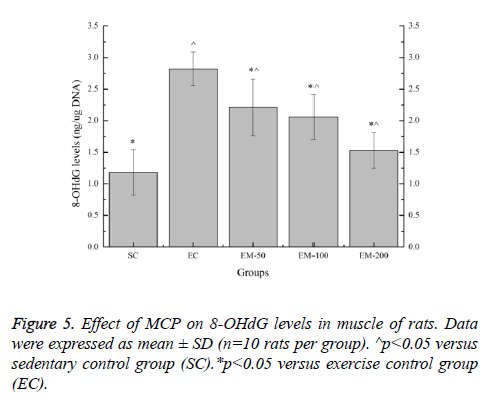
Effect of MCP on 8-OHdG levels in muscle of rats
Effect of MCP on 8-OHdG levels in muscle of rats are shown in Figure 5. Compared with the SC group, the 8-OHdG levels in the EC, EM-50, EM-100 and M-200 groups were significantly increased (p<0.05) by 138.98%, 87.29%, 74.58% and 29.66%, respectively. Compared with the EC group, the 8- OHdG levels in the E-100 and M-200 groups were significantly decreased (p<0.05) by 27.60%, 36.89% and 84.31%, respectively.
Discussion
Over the past few decades, ROS have been identified as likely components in cellular differentiation, aging, mutagenesis, carcinogenesis, pathophysiology of many diseases and oxidative damage during strenuous exercise [22]. Numerous studies have demonstrated that an increased dietary intake of natural antioxidant compounds, such as vitamin C, vitamin E, polysaccharides, polyphenols, resveratrol, curcumin, carotenoids, flavonoid, and saponins, may prevent and/or reduce ROS-related diseases in human and animals [23]. Scicchitano et al. [24] reported that resveratrol, curcumin, and carotenoids play a peculiar role in ameliorating human dyslipidaemia. Sahin et al. [25] discovered that lycopene protects against diethylnitrosamine-induced hepatocarcinogenesis. Li et al. [26] found that Ganoderma Lucidum Polysaccharides has an antihyperglycemic effect. Ciccone et al. [27] demonstrated the anti-inflammatory action of carotenoids (i.e., lutein, lycopene, and α- and β- carotene) and their protective effect on cardiovascular events. Qi et al. [28] found that ginsenosides-Rb1 possesses protective effects on swimming exercise-induced oxidative stress. The present study was designed to investigate the effects of MCP, a natural antioxidant compounds and principal active ingredients of Maca, on exhaustive exercise-induced oxidative damage in rats.
Exercise-induced oxidative stress can significantly increase the risk of muscle damage, and ROS play an important role in muscle damage [29], which leads to a temporary loss of the exercising capacity of muscle for force production and an increases in muscle soreness postexercise [30]. LDH and CK are released into blood as the result of the destruction of the cell membrane by oxidative stress or muscle damage, as well as the direct destruction of the cell wall and tissue necrosis [31]. Therefore, serum LDH and CK have been used as accurate indicators of muscle damage after strenuous exercise. It has also been reported that muscle damage is mostly associated with release of AST enzymes into the blood stream. AST is present in both cytoplasm and mitochondria and is released into the blood after injury or death of cells [32]. Many studies have indicated that strenuous exercise elevates the levels of LDH, CK and AST in blood [33]. The results of this study showed that MCP could protect muscle damage after exhaustive exercise by decreasing the LDH, CK and AST levels in blood.
ROS generation during strenuous exercise evokes changes in the activity of the antioxidant defense systems of the living body, both enzymatic and nonenzymatic [34]. The main antioxidant enzymes include SOD, GPx, CAT and GR. SOD converts superoxide radicals to hydrogen peroxide, which then can be further converted to water through GPX or CAT. CAT catalyzes the breakdown of hydrogen peroxide to form water. GPX utilizes GSH as a hydrogen donor for the removal of peroxides. The produced GSSG may in the presence of NADPH be GSH through GR [35]. GR plays a role in the antioxidant defence processes, by reducing GSSG to GSH with consumption of NADPH, thus maintaining a high intracellular GSH/GSSG ratio [36]. In recent years, substantial evidence indicates that strenuous exercise causes a dramatic drop in antioxidant enzymes activities in various tissues [28]. The decrease in antioxidant enzymes activities after strenuous exercise may be possibly due to their use against the free radicals and their inhibition by free radical species [37]. The results of this study showed that MCP could enhance antioxidant enzymes activities to protect against ROS mediated oxidative damage.
Glutathione is one of the most important non-enzymatic antioxidants in antioxidant defense system, which can exist intracellularly in either an oxidized (GSSG) or reduced (GSH) state [38]. GSH is oxidized to GSSG in cells in response to an increase in ROS. When the rate of oxidation is low, much of the GSSG produced may be reduced to GSH by GR with a consumption of one NADPH. However, with a more severe oxidative stress, the rate of GSSG reduction cannot match that of its formation, thus resulting in the accumulation of intracellular GSSG [39]. Therefore, GSH/GSSG ratio is a sensitive index of cellular redox status. It has been shown that strenuous exercise can promote GSH oxidation to GSSG, cause disturbances in GSH homeostasis, such as decreasing its concentration, degrading its cellular redox status, and interfere with GSH transport [40], as a consequence, lead to an decreased GSH/GSSG ratio in various tissues. The results of this study showed that MCP could protect exercise-induced oxidative damage by enhancing GSH/GSSG ratio and GSH levels in muscle. It is possible that MCP sntimulate the production of GSH from GSSG by induction of the GR, and additional studies are needed to confirm this hypothesis.
Strenuous exercise is known to induce the generation of ROS, and this increased level of ROS as a result of increase in mitochondrial oxygen consumption and electron transport flux, inducing lipid peroxidation [41]. MDA is a three carbon chain aldehyde produced during decomposition of a lipid hydroperoxide, and the change in MDA concentration can be used as an index of oxidative damage [42,43]. The results of this study showed that MCP could effectively diminish lipid peroxidation and might attenuate exercise-induced oxidative damage.
Previous studies provided evidence that ROS produced during strenuous exercise could cause extensive DNA damage in multiple tissues, including single-strand breaks and the formation of modified bases [44]. 8-OHdG is a product of oxidative DNA damage following specific enzymatic cleavage after ROS induced 8-hydroxylation of the guanine base in mitochondria and nuclear DNA [45]. It has been reported that 8-OHdG is extensively investigated in human and animal exercise studies, which is attributed to its high sensitivity and considerable stability as it cannot be metabolized once generated by free radicals [46,47]. Therefore, 8-OHdG has been the most widely used markers of -induced oxidative DNA damage. In this study, the results of this study showed MCP could attenuate oxidative DNA damage induced by exhaustive exercise. However, further studies are needed to elucidate the mechanism of attenuate effects.
Conclusion
In conclusion, our data demonstrates that MCP could increase the levels of SOD, CAT, GPx, GR and GSH, and GSH/GSSG ratio in muscle, and decrease the levels of LDH, CK and AST in serum, as well as the levels of GSSG, MDA and 8-OHdGs in muscle. These results suggest that MCP might have protective effects on exhaustive exercise-induced oxidative damage. However, the detailed mechanisms underlying of this protective effect have not yet been clarified. Further investigation using other models including humans of different sporting backgrounds is warranted to extend these findings and elucidate its detailed mechanisms.
Acknowledgements
This study was supported by grants from the Research Project of Yunnan University of Finance and Economics (No. YNU2014132x).
References
- Ji LL. Oxidative stress during exercise: implication of antioxidant nutrients. Free Radic Biol Med 1995; 18: 1079-1086.
- Silva LA, Pinho CA, Scarabelot KS, Fraga DB, Volpato AM, Boeck CR, De Souza CT, Streck EL, Pinho RA. Physical exercise increases mitochondrial function and reduces oxidative damage in skeletal muscle. Eur J Appl Physiol 2009; 105: 861-867.
- Akimoto AK, Miranda-Vilela AL, Alves PC, Pereira LC, Lordelo GS, Hiragi Cde O, da Silva IC, Grisolia CK, Klautau-Guimarães Mde N. Evaluation of gene polymorphisms in exercise-induced oxidative stress and damage. Free Radic Res 2010; 44:322-331.
- Aguiar AS Jr, Tuon T, Soares FS, da Rocha LG, Silveira PC, Pinho RA. The effect of n-acetylcysteine and deferoxamine on exercise-induced oxidative damage in striatum and hippocampus of mice. Neurochem Res 2008; 33:729-736.
- Radák Z, Naito H, Kaneko T, Tahara S, Nakamoto H, Takahashi R, Cardozo-Pelaez F, Goto S. Exercise training decreases DNA damage and increases DNA repair and resistance against oxidative stress of proteins in aged rat skeletal muscle. Pflugers Arch 2002; 445:273-278.
- Belviranlı M, Gökbel H, Okudan N, Büyükbaş S. Effects of grape seed polyphenols on oxidative damage in liver tissue of acutely and chronically exercised rats. Phytother 2013; 27: 672-677.
- Zhang Y, Yu L, Ao M, Jin W. Effect of ethanol extract of Lepidium meyenii Walp. on osteoporosis in ovariectomized rat. J Ethnopharmacol 2006; 105: 274-279.
- Liu H, Jin WW, Fu CH, Dai PF, Yu YT, Huo Q, Yu LJ. Discovering anti-osteoporosis constituents of maca (Lepidium meyenii) by combined virtual screening and activity verification. Food Res Int 2015; 77: 215-220.
- Sandoval M, Okuhama NN, Angeles FM, Melchor VV, Condezo LA, Lao J, Miller MJS. Antioxidant activity of the cruciferous vegetable Maca (Lepidium meyenii). Food Chem 2002; 79: 207-213.
- Lee KJ, Dabrowski K, Sandoval M, Miller MJS. Activity-guided fractionation of phytochemicals of Maca meal, their antioxidant activities and effects on growth, feed utilization and survival in rainbow trout (Oncorhynchus mykiss) juveniles. Aquaculture 2005; 244: 293-301.
- Vecera R, Orolin J, Skottová N, Kazdová L, Oliyarnik O, Ulrichová J, Simánek V. The influence of maca (Lepidium meyenii) on antioxidant status, lipid and glucose metabolism in rat. Plant Foods Hum Nutr 2007; 62: 59-63.
- Campos D, Chirinos R, Barreto O, Noratto G, Pedreschia R. Optimized methodology for the simultaneous extraction of glucosinolates, phenolic compounds and antioxidant capacity from maca (Lepidium meyenii). Ind Crop Prod 2013; 49: 747-754.
- He Z, Feng Y, Xu LF, Sun L, Shi L, Chen XM. In vitro antioxidant activity of ethanol extract of maca (Lepidium meyenii Walpers) cultivated in Yunnan. Food Sci 2010; 31: 39-43.
- Bai N, He K, Roller M, Lai CS, Bai L, Pan MH. Flavonolignans and other constituents from Lepidium meyenii with activities in anti-inflammation and human cancer cell lines. J Agric Food Chem 2015; 63: 2458-2463.
- Buckiová D, Křen V, Pěknicová J, Ulrichová J, Šimánek V. The in vitro biological activity of Lepidium meyenii extracts. Cell Biol Toxicol 2006; 22: 91-99.
- Zha SH, Zhao QS, Chen JJ, Wang LW, Zhang GF, Zhang H, Zhao B. Extraction, purification and antioxidant activities of the polysaccharides from maca (Lepidium meyenii). Carbohyd Polym 2014; 111: 584-587.
- Zhang Y, Yu L, Jing W, Li S. Study on antioxidative activity of polysaccharide from maca (lepidium meyenii walp.) in vitro. Food Sci Tech 2005; 8: 97-99.
- Sun XD, Tang H, Du P, Yang J, Shan Y, Zhou RF, Yang YW. Update Nutritional Components and Antioxidative Activity of Polysaccharidein Vitro from Maca Cultivated in Lijiang. Chin J Spectrosc Lab 2013; 30: 2365-2370.
- Teixeira AM, Trevizol F, Colpo G, Garcia SC, Charão M, Pereira RP, Fachinetto R, Rocha JB, Bürger ME. Influence of chronic exercise on reserpine-induced oxidative stress in rats: behavioral and antioxidant evaluations. Pharmacol Biochem Behav 2008; 88: 465-472.
- Chen QP, Wei P. Icariin supplementation protects mice from exercise-induced oxidant stress in liver. Food Sci Biotechnol 2013; 22: 1-5.
- Yan F, Wang B, Zhang Y. Polysaccharides from Cordyceps sinensis mycelium ameliorate exhaustive swimming exercise-induced oxidative stress. Pharm Biol 2014; 52: 157-161.
- McAnulty SR, McAnulty LS, Nieman DC, Dumke CL, Morrow JD, Utter AC, Henson DA, Proulx WR, George GL. Consumption of blueberry polyphenols reduces exerciseinduced oxidative stress compared to vitamin C. Nutr Res 2004; 24: 209-221.
- Jówko E, Sacharuk J, Balasińska B, Ostaszewski P, Charmas M, Charmas R. Green tea extract supplementation gives protection against exercise-induced oxidative damage in healthy men. Nutr Res 2011; 31: 813-821.
- Scicchitano P, Cameli M, Maiello M, Modesti PA, Muiesan ML, Novo S, Palmiero P, Saba PS, Pedrinelli R, Ciccone MM. Nutraceuticals and dyslipidaemia: Beyond the common therapeutics. J Funct Foods 2014; 6: 11-32.
- Sahin K, Orhan C, Tuzcu M, Sahin N, Ali S, Bahcecioglu IH, Guler O, Ozercan I, Ilhan N, Kucuk O. Orally administered lycopene attenuates diethylnitrosamine-induced hepatocarcinogenesis in rats by modulating Nrf-2/HO-1 and Akt/mTOR pathways. Nutr Cancer 2014; 66: 590-598.
- Li FL, Zhang YM, Zhong ZJ. Antihyperglycemic effect of ganoderma lucidum polysaccharides on streptozotocin-induced diabetic mice. Int J Mol Sci 2011; 12: 6135-6145.
- Ciccone MM, Cortese F, Gesualdo M, Carbonara S, Zito A, Ricci G, De Pascalis F, Scicchitano P, Riccioni G. Dietary intake of carotenoids and their antioxidant and anti-inflammatory effects in cardiovascular care. Mediators Inflamm 2013; 2013: 782137.
- Qi B, Zhang L, Zhang Z, Ouyang J, Huang H. Effects of ginsenosides-Rb1 on exercise-induced oxidative stress in forced swimming mice. Pharmacogn Mag 2014; 10: 458-463.
- Yavari A, Javadi M, Mirmiran P, Bahadoran Z. Exercise-induced oxidative stress and dietary antioxidants. Asian J Sports Med 2015; 6: e24898.
- Huang KC, Wu WT, Yang FL, Chiu YH, Peng TC, Hsu BG, Liao KW, Lee RP. Effects of freshwater clam extract supplementation on time to exhaustion, muscle damage, pro/anti-inflammatory cytokines, and liver injury in rats after exhaustive exercise. Molecules 2013; 18: 3825-3838.
- Kim H, Chae C. Effect of exercise and alpha-lipoic acid supplementation on oxidative stress in rats. Biology of Sport 2006; 23: 143-154.
- Salahshoor T, Farzanegi P, Habibian M. Synergistic effects of omega 3 supplementation and exercise on markers of liver (ALP, AST, and ALT) and muscle (LDH and CK) damage in male karate athletes. J App Sci Agric 2014; 9: 245-249.
- Townsend DM, Tew KD, Tapiero H. The importance of glutathione in human disease. Biomed Pharmacother 2003; 57: 145-155.
- Jówko E, Sacharuk J, Balasińska B, Ostaszewski P, Charmas M, Charmas R. Green tea extract supplementation gives protection against exercise-induced oxidative damage in healthy men. Nutr Res 2011; 31: 813-821.
- Kozakowska M, Pietraszek-Gremplewicz K, Jozkowicz A, Dulak J. The role of oxidative stress in skeletal muscle injury and regeneration: focus on antioxidant enzymes. J Muscle Res Cell Motil 2015; 36: 377-393.
- Hao HT. Effect of Auricularia auricula polysaccharides on exhaustive swimming exercise-induced oxidative stress in mice. Trop J Pharm Res 2014; 13: 1319-1326.
- Huang SC, Lee FT, Kuo TY, Yang JH, Chien CT. Attenuation of long-term Rhodiola rosea supplementation on exhaustive swimming-evoked oxidative stress in the rat. Chin J Physiol 2009; 52: 316-324.
- Tauler P, Sureda A, Cases N, Aguiló A, Rodríguez-Marroyo JA, Villa G, Tur JA, Pons A. Increased lymphocyte antioxidant defences in response to exhaustive exercise does not prevent oxidative damage. J Nutr Biochem 2006; 17: 665-671.
- Pittaluga M, Sgadari A, Dimauro I, Tavazzi B, Parisi P, Caporossi D. Physical exercise and redox balance in type 2 diabetics: effects of moderate training on biomarkers of oxidative stress and DNA damage evaluated through comet assay. Oxid Med Cell Longev 2015; 2015: 981242.
- Voces J, Cabral de Oliveira AC, Prieto JG, Vila L, Perez AC, Duarte ID, Alvarez AI. Ginseng administration protects skeletal muscle from oxidative stress induced by acute exercise in rats. Braz J Med Biol Res 2004; 37: 1863-1871.
- Huang CC, Tsai SC, Lin WT. Potential ergogenic effects of L-arginine against oxidative and inflammatory stress induced by acute exercise in aging rats. Exp Gerontol 2008; 43: 571-577.
- Fisher-Wellman K, Bloomer RJ. Acute exercise and oxidative stress: a 30 year history. Dyn Med 2009; 8:1.
- Kim H, Chae C. Effect of exercise and alpha-lipoic acid supplementation on oxidative stress in rats. Biol Sport 2006; 23: 143-152.
- Rahimi R. Creatine supplementation decreases oxidative DNA damage and lipid peroxidation induced by a single bout of resistance exercise. J Strength Cond Res 2011; 25: 3448-3455.
- Xu GW, Yao QH, Weng QF, Su BL, Zhang X, Xiong JH. Study of urinary 8-hydroxydeoxyguanosine as a biomarker of oxidative DNA damage in diabetic nephropathy patients. J Pharm Biomed Anal. 2004; 36:101-104.
- Hamurcu Z, Saritas N, Baskol G, Akpinar N. Effect of wrestling exercise on oxidative DNA damage, nitric oxide level and paraoxonase activity in adolescent boys. Pediatr Exerc Sci 2010; 22: 60-68.
- Yue J, Wang P, Liu YH, Wu JY, Chen J, Peng RX. Fast evaluation of oxidative DNA damage by liquid chromatography-electrospray tandem mass spectrometry coupled with precision-cut rat liver slices. Biomed Environ Sci 2007; 20: 386-391.