ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2016) Volume 27, Issue 4
The effects of dexmedetomidine on renal injury induced by intra-abdominal hypertension in rats
1Department of Anaesthesiology and Reanimation, Kirikkale University School of Medicine, Kirikkale, Turkey
2Department of Pediatric Surgery, Kirikkale University School of Medicine, Kirikkale, Turkey
3Department of Anaesthesiology and Reanimation, Medipol University, Istanbul, Turkey
4Department of Biochemistry, Kirikkale University School of Medicine, Kirikkale, Turkey
5Department of Pathology, Kirikkale University School of Medicine, Kirikkale, Turkey
6Department of Anaesthesiology and Reanimation, Giresun University School of Medicine, Giresun, Turkey
- *Corresponding Author:
- Ferda Yaman
Department of Anaesthesiology and Reanimation
Kirikkale University, Faculty of Medicine
Kirikkale-71451, Turkey
Accepted date: April 04, 2016
Introduction: Intra-abdominal hypertension (IAH) and abdominal compartment syndrome (ACS) are potentially life-threatening conditions in critically ill patients. Laparascopic surgery is the gold standard and has been widely performed for many procedures since its inception in the early 1980s. Pneumoperitoneum is essential for laparascopic surgery. Dexmedetomidine is a potent and highly selective α-2 adrenoceptor agonist with sympatholytic, sedative, amnestic and analgesic properties without respiratory depression. There is increasing evidence of its organ protective effects against ischemic and hypoxic injury, including neuroprotection, cardioprotection and renoprotection. The aim of this experimental study was to investigate the effects of the α-2 adrenoceptor agonist, dexmedetomidine on IAH induced by renal injury.
Materials and methods: A total of 24 male Wistar-albino rats were randomly separated into 4 groups as the control group (CG, n=6), sham group (SG, n=6), low-dose group (DXLD, n=6) and high-dose group (DXHD, n=6). In CG, no intervention was made. IAH was obtained by insufflating atmospheric air with percutaneous intraperitoneal needle using a manual insufflator of manometer up to 15 mmHg. At the 60th min, in SG, 1.5 ml/100 gr/hr saline was infused. In DXLD, 0.5 μg/kg/hr, and in DXHD, 1 μg/kg/hr dexmedetomidine (Precedex, 100 μg/ml; Abbott, Istanbul, Turkey) was infused intravenously. At the 90th min, a midline incision was made and the left kidney was harvested by median laparatomy for the measurement of tissue nitric oxide (NO), malondialdehyde (MDA) level and histopathological examination for proximal tubule injury by light microscopy.
Results: No significant difference was determined between the groups either biochemically or histopathologically (p>0.05).
Conclusion: Dexmedetomidine may not provide renoprotective effects within the clinical infusion doses of 0.5 μg/kg/hr, and 1 μg/kg/hr.
Keywords
Dexmedetomidine, Intra-abdominal pressure, Renal injury.
Introduction
Intra-abdominal hypertension (IAH) and abdominal compartment syndrome (ACS) contribute to multiorgan dysfunction syndrome, failure and death in surgical critically ill patient populations [1,2]. IAH and ACS are defined as intraabdominal pressure (IAP) exceeding 12 mmHg and 20 mmHg respectively by The World Society of Abdominal Compartment Syndrome (WSAC) [3]. Although multiorgan failure is a known fact in ACS, it is less anticipated that the kidneys may be at risk with much lower levels of IAP than in fully established ACS [4,5]. Laparascopic surgery is the gold standard and has been widely performed for many procedures since its inception in the early 1980s. Pneumoperitoneum is essential for laparascopic surgery [6]. Several studies have demonstrated that the increase in intra-abdominal pressure can cause hypoperfusion of intra-abdominal organs [7,8]. Experimental studies have shown oxidative stress injury and histopathological changes in renal tissue with intra-abdominal pressure of 12-15 mmHg [9,10].
Dexmedetomidine is a potent and highly selective α-2 adrenoceptor agonist with sympatholytic, sedative, amnestic and analgesic properties without respiratory depression [11,12]. There is increasing evidence of its organ protective effects against ischemic and hypoxic injury, including neuroprotection, cardioprotection and renoprotection [13]. It has also been reported to have a protective effect in radiocontrast-induced nephropathy [14]. Several studies have shown that dexmedetomidine decreases the oxidative stress caused by pneumoperitoneum and ischemia-reperfusion injuries [15,16].
The aim of this study was to investigate the possible protective effects of dexmedetomidine in IAH-induced renal inflammation and oxidative damage in an experimental rat model.
Materials and Methods
Approval for this study was granted by the Experimental Animal Studies Ethics Board of Ankara Training and Research Hospital, (approval no 04.06.2013/0014-213). A total of 24 adult, male Wistar albino rats, each weighing 200-250 gr, were used in the study. The animals were allowed free access to standard rodent chow and water at 22 0C room temperature under a 12 hour day-night cycle, until 12 hrs. prior to surgery when food (but not water) was removed.
Experimental groups
• The rats were randomly allocated to one of 4 groups as follows:
• Control group (no intervention was made, n=6),
• Sham group (IAH was performed and 1.5 ml/100 gr/hr saline was infused, n=6),
• Low dose dexmedetomidine group (DXLD) (IAH was performed and 0.5 μg/kg/hr dexmedetomidine (Precedex, Abbott, Istanbul, Turkey), was infused intravenously, n=6),
• High dose dexmedetomidine group (DXHD) (1 μg/kg/hr dexmedetomidine was infused intravenously, n=6).
Anaesthetic procedure
All rats were sedated with an intraperitoneal injection of ketamine hydrochloride (40 mg/kg, Ketalar, Pfizer Warner Lambert) and Xylazine hydrochloride (5 mg/kg, Alfasan Int BV, Woerden, Holland). Spontaneous breathing with 100% oxygen was maintained during the experiments. The animals were placed in a supine position. A 20 G, 32 mm needle was inserted percutaneously into the peritoneal cavity. Atmospheric air was insufflated into the abdominal cavity using a manual insufflator of manometer (Figure 1). IAP was maintained at the level of 15 mmHg throughout the experiment in all groups except the Control group. After 60 minutes, 1.5 ml/100 gr/hr saline in the Sham group, 0.5 μg/kg/hr dexmedetomidine in the DXLD group and 1 μg/kg/hr dexmedetomidine in the DXHD group was infused intravenously during 30 minutes through the tail veins of the rats using a 24 G branule. The same volume was infused in all groups. At 90 minutes after the achievement of IAH, a median laparatomy was performed on all the rats and the left kidney was harvested for histopathological and biochemical analyses. All the animals were then sacrified by exsanguination. Part of the renal tissues was stored in 10% buffered formaldehyde solution at room temperature for future histopathological examination. The remainder of the renal tissues were immediately stored at -80°C in dry air until biochemical examination.
Histopathological examination
The renal tissues fixed in 10% formalin were dehydrated with ethanol solution and cleared with xylene, then all tissues were embedded in paraffin. Serial 5 μm sections of the tissue were obtained and stained with hematoxylin-eosin (HE). The histopathological sections were examined under a light microscope (Olympus CX31, Ireland) at 200x and 400x magnification by an experienced pathologist blinded to the groups, and test materials. Renal tissue damage was determined using a renal proximal tubule injury grading scale which was modified from that of Houghton et al. [17] (Table 1).
| Criterias for the Grading of the Renal Proximal Tubule Injury | |
|---|---|
| Grade 0 | Normal |
| Grade 1 | Desquamation of tubular epithelial cells in small foci (less than 1% of total tubule population involved). Areas of focal granulovacuolar epithelial cell degeneration and granular debris in tubular lumina with or without evidence of desquamation |
| Grade 2 | Tubular epithelial necrosis and desquamation are prominent but involve less than half of cortical tubules. |
| Grade 3 | More than half of proximal tubules are undergoing necrosis and desquamation, but intact tubules are easily identified |
| Grade 4 | Total or near total proximal tubular necrosis |
Table 1: Descriptive table of the criteria for grading of the renal proximal tubule injury.
Biochemical examination
The renal tissues were kept at -80°C and washed with 0.9 % NaCl. The tissues were homogenized (Labor Technique, Müllheim, Germany) with 0.9% NaCl solution 1 ml in ice, and centrifuged at 1.500 g for 10 minutes at 4°C. The aliquot of the supernatants were used to determine the malondialdehyde (MDA) and total nitrite/nitrate (NO) protein levels. The protein levels were measured using the Lowry et al. method [18].
NO levels were measured using a spectrophotometric method with the techniques described by Miranda et al. [19]. Nitrate was reduced to nitrite with vanadium (III) and then the nitrite level was measured using Griess reagents. The results were expressed as μM/mg protein.
MDA levels were measured using the modified Yagi method as described by Armstrong and Al-Awadi [20]. The calibration curve was prepared with 1,1,3,3-tetraethoxypropane (Sigma, St Louis, MO) standards of 1-25 nmol/L dilutions. The results were expressed as μM/mg protein.
Statistical analysis
The statistical analyses were performed using the Statistical Package for the Social Sciences version 15.0 for Windows (SPSS, Chicago, IL).
The histopathological grading score values and MDA values were distributed normally (Kolmogorov-Smirnov test; p>0.05) but variations were not homogenous (Levene’s test, p<0.05) between all the groups. Therefore, the Kruskall Wallis test was applied to all values. A value of p<0.05 was considered statistically significant.
NO values were distributed normally (Kolmogorov-Smirnov test; p>0.05) and variations were homogenous (Levene’s test, p>0.05) between all the groups. Therefore, the one-way analysis of variance (ANOVA) was applied to all values. A value of p<0.05 was considered statistically significant.
Results
The biochemical and histopathological results are shown in Tables 2-4 respectively.
| Variable | Groups | N | Min | Max | Mean#/Median* | SD |
|---|---|---|---|---|---|---|
| Histopathological grading score* | DXLD | 6 | 1 | 2 | 1.5 | 0.55 |
| DXHD | 6 | 0 | 3 | 1 | 1.17 | |
| CONTROL | 6 | 0 | 3 | 1 | 1.17 | |
| SHAM | 5 | 0 | 4 | 1 | 1.64 | |
| MDA* | DXLD | 6 | 5.39 | 12.97 | 8.12 | 2.96 |
| DXHD | 6 | 7.38 | 12.74 | 10 | 2.47 | |
| CONTROL | 6 | 8.65 | 11.54 | 8.88 | 1.12 | |
| SHAM | 5 | 8.46 | 18.79 | 11.69 | 4.58 | |
| NO# | DXLD | 6 | 2.8 | 6.53 | 4.58 | 1.43 |
| DXHD | 6 | 3.21 | 9.55 | 5.76 | 2.53 | |
| CONTROL | 6 | 1.09 | 7.7 | 4.03 | 2.48 | |
| SHAM | 5 | 2.11 | 10.3 | 6.67 | 3.28 |
Table 2: Descriptive table of the histopathological grade scores, MDA and NO values (MDA: malondialdehyde; NO: nitric oxide; min: minimum; max: maximum; N: number of the subjects; SD: standard deviation).
| Variable | X2 | df | p |
|---|---|---|---|
| Histopathological grading score | 1.295 | 3 | 0.73 |
| MDA | 3.022 | 3 | 0.388 |
Table 3: The histopathological grade scores and malondialdehyde (MDA) values were not statistically different between the groups (Kruskall Wallis test, p<0.05).
| Variable | df | F | p |
|---|---|---|---|
| NO | 3 | 1.271 | 0.313 |
Table 4: The nitric oxide (NO) values were not statistically different between the groups (One-Way Analysis of Variance (ANOVA) test, p<0.05).
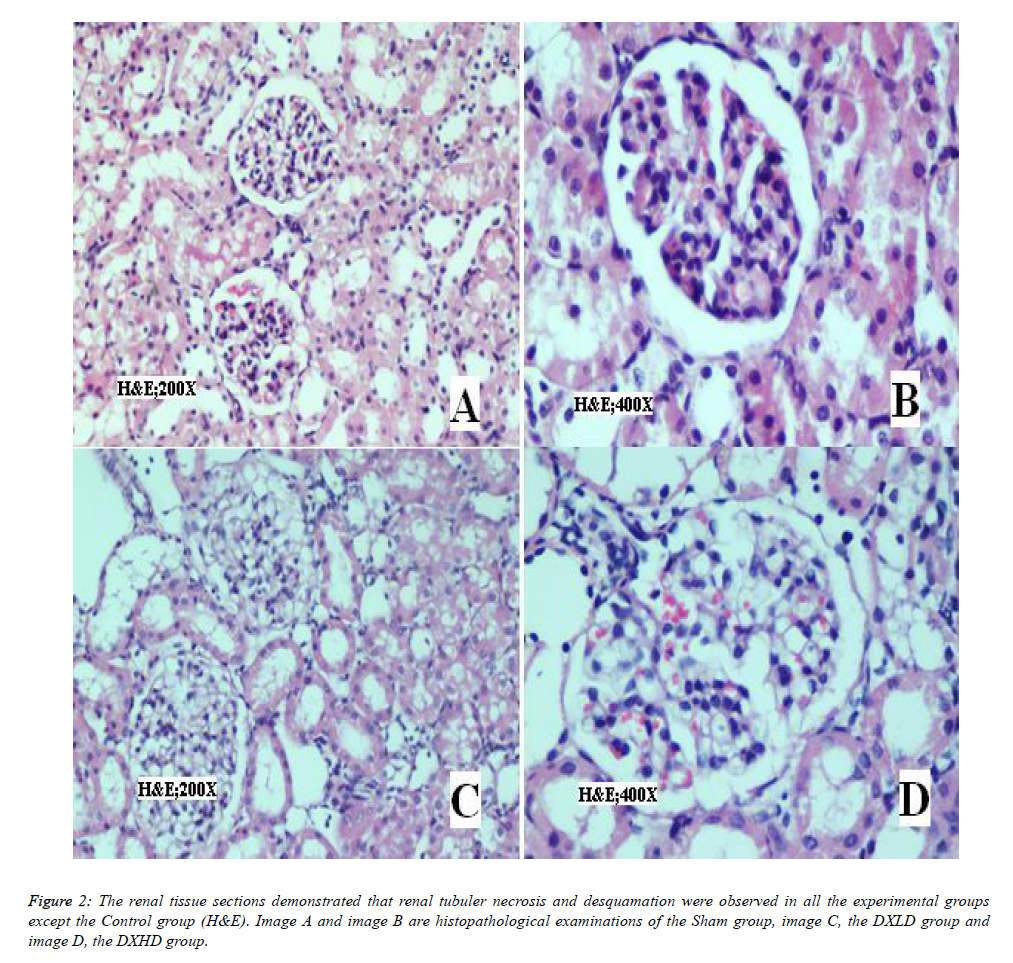
In H&E staining of the kidney tissue sections (Figure 2), renal tubuler necrosis and desquamation were observed in all experimental groups except the Control group.
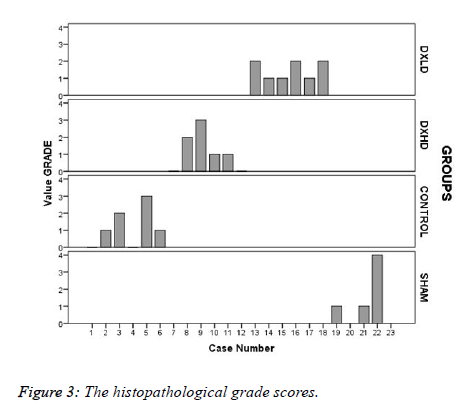
The histopathological grade scores (Figure 3) were numerically high in the Sham group, but there was no statistically significant difference between the groups (X2=1.295, p=0.730).
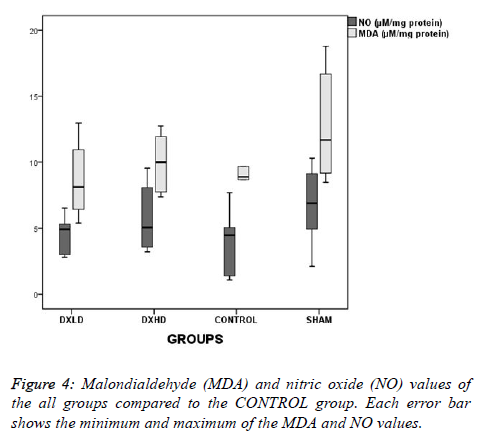
Although the NO levels were slightly lower and the MDA levels were higher as numerical values in the SHAM group than in the CONTROL group (Figure 4), there was no statistically significant difference between the groups (F=1.271, p=0.311; and X2=3.022, p=0.388, respectively).
Discussion
Intra-abdominal hypertension, and ACS have been defined since the 1980s and the physiological complications of IAH and ACS have been known and reported for the last 15 years [21,22]. IAH represents a potentially serious condition that is frequently underdiagnosed despite a high estimated prevalence of 18%-81% in the ICU [23]. Although the procedure time is short, laparascopic nephrectomy leads to increased inflammation and signs of renal injury with 12 mmHg of IAP [24]. IAH decreases renal perfusion pressure, the filtration gradient, and renal blood flow. Therefore, oliguria develops, tubuler dysfunction increases, the glomerular filtration rate drops, renal vascular resistance increases, renal vein and ureter compression increases, renin, aldosteron and anti-diuretic hormone levels increase, while the adrenal blood flow usually remains preserved [25].
In this study, the results are presented of a significant renal injury at 15 mmHg pressure of IAP for 90 minutes in the rat kidney. The MDA level was used as proof of lipid peroxidation to determine oxidative stress [26]. NO plays a fundamental biological role in protecting the kidney against ischemia/ reperfusion injury [9]. The level of NO was used to determine the protective properties of dexmedetomidine. The results showed that the NO and MDA levels of renal tissue were increased in the sham, DXLD and DXHD groups, but no statistically significant difference was found between the groups. Dexmedetomidine is a potent and highly selective α-2 adrenoceptor agonist, which is used as an adjunct to anaesthesia, analgesia and intensive care unit sedation [27]. In addition, organoprotective effects of dexmedetomidine have been shown in several inflammatory models [28-30]. The effects of dexmedetomidine on renal function are multiple, including diuresis by inhibiting the antidiuretic action of vasopressin at the collecting duct and enhancing osmolal clearance through non-antidiuretic action of vasopressin dependent pathways [27]. It has been shown that renal function and renal blood flow improved in a model of radiocontrastinduced nephropathy within the dose of 3 μg/kg bolus and 4 μg/kg/h subcutaneous infusion [14]. Koca et al. demonstrated that dexmedetomidine attenuates sepsis-induced lung and kidney injuries and reduced apoptosis in a rat model of sepsis at the dose of 50 μg/kg [31].
The infusion rate of dexmedetomidine is crucial because dexmedetomidine may cause significant hemodynamic changes that affect renal blood flow. In clinical practice, the doses of dexmedetomidine were established on the basis of the recommended dose 0.2 to 0.7 μg/kg/h [32]. Sugita et al showed that continuous infusion of dexmedetomidine at 10 and 20 μg/kg/h and 1 μg/kg/h in the reperfusion period reduces renal dysfunction and it was also demonstrated that dexmedetomidine inhibits increased expression of IL-6, ICAM-1, and iNOs mRNA caused by renal IR. It is noticeable that according to the results of that study, a dose of 1 μg/kg/h reduces renal dysfunction [33]. In the current study, clinically relevant doses of dexmedetomidine were administered when the rats were still insufflated at the pressure level of 15 mmHg. This may have affected the results of this study as no beneficial effects of dexmedetomidine were determined. Kocoglu et al. studied the the role of dexmedetomidine on renal histological alterations after ischemia reperfusion injury (IRI) with single dose of 100 μg/kg, at the starting time of reperfusion and found normal glomeruli and slight edema of the tubular cells. According to that study, dexmedetomidine could reduce the renal injury caused by IRI of the kidney [16]. In the present study, dexmedetomidine was infused at clinically relevant doses after IAP continuous IAP, as would happen in clinical practice and the results showed no beneficial effects in the DXLD and DXHD groups.
Curtis et al. studied the effects of dexmedetomidine on renal histological changes and the blood Cr level after ischemiareperfusion injury in rats anesthetized with ketamine. The dose of dexmedetomidine administered was similar to that of the current study at 1 μg/kg intravenously for 10 min and this was continued with a dose of 1 μg/kg/h before the period of ischemia. In the evaluation of histological changes, it was concluded that dexmedetomidine may have not totally protected the kidneys from these injuries, despite the better results of blood creatinine [34]. In the current study, serum creatinine was not determined because only the acute effects of renal injury were investigated and this is not a reliable biomarker at this phase of renal injury [35]. Dexmedetomidine was administered at the same doses but the period of the administration of dexmedetomidine was different from that of the current study. Cekic et al. demonstrated the protective effects of dexmedetomidine in pneumoperitoneum-related ischaemia-reperfusion injury in rat ovarian tissue. Pneumoperitoneum was established in 60 min under 12 mmHg pressure and 100 μg dexmedetomidine was administered in that experimental study [36]. The period of administration and dose of dexmedetomidine differed from the current study as dexmedetomidine was administered before the insufflation period.
In a clinical study dexmedetomidine administration during laparoscopic cholecystectomy was shown to reduce intraoperative and post-operative secretion of cytokines, as well as post-operative leukocyte count and CRP level. In that study, it was crucial that the dexmedetomidine infusion was begun after induction of anaesthesia before the insufflation period [37]. The limitations of the current study could be said to be that mean arterial pressure and renal blood flow could not be measured as important effects of the model. The results of the present study showed no statistical significance between the groups. The reasons for this may be the dosage of dexmedetomidine administered or the small number of animals used in the experiment. Further studies with a greater number of animals at different doses of dexmedetomidine and different periods of the experiment may yield more comprehensive results.
Conclusions
In conclusion, the administration of clinical doses of dexmedetomidine infusion does not seem to improve IAH-induced renal injury in the period of insufflation.
References
- Ameloot K, Gillebert C, Desie N, Malbrain ML. Hypoperfusion, shock states, and abdominal compartment syndrome (ACS). Surg Clin North Am 2012; 92: 207-220.
- Balogh Z, McKinley BA, Holcomb JB, Miller CC, Cocanour CS, Kozar RA, Valdivia A, Ware DN, Moore FA. Both primary and secondary abdominal compartment syndrome can be predicted early and are harbingers of multiple organ failure. J Trauma 2003; 54: 848-859.
- Kirkpatrick AW, Roberts DJ, De Waele J, Jaeschke R, Malbrain ML, De Keulenaer B, Duchesne J, Bjorck M, Leppaniemi A, Ejike JC, Sugrue M, Cheatham M, Ivatury R, Ball CG, Reintam Blaser A, Regli A, Balogh ZJ, D'Amours S, Debergh D, Kaplan M, Kimball E, Olvera C; Pediatric Guidelines Sub-Committee for the World Society of the Abdominal Compartment Syndrome. Intra-abdominal hypertension and the abdominal compartment syndrome: updated consensus definitions and clinical practice guidelines from the World Society of the Abdominal Compartment Syndrome. Intensive Care Med 2013; 39: 1190-1206.
- Malbrain ML, Chiumello D, Pelosi P, Wilmer A, Brienza N, Malcangi V, Bihari D, Innes R, Cohen J, Singer P, Japiassu A, Kurtop E, De Keulenaer BL, Daelemans R, Del Turco M, Cosimini P, Ranieri M, Jacquet L, Laterre PF, Gattinoni L. Prevalence of intra-abdominal hypertension in critically ill patients: a multicentre epidemiological study. Intensive Care Med 2004; 30: 822-829.
- Mullens W, Abrahams Z, Skouri HN, Francis GS, Taylor DO, Starling RC, Paganini E, Tang WH. Elevated intra-abdominal pressure in acute decompensated heart failure: a potential contributor to worsening renal function? J Am Coll Cardiol 2008; 51: 300-306.
- Grabowski JE, Talamini MA. Physiological effects of pneumoperitoneum. J Gastrointest Surg 2009; 13: 1009-1016.
- Mogilner JG, Bitterman H, Hayari L, Brod V, Coran AG, Shaoul R, Lurie M, Eldar S, Sukhotnik I. Effect of elevated intra-abdominal pressure and hyperoxia on portal vein blood flow, hepatocyte proliferation and apoptosis in a rat model. Eur J Pediatr Surg 2008; 18: 380-386.
- Wiesenthal JD, Fazio LM, Perks AE, Blew BD, Mazer D, Hare G, Honey RJ, Pace KT. Effect of pneumoperitoneum on renal tissue oxygenation and blood flow in a rat model. Urology 2011; 77: 1508-1515.
- Abassi Z, Bishara B, Karram T, Khatib S, Winaver J, Hoffman A. Adverse effects of pneumoperitoneum on renal function: involvement of the endothelin and nitric oxide systems. Am J Physiol Regul Integr Comp Physiol 2008; 294: 842-850.
- Akbulut G, Polat C, Aktepe F, Yilmaz S, Kahraman A, Serteser M, Gökçe C, Gökçe O. The oxidative effect of prolonged CO2 pneumoperitoneum on renal tissue of rats. Surg Endosc 2004; 18: 1384-1388.
- Piao G, Wu J. Systematic assessment of dexmedetomidine as an anesthetic agent: a meta-analysis of randomized controlled trials. Arch Med Sci 2014; 10: 19-24.
- Venn RM, Bradshaw CJ, Spencer R, Brealey D, Caudwell E, Naughton C, Vedio A, Singer M, Feneck R, Treacher D, Willatts SM, Grounds RM. Preliminary UK experience of dexmedetomidine, a novel agent for postoperative sedation in the intensive care unit. Anaesthesia 1999; 54: 1136-1142.
- Panzer O, Moitra V, Sladen RN. Pharmacology of sedative-analgesic agents: dexmedetomidine, remifentanil, ketamine, volatile anesthetics, and the role of peripheral mu antagonists. Crit Care Clin 2009; 25: 451-469.
- Billings FT 4th, Chen SW, Kim M, Park SW, Song JH, Wang S, Herman J, D'Agati V, Lee HT. alpha2-Adrenergic agonists protect against radiocontrast-induced nephropathy in mice. Am J Physiol Renal Physiol 2008; 295: 741-748.
- Cekic B, Geze S, Ozkan G, Besir A, Sonmez M, Karahan SC, Mentese A. The effect of dexmedetomidine on oxidative stress during pneumoperitoneum. Biomed Res Int 2014; 2014: 760323.
- Kocoglu H, Ozturk H, Ozturk H, Yilmaz F, Gulcu N. Effect of dexmedetomidine on ischemia-reperfusion injury in rat kidney: a histopathologic study. Ren Fail 2009; 31: 70-74.
- Houghton DC, Plamp CE 3rd, DeFehr JM, Bennett WM, Porter G, Gilbert D. Gentamicin and tobramycin nephrotoxicity. A morphologic and functional comparison in the rat. Am J Pathol 1978; 93: 137-152.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 1951; 193: 265-275.
- Miranda KM, Espey MG, Wink DA. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide 2001; 5: 62-71.
- Armstrong D, al-Awadi F. Lipid peroxidation and retinopathy in streptozotocin-induced diabetes. Free Radic Biol Med 1991; 11: 433-436.
- Cheatham ML, Safcsak K. Is the evolving management of intra-abdominal hypertension and abdominal compartment syndrome improving survival? Crit Care Med 2010; 38: 402-407.
- Cheatham ML. Abdominal compartment syndrome. Curr Opin Crit Care 2009; 15: 154-162.
- Malbrain ML, Chiumello D, Pelosi P, Bihari D, Innes R, Ranieri VM, Del Turco M, Wilmer A, Brienza N, Malcangi V, Cohen J, Japiassu A, De Keulenaer BL, Daelemans R, Jacquet L, Laterre PF, Frank G, de Souza P, Cesana B, Gattinoni L. Incidence and prognosis of intraabdominal hypertension in a mixed population of critically ill patients: a multiple-center epidemiological study. Crit Care Med 2005; 33: 315-322.
- Hofker HS, Nijboer WN, Niesing J, Krikke C, Seelen MA, van Son WJ, van Wijhe M, Groen H, Vd Heide JJ, Ploeg RJ. A randomized clinical trial of living donor nephrectomy: a plea for a differentiated appraisal of mini-open muscle splitting incision and hand-assisted laparoscopic donor nephrectomy. Transpl Int 2012; 25: 976-986.
- Malbrain ML, Deeren D, De Potter TJ. Intra-abdominal hypertension in the critically ill: it is time to pay attention. Curr Opin Crit Care 2005; 11: 156-171.
- Del Rio D, Stewart AJ, Pellegrini N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr Metab Cardiovasc Dis 2005; 15: 316-328.
- Afonso J, Reis F. Dexmedetomidine: current role in anaesthesia and intensive care. Rev Bras Anestesiol 2012; 62: 118-133.
- Engelhard K, Werner C, Eberspächer E, Bachl M, Blobner M, Hildt E, Hutzler P, Kochs E. The effect of the alpha 2-agonist dexmedetomidine and the N-methyl-D-aspartate antagonist S (+)-ketamine on the expression of apoptosis-regulating proteins after incomplete cerebral ischemia and reperfusion in rats. Anesth Analg 2003; 96: 524-531.
- Okada H, Kurita T, Mochizuki T, Morita K, Sato S. The cardioprotective effect of dexmedetomidine on global ischaemia in isolated rat hearts. Resuscitation 2007; 74: 538-545.
- Gu J, Chen J, Xia P, Tao G, Zhao H, Ma D. Dexmedetomidine attenuates remote lung injury induced by renal ischemia-reperfusion in mice. Acta Anaesthesiol Scand 2011; 55: 1272-1278.
- Koca U, Olguner ÇG, Ergür BU, Altekin E, Taşdöğen A, Duru S, Girgin P, Gündüz K, Cilaker Mıcılı S, Güzeldağ S, Akkuş M. The effects of dexmedetomidine on secondary acute lung and kidney injuries in the rat model of intra-abdominal sepsis. ScientificWorldJournal 2013; 2013: 292687.
- Dyck JB, Maze M, Haack C, Azarnoff DL, Vuorilehto L, Shafer SL. Computer-controlled infusion of intravenous dexmedetomidine hydrochloride in adult human volunteers. Anesthesiology 1993; 78: 821-828.
- Sugita S, Okabe T, Sakamoto A. Continuous infusion of dexmedetomidine improves renal ischemia-reperfusion injury in rat kidney. J Nippon Med Sch 2013; 80: 131-139.
- Curtis FG, Vianna PT, Viero RM, Fiorio PM, Silva LM, Braz JR, Oliveira C, Castiglia YM. Dexmedetomidine and S (+)-ketamine in ischemia and reperfusion injury in the rat kidney. Acta Cir Bras 2011; 26: 202-206.
- Nguyen MT, Devarajan P. Biomarkers for the early detection of acute kidney injury. Pediatr Nephrol 2008; 23: 2151-2157.
- Cekic B, Besir A, Yulug E, Geze S, Alkanat M. Protective effects of dexmedetomidine in pneumoperitoneum-related ischaemia-reperfusion injury in rat ovarian tissue. Eur J Obstet Gynecol Reprod Biol 2013; 169: 343-346.
- Kang SH, Kim YS, Hong TH, Chae MS, Cho ML, Her YM, Lee J. Effects of dexmedetomidine on inflammatory responses in patients undergoing laparoscopic cholecystectomy. Acta Anaesthesiol Scand 2013; 57: 480-487.