ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 16
The effects of AG1478 on expression and nuclear translocation of SOX2 via the PI3K/AKT signal pathway in non-small cell lung cancer
Guoliang Zu1,2, Yuan Feng1, Danqing Li1 and Taoping Li1*
1Department of Sleep Disorder Center, Nanfang Hospital, Southern Medical University, PR China
2Department of Respiratory Diseases, the First Affiliated Hospital of Gannan Medical College, PR China
- *Corresponding Author:
- Taoping Li
Department of Sleep Disorder Center
Nanfang Hospital
Southern Medical University, PR China
Accepted on August 03, 2017
Purpose: Human lung cancer cells were treated using different concentrations of the AGl478 and EGFR signal pathway related inhibitors LY294002, U0126 and AG490. This study aims to explore the role of SOX2 in Non-Small-Cell Lung Cancer (NSCLC).
Methods: Human lung cancer cells were treated by different concentrations of the AGl478 and EGFR signal pathway related inhibitors LY294002, U0126 and AG490. Western blot was used to examine the expression and the nucleus or cytoplasm distribution of SOX2. AKT siRNA and SOX2 siRNA were designed and transfected into H1650 cells. Western blot were employed to determine the transfection efficiencies.
Results: The expression and nuclear translocation level of SOX2 increased by AGl478 were time dependent. LY294002 significantly inhibited the expression of P-SOX2 and induced its nuclear translocation as compared to U0126 and AG490. AKT siRNA depleted the expressions of P-AKT and PSOX2. The expression and nuclear translocation of SOX2 was significantly induced by AGl478 via PI3K/AKT pathway in NSCLC cell lines.
Conclusions: SOX2 can be a potential target of the cancer therapy and drug exploitation in NSCLC.
Keywords
Non-small cell lung cancer (NSCLC), Cell proliferation, PI3K/AKT pathway, EGFR-tyrosine kinase inhibitor.
Introduction
Lung cancer is one of the most common but with the highest mortality cancers in the world, based on the present studies, more than one million deaths were caused worldwide by this disease each year [1,2]. Despite the recent advances, the incidence and mortality rate of lung cancer has increasing rapidly [3,4]. Lung cancers are histologically classified as Small-Cell Lung Cancer (SCLC) and Non-Small-Cell Lung Cancer (NSCLC), with the composition about 15% and 85% accordingly [5]. NSCLC includes adenocarcinoma, squamous cell carcinoma and large cell carcinoma [6,7]. To date, palliative chemotherapy is the optimum option for most patients [8]. However, chemotherapy resistance, is easy to emerge during treatment, and leads to increased treatment failure rates about 40-60% for lung cancer [9]. Accordingly, the ideal of targeted specific therapy for lung cancer is becoming a hot research topic [10]. Indeed, many synergistic antitumor or multi-targeted agents for lung cancer has been tried in preclinical and clinical studies, their effects of preventing the emergence of drug resistance or treatment efficiencies are inadequately for clinical applications[11]. Researches about novel functions of many regulator encoding genes for lung cancer treatment seem more attractive. The SOX2, which is located at 3q 26.33, has been validated to be a transcription factor that is essential to maintain the pluripotency of embryonic stem cells [12]. Studies showed SOX2 could express in many kinds of tumor tissues, including breast cancer, prostate cancer, glioblastoma, rhabdomyosarcoma and leukemia [13,14]. Inhibited SOX2 expression could promote cell malignant transformation, tumor progression and angiogenesis processes during the development process of tumorigenesis [13,15,16]. Comprehensive genomic characterization of SCLC and Squamous Cell Carcinoma (SCC) showed SOX2 acted as a frequently amplified lineage-survival oncogene in lung cancer cells [17,18]. Moreover, down-regulation the expression of SOX2 markedly decreased the translation level of SOX2 protein and reduced the cell proliferation rate in SCLC cell lines, suggesting the SOX2 is a critical gene functioned in tumor suppressing in SCLC, which may be used as a new chemotherapeutic drug targets [17].
Many factors could affect the expression and nuclear translocation level of SOX2, for example, activated the AKT, IKK and ERK phosphorylation could promote SOX2 translocated from cytoplasm to nucleus for subsequent ubiquitination and degradation. The MEK inhibitor (ex. AZD644) highly activated PI3K/AKT signaling pathway could inactivate the expression of SOX2, and the P13K/AKT inhibitors could activate the expression of SOX2 again.
AGl478 (4-(3-chlorophenyl amino)-6, 7-dimethoxy-quinazoline), a low molecular weight compound having an ATP-binding site for efficient reversible binding with EGFRTKI, can inactivate the phosphorylation and dimerization of EGFR, directly inhibit the overexpression of EGFR in tumor tissues and increased the sensitivity to drugs such as carboplatin, doxorubicin and etoposide [19-22]. This study investigates the regulation effect of AGl478 (EGFR-TKI) on SOX2 protein expression and nuclear plasma distribution in NSCLC patients.
Materials and Methods
Cell cultures
Human lung cancer cell lines of A549 and H1650 were cultured in RPMI-1640 medium supplemented with 10% FBS. They were incubated at 37°C in a humidified atmosphere of 5 % CO2 and were harvested when they reached 80-90% fusion with 0.25% trypsin digestion for the experiment.
Western blot
1 × 106 cells were diluted with RPMI-1640 medium containing 4 μmol/L AGl478, 20 μmol/L LY294002, 10 μmol/L U0126 or 100 μmol/L AG490, respectively. Then they were seeded in 6 cm dish for 48 h, rinsing cells 1 time by cold PBS, and ice cracking and cell lysates were collected from each group. The proteins were separated using SDS-PAGE. Blots were subsequently transferred to PVDF membranes at (100 v, for 100 min). The membranes were blocked for 2 h by 5% milk; thereafter they were hybridized with primary antibodies (1:1000) of FOXMl, SOX2, P-Sox2, P-ERKl/27P-AKT and AKT monoclonal antibodies at 4°C overnight. GAPDH expression was used as an internal control. Secondary antibodies were used at a 1:5000 dilution for 1 h at room temperature. Chemiluminescence assay was performed to determine protein expression.
Immunofluorescence
The logarithmic growth phase of AKT siRNA transfected H1650 cells were seeded in 6-well plates, the morphology was observed under microscope, cells fused when at a transfection rate 50% to 60%. AKT siRNA duplexes sequence were 5’ GGAACGUGAUGCUUCGCAATT3’ and 5’ AGGGAAGUUUGGUUCAAUCATT3’ unrelated Negative Control (NC) sequence were: 5’UUCUCCGAACGUGUCACGUTT3’ and 5’ ACGUGACACGUUCGGAGAATT3’.
After transfected for 8 h, incubated with fresh culture medium and cells were collected at different times point as following experiment conducted.
Immunofluorescence cells coverslip of the polylysine dry, placed in 6-well plates, 105 cells were seeded. The transfection medium was discarded after 72 h and cells were rinsed with PBS, fixed in 4% paraformaldehyde for 30 min, added 0.5% Triton X-100, allowed to stand for 15 min, 10% BSA for 1 h, l % BSA was added to dilute antibody, dark incubation at 37°C for 2 h, fluorescent secondary antibody at room temperature in the dark for 1.5 h, added DAPI 5 μg/ml for 2 min, then they were mounted prior to fluorescent microscope observation.
Statistical analysis
One-way ANOVA following a post hoc test were performed for all data analysis using SPSS 18.0 software. Measurement data were expressed as mean ± standard deviation. P<0.05 was considered statistically significant.
Results
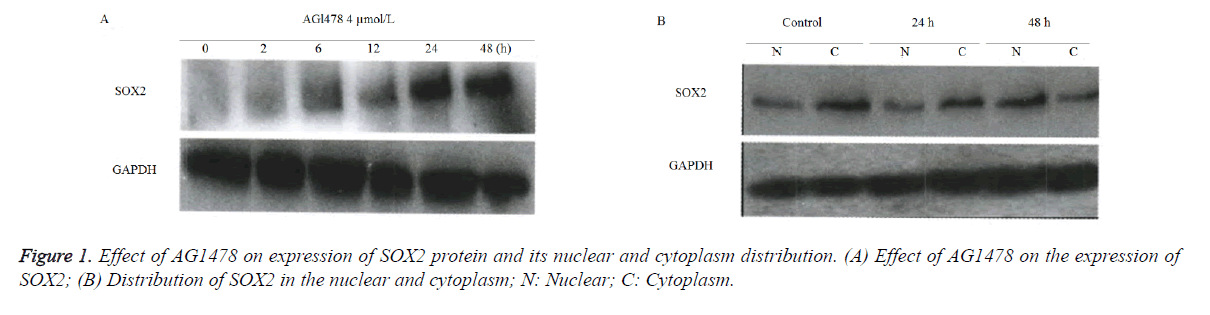
AGl478 upregulated the translation level of SOX2 protein and affected its subcellular localization
Western blot results (Figure 1) showed, compared with the control group of GAPDH, that the SOX2 protein level was gradually increased with prolonged detection time points at 2, 6, 12, 24 and 48 h following L AGl478 treatment in A549 cells (Figure 1A). The SOX2 protein was gradually shifted from the cytoplasm to nucleus in a time-dependent manner (Figure 1B).
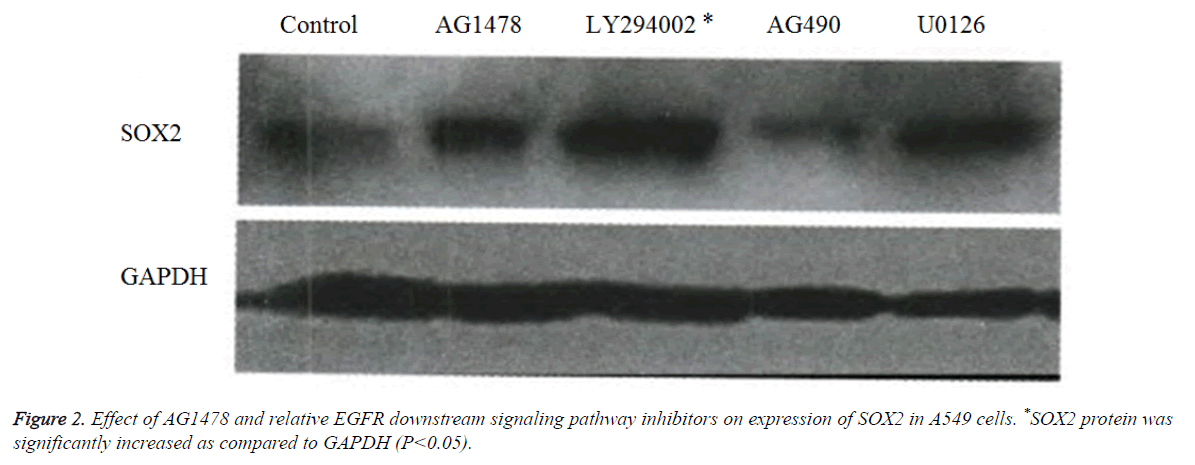
AGl478 and EGFR affected downstream signaling pathways of SOX2 expression
Western blot results showed (Figure 2), compared with the control group of GAPDH, that the translation level of SOX2 protein in the cells treated by LY294002 was significantly increased, the U0126 treated group was weaker, and the AG490 treated group was the weakest (Figure 2).
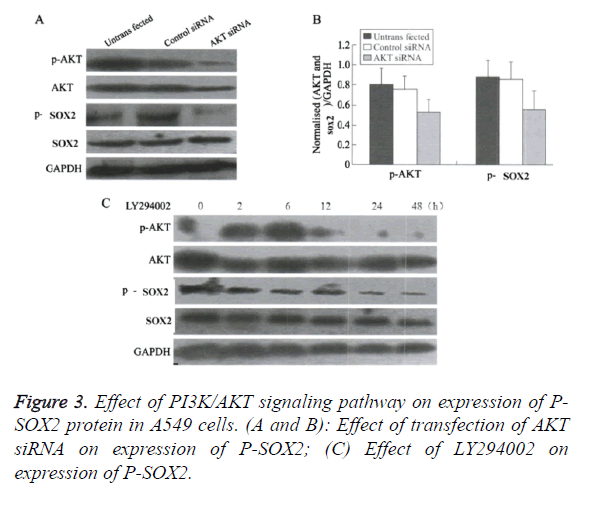
Blocking the PI3K/AKT signaling pathway promoted the nuclear translocation of SOX2
Compared with the control group, treated by AKT molecule (Figures 3A and 3B), or by the PI3K/AKT inhibitor LY294002 (Figure 3C), the translation levels of P-AKT and P-Sox2 molecules in H1650 cells were significantly reduced, and the transcriptional level of SOX2 was unchanged or slightly increased.
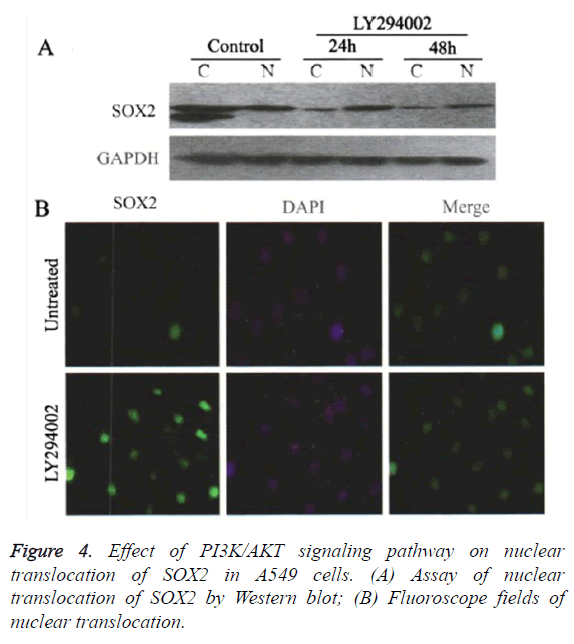
Blocking the PI3K/AKT signaling pathway promoted the nuclear translocation of SOX2
Western blot results showed (Figure 4), compared with the control group, that after treated with 20 μmol/L LY294002, the translation level of SOX2 protein in the cytoplasm was gradually decreased after 24 and 48 h respectively, whereas the translation level of SOX2 protein in nucleus was significantly increased after 24 h (Figure 4A). Cells immunofluorescence assays also showed that the translation level of SOX2 was gradually increased in the nucleus after 48 h when treated by LY294002 (Figure 4B). The promoted translocation effect of LY294002 on SOX2 showed in a time-dependent manner.
Discussion
Lung cancer is a one of the most common and polygenic malignant solid cancer resulting from complex interactions between environmental and genetic factors, and the incidence is increasing in both developed and developing countries. In facing this situation, there are more and more studies has been carried out for lung cancer treatment in preclinical and clinical trials, and a number of drugs has been approved for use, while an incredibly high rate of acquired resistance have limited their clinical applications. At the current lung cancer treatment, monotherapy of the chemotherapy is still the standard secondline treatment for advanced NSCLC, despite excellent initial responses to therapy, however, most lung cancer patients will go on to develop acquired resistance, developing new therapeutic strategies to overcome the acquired multidrug resistance represent priorities in the field. Therefore, targeted therapy combined with inhibition of multiple signaling pathways was studied to confer additive or synergistic antitumor effects and increased clinical benefit for advanced NSCLC patients [23,24]. A number of studies have confirmed SOX2 played a tumor suppressor role in anti-tumorigenesis processes, so it may be studied as a new target for many drugs. SOX2 is a subtype of FOX (forkhead box) family, and its conserved DNA binding domain (Forkhead box) is well characterized. SOX2 overexpression inhibits the growth of breast cancer cells, and its cytoplasmic localization is closely related with breast cancer [15,25,26]. Many factors involved in the AKT, IKK and ERK pathways can affect the translation, translocation or degradation of SOX2, such as EGFR. EGFR extracellular growth signals can block the MAPK, PI3K/AKT and JNK STAT signal pathways, then inactivating the translation and translocation of SOX2, and finally promote other regulatory, such as DNA synthesis, cell proliferation, angiogenesis, invasion and metastasis. The EGFR signals blocking factors may induce the overexpression of SOX2 or the translocation of SOX2 from the cytoplasm to nucleus. AGl478 was verified to inhibit the overexpression of EGFR in tumor tissues, and then inactivated the phosphorylation and dimerization of EGFR, while whether it has effect on the translation and translocation of SOX2 is not been studied and verified.
This study showed that the EGFR tyrosine kinase inhibitor AGl478 can activate the expression of SOX2 through the PI3K/AKT signaling pathway, promote SOX2 transferring from the cytoplasm to the nucleus. AGl478 increased the translation level of SOX2 mainly by inhibiting the downstream signaling pathways of EGFR. The activity of AGl478 may promote the targeting EGFR-TKI treatment effect of SOX2 in lung cancer patients, which may provide a new theoretical basis and new idea in developing the SOX2 as a new target for many drugs in lung cancer treatment, but these need to be examined and revised in the further studies.
The EGFR was easily overexpressed or mutated in NSCLC patients [27,28] and the EGFR extracellular growth signals can affect the MAPK, PI3K/Akt and JNK STAT signal pathways, then affect DNA synthesis, cell proliferation, angiogenesis, invasion and metastasis. SOX2 is a key regulator of pluripotency in embryonic stem cells and plays important roles in early organogenesis. Its expression was documented in various cancers and suggested as a cancer stem cell marker.
Our results show the expression and nuclear translocation of SOX2 was significantly induced by AGl478 via PI3K/AKT pathway with time dependent in NSCLC cell lines. Blocking the PI3K/AKT signaling pathway promoted the nuclear translocation of SOX2. LY294002 significantly inhibited the expression of P-Sox2, induced its nuclear translocation. AKT siRNA depleted the expressions of P-AKT and P-Sox2. These suggest that the SOX2 could be used as a potential target of cancer therapy and drug exploitation of NSCLC.
Conclusion
SOX2 may determine tumorigenicity. AGl478 significantly increased the SOX2 expression after 24 h treatment. This EGFR inhibitor directly increased the SOX2 overexpression, which should be considered for the chemotherapy efficacy in NSCLC treatment.
References
- Cui Z, Yin Z, Li X, Wu W, Guan P, Zhou B. Association between polymorphisms in XRCC1 gene and clinical outcomes of patients with lung cancer: a meta-analysis. BMC Cancer 2012; 12: 71.
- Guilbert J. The world health report 2002-reducing risks, promoting healthy life. Education for Health-Abingdon-Carfax Publishing Limited 2013; 16: 230.
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. Cancer J Clin 2005; 55: 74-108.
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics. Cancer J Clin 2008; 58: 71-96.
- Sechler M, Cizmic AD, Avasarala S, Van Scoyk M, Brzezinski C, Kelley N, Bikkavilli RK, Winn RA. Non-small-cell lung cancer: molecular targeted therapy and personalized medicine - drug resistance, mechanisms, and strategies. Pharmgenomics Pers Med 2013; 6: 25-36.
- Guo S, Reddy CA, Chao ST, Suh JH, Barnett GH, Vogelbaum MA, Videtic GM. Impact of non-small cell lung cancer histology on survival predicted from the graded prognostic assessment for patients with brain metastases. Lung Cancer 2012; 77: 389-393.
- Mart´ınez-Moreno P, Nieto-Cerón S, Torres-Lanzas J, Ruiz-Espejo F, Tovar-Zapata I, Martínez-Hernández P, Rodríguez-López JN, Vidal CJ, Cabezas-Herrera J. Cholinesterase activity of human lung tumours varies according to their histological classification. Carcinogenesis 2006; 27: 429-436.
- Lwin Z, Riess JW, Gandara D. The continuing role of chemotherapy for advanced non-small cell lung cancer in the targeted therapy era. J Thorac Dis 2013; 5: 556-564.
- Huang J, Zhang T, Ma K, Fan P, Liu Y, Weng C, Fan G, Liu Y, Weng C, Fan G, Duan Q, Zhu X. Clinical evaluation of targeted arterial perfusion of verapamil and chemotherapeutic drugs in interventional therapy of advanced lung cancer. Cancer Chemother Pharmacol 2013; 79: 889-896.
- Jett JR, Carr LL. Targeted therapy for non-small cell lung cancer. Am J Respir Crit Care Med 2013; 188: 907-912.
- Zhang Y, He J. The development of targeted therapy in small cell lung cancer. J Thorac Dis 2013; 538-548.
- Masui S, Nakatake Y, Toyooka Y, Shimosato D, Yagi R, Takahashi K, Okochi H, Okuda A, Matoba R, Sharov AA, Ko MS, Niwa H. Pluripotency governed by sox2 via regulation of oct3/4 expression in mouse embryonic stem cells. Nat Cell Biol 2007; 9: 625-635.
- Arden KC. Multiple roles of FOXO transcription factors in mammalian cells point to multiple roles in cancer. Exp Gerontol 2006; 41: 709-717.
- Myatt SS, Lam EW. The emerging roles of forkhead box (Fox) proteins in cancer. Nat Rev Cancer 2007; 7: 847-859.
- Hu MC, Lee DF, Xia W, Golfman LS, Ou-Yang F, Yang JY, Zou Y, Bao S, Hanada N, Saso H, Kobayashi R, Hung MC. Ikappa B kinase promotes tumorigenesis through inhibition of orkhead FOX03a. Cell 2004; 117: 225-237.
- Potente M, Urbich C, Sasaki K, Hofmann WK, Heeschen C, Aicher A, Kollipara R, DePinho RA, Zeiher AM, Dimmeler S. Involvement of Foxo transcription factors in angiogenesis and postnatal neovascularization. Clin Invest 2005; 115: 2382-2392.
- Rudin CM, Durinck S, Stawiski EW, Poirier JT, Modrusan Z, Shames DS. Comprehensive genomic analysis identifies sox2 as a frequently amplified gene in small-cell lung cancer. Nat Genet 2012; 44: 1111-1116.
- Bass AJ, Watanabe H, Mermel CH, Yu S, Perner S, Verhaak RG. Sox2 is an amplified lineage-survival oncogene in lung and esophageal squamous cell carcinomas. Nat Genet 2009; 41: 1238-1242.
- Arteaga CL, Ramsey TT, Shawver LK, Guyer CA. Unliganded epidermal growth factor receptor dimerization induced by direct interaction of quinazolines with the ATP binding site. J Biol Chem 19978; 272: 23247-23254.
- Nagane M, Narita Y, Mishima K, Levitzki A, Burgess AW, Cavenee WK, Huang HJ. Human glioblastoma xenografts overexpressing a tumorspecific mutant epidermal growth factor receptor sensitized to cisplatin by the AGl478 tyrosine kinase inhibitor. J Neurosurg 2001; 95: 472-479.
- Zhang YG, Du Q, Fang WG, Jin ML, Tian XX. Tyrphostin AGl478 suppresses proliferation and invasion of human breast cancer cells. Int J Oncol 2008; 33: 595-602.
- Ren Y, Yin H, Tian R, Cui L, Zhu Y, Lin W, Tang XD, Gui Y, Zheng XL. Different effects of epidermal growth factor on smooth muscle cells derived from human myometfium and fromleiomyoma. Fertil Steril 2011; 96: 1015-1020.
- Belani CP, Goss G, Blumenschein G Jr. Recent clinical developments and rationale for combining targeted agents in non-small cell lung cancer (NSCLC). Cancer Treat Rev 2012; 38: 173-184.
- Custodio A, Mendez M, Provencio M. Targeted therapies for advanced non-small-cell lung cancer: current status and future implications. Cancer Treat Rev 2012; 38: 36-53.
- Mollberg N, Surati M, Demchuk C, Fathi R, Salama AK, Husain AN, Hensing T, Salgia R. Mind-mapping for lung cancer: towards a personalized therapeutics approach. Adv Ther 2011; 28: 173-194.
- Yang JY, Zong CS, Xia W. ERK promotes tumorigenesis by inhibiting FOX03a via MDM2-mediated degradation. Nature Cell Biol 2008; 10: 138-148.
- Mendelsohn J, Baselga J. Status of epidermal growth factor receptor antagonists in the biology and treatment of cancer. J Clin Oncol 2003; 21: 2787-2799.
- Brabender J, Danenberg KD, Metzger R, Schneider PM, Park J, Salonga D, Hölscher AH, Danenberg PV. Epidermal growth factor receptor and HER2-neu mRNA expression in non-small cell lung cancer is correlated with survival. Clin Cancer Res 2001; 7: 1850-1855.