ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2022) Volume 33, Issue 6
The effect of human adipose tissue derived stem cells-conditioned medium and Rosmarinus officinalis L.'s extract on the survival of breast cancer cells (MCF7) in vitro.
Motaghi Moridani Fatemeh, Haji Ghasem Kashani Maryam*
Department of Cellular and Molecular Biology, School of Biology and Institute of Biological Sciences, Damghan University, Damghan, Iran
- Corresponding Author:
- Haji Ghasem Kashani Maryam
Department of Cellular and Molecular Biology
School of Biology and Institute of Biological Sciences
Damghan University
Damghan
Iran
Accepted date: 19 July, 2022
Cultured Human Adipose Tissue Derived Stem Cells (hADSCs) secreted many growth factors, which called Conditioned Medium (CM). CM has antioxidant and antitumor activities. Recent studies have demonstrated antiproliferative and apoptotic features of Rosmarinus officinalis L.'s Extract (RE) on cancer cells.
In this study, CM was prepared from fourth passage of cultured hADSCs in serum-free medium for 48 h. MCF7 cells proliferation rate, survival and also expression of apoptotic genes treated by RE (50, 100, and 200 μg/ml) and CM for 24, 48, and 72 h were assessed by MTT, and RT-PCR.
Treated MCF7 cells by RE at concentration of 50 μg/ml at all times and CM at 24 and 48 h post induction have shown significant decrease of cell proliferation rate as compared to un-induced cells (control). Caspase 3 gene expression in cells treated by RE50 (24 and 72 h), RE100 or CM for 24 and 48 h was increased significantly as compared to control. In addition, significant increase of Caspase 9 gene expression was observed in 50 μg/ml RE- treated cells after 24, 48 and 72 h and 100 μg/ml RE- treated cells for 48 h as compared to control. While CM-induced cells showed a significant increase in the mRNA level of Caspase 9 gene at 24 h. Incubation with RE at 50 μg/ml for 24, 48 and 72 h or CM for 24 h were efficient for apoptosis induction of MCF7 cells. RE and CM treatment showed different effects on MCF7 cells in the absence or presence of serum, like a double-edged sword.
Keywords
MCF7, Conditioned medium, Rosmarinus officinalis L, Caspase.
Introduction
Human Adipose Tissue Derived Stem Cells (hADSCs) were first identified by Zuk and colleagues in 2001[1]. Human adipose tissue collection is easy and safe [2]. Abdominal fat obtained by liposuction is the most abundant and accessible source of stem cells [3,4]. Recent studies have shown that adipose tissue possesses more stem cells per gram compared to bone marrow or umbilical cord. ADSCs are multipotent with high proliferation rate and easily extracted, retaining their stem cell [5-7]. Also they have adherent property to the culture dish [8]. ADSCs are adipogenic, chondrogenic, osteogenic and neurogenic precursor cells [3,7]. Growth factors including Platelet- Derived Growth Factor (PDGF), Vascular Endothelial Growth Factor (VEGF), basic Fibroblast Growth Factor (bFGF), insulin-like growth factor 1, Hepatocyte Growth Factor (HGF), and Transforming Growth Factor (TGF)-β1 are secreted by ADSCs which act as paracrine hormones [8-11]. These factors are microvesicles, secretome or exosome secreted by cultured cells and called Conditioned Medium (CM). Since CM is cell-free, it prevents occurrence of immune responses and transplantation rejection. CM has anti-oxidant and anti-tumor effects [12]. Cell number, cell type, culture conditions, medium type and cell passage number have affected CM quality. CM has several advantages, as it can be prepared, freeze-dried, packaged, and transported more easily. CM could be used in drug manufacturing. Unlike stem cells, CM doesn’t need special preservation [12]. Furthermore, unlike stem cells that have low-rate transplantation and high risk of metastasis, CM does not have these defects and is available to precede treatment without any need to isolate patient's stem cells. To obtain an appropriate CM for clinical use, it is better to use culture medium containing vitamins, amino acids, glucose and serum derived from human blood [13]. Short lifespan is the only defect of CM [12]. Wound healing [14], improvement of wrinkles, premature skin caused by UV [4], Alopecia, hair growth, nervous disorders, skeletal and lung defect, kidney diseases, liver injuries, neuronal infections [12], stroke [2], myocardial infarct [15] are the therapeutic applications of CM. CM is also effective for ovarian oocyte maturation [16] and the treatment of some cancers, including breast cancer. Recently, the differentiation of embryonic cancer stem cells into insulin-producing cells was confirmed by using pancreatic conditioned medium as inducer [17]. Neurotrophic factors such as NGF and BDNF of CM has been reported to cause glial cells survival and protection in Friedreich's ataxia [10]. It was reported that ADSCs-CM has the inhibitory effect on breast and pancreas cancer cells proliferation by blocking the cell cycle in G0/G1 stages. In addition, CM inhibited the survival of colon and prostate cancer cells [18]. CM treatment can reduce proliferation rate and survival of liver cancer cells and induce apoptosis [18,19].
ADSCs-CM inhibits breast cancer cells (MCF7) proliferation more rapidly than CM of umbilical cordderived mesenchymal stem cells [20]. CM of bone marrow-derived mesenchymal stromal cells can increase proliferation rate of MCF7 [21]. Nowadays, herbal medicines are being used to improve the efficacy of cancer therapy [22]. Rosemary is a Mediterranean herbaceous plant belonging to the Laminaceae family [23]. Rosemary has many therapeutic uses, including blood flow and blood pressure improvement in cardiovascular disease, memory improvement of neurodegenerative diseases, headache, migraine, depression, anxiety, asthma and liver function improvement [23]. Many in vitro studies have proven the anti-cancer effect of Rosemary [24] such as oral cancer [25].
Most studies revealed that Rosemary Extract (RE) has anti-cancer effect by inducing apoptosis [26]. Cheng and colleagues have shown the suppressive role of RE related to its major components, including Carnosic Acid (CA), Rosmarinic Acid (RA), carnosol, rosmanol and ursolic acid [27]. RA was originally isolated from Rosemary in 1958 [28]. RA has several biological activities, including: anti-angiogenic and anti-depressant [29,30], antiviral, antimicrobial, antimutagenic, anti-metastatic, neuroprotective, immune system modulator, melanogenic, anticancer and antioxidant [28,31,32]. CA extracted from rosemary leaves and constituted 0.1% to 0.5% of Rosemary extract. CA has anti-proliferative, anti-inflammatory, anti-tumor and neuroprotective effects [25,33]. Chemoherbal combinations provided a promising tool for cancer treatment in the near future [26]. The anti-inflammatory and anti-proliferative effects of RA on colon cancer has been demonstrated [27,28,34].
Previous studies have shown the role of RE in inhibiting pancreatic cancer cells viability and proliferation and inducing apoptosis [26]. In gastric cancer cells, RA reduces cell viability and drug resistance. In skin cancer, CA inhibits cancer cells survival and cell migration by increasing cell cycle arrest and inducing apoptosis [26]. Anti-inflammatory and antioxidant features of RA play significant role against skin tumors in carcinomas and melanomas. In melanoma cells, RA as a radio sensitizer, increasing cellular death by 42%. Patients with lung cancer treated by RA have shown 50% reduction of cancer cells [28]. Dorrie and colleagues reported that carnosol induced apoptosis in leukemia cells [35]. CA induces apoptosis by activating caspases and endoplasmic reticulum stress mediated by ROS in human renal cancer cells. Also CA induced apoptosis in liver cancer cells [36]. Incubation of MCF7 with carnosol reduced the expression of Estrogen Receptor alpha (ERα), so that it can be effective against the progression of breast cancer [24]. Description of the estrogen receptor of MCF7 cells in 1973 was the key in breast cancer [37]. MCF7 cells have various hormone receptors, including the estrogen receptor responded to estrogen. It has been shown that estrogen can directly stimulate tumor growth. These cancer cells have many features, which distinguish them from the breast epithelium, also they have genomic instability. MCF7 is a weak, noninvasive cell line with low metastatic potential. MCF7 cell spheroids were described as a mass of cells with inactive nucleus, strong cell-to-cell junctions, and the absence of lumen formation [37-39]. CA presented inhibition more than 2-fold in the estrogen-independent breast cancer cell line (MDA-MB-231) as compared to the estrogen-dependent carcinoma cell line (MCF7) so that CA can induce G2/M cell cycle arrest [35]. Skeletal abnormalities are another complication, caused by breast cancer metastasis. RA treatment inhibits metastasis of breast cancer and also protects bones from damage [40]. Cognitive function improvement, Alzheimer's disease prevention, heart protection, reducing the severity of kidney disease, antioxidant, anti-allergy and chemical prevention of cancer are another effects of the RA that scientists have come to realize [28]. The prevalence of breast cancer in modern society is a serious threat to women's health, so that research and treatment of targeted cancer cells are very important. Recent studies have shown that MCF7 cancer cell line is a great advantage and the conditioned medium may offer a new way to treat cancer. In this study, the effects of RE and CM on MCF7 cells were evaluated to create advances in the treatment of breast cancer. It can be used as an anticancer agent either alone or in combination with herbal medicine and chemotherapy.
Materials and Methods
Preparation of MCF7 cells and conditioned medium
MCF7 cells (purchased from the Pasteur Institute of Iran) were transferred into 25 cm2 flasks and incubated (at 37°C, 95% humidity and 5% CO2) in DMEM/F-12 (Dulbecco’s Modified Eagle’s Medium-F12; Gibco, USA) containing 10% FBS (Gibco) [39,41]. The cells were subcultured when the confluency reached to 70%-80%. Liposuction samples were collected from women (25-35 years old) after obtaining written informed consent with the approval of the local Ethics Committee (IR.DU.REC.1400.009). Firstly, adipose tissue was cut into very tiny pieces and then 0.2% collagenase (Gibco, 17100-017) added and incubated at 37°C for 2 h. To stop digestion, DMEM (Invitrogen, USA) containing 10% FBS was added to the suspension and centrifuged with 1200 R.P.M at 37°C for 5 min and incubated. The cells at passage four were washed by PBS for three times and cultured in serum-free medium for 72 h to allow secretion of neurotrophic factors. The conditioned medium (hADSCs-CM) was then collected, centrifuged at 2000 rpm for 5 min, filtered through a 0.22 mm syringe filter and stored at -80°C [7].
Experimental groups
The studied groups were divided into: cultured MCF7 in medium containing 10% FBS (Control), in hADSCs-CM (CM), in CM containing 10% FBS (CM+FBS). Cultured MCF7 cells in serum-free medium containing RE (40% CA, carnosic acid powder CAP25-110401, purchased from Hunan Geneham Biomedical Technology, China) at doses 50 μg/ml (RE50), 100 μg/ml (RE100), 200 μg/ ml (RE200). Cultured MCF7 cells in medium containing serum and RE at doses of 50 μg/ml (RE50+FBS), 100 μg/ ml (RE100+FBS) and 200 μg/ml (RE200+FBS).
Evaluation of cell doubling time using MTT assay
MTT assay kit was purchased from Sigma and performed according to the standard protocol. The P4 cells were seeded at a density of 2 × 104 cells per well of 96-well plates and treated by CM and RE for 24, 48 and 72 hours. The media was discarded and replaced with 100 μL of fresh culture media. The MTT solution (50 μL) was deposited in each well. After 3 hours of incubation at 37°C, the previous medium was replaced with 250 μL of DMSO. Cell viability was detected by measuring the absorbance at 540 nm using a micro plate reader (BioTek, USA).
Reverse Transcription Polymerase Chain Reaction (RTPCR)
Reverse Transcription Polymerase Chain Reaction (RT-PCR) was performed to evaluate the expression of Caspase 3 and 9 genes. Briefly, after induction, total RNA extraction and cDNA synthesis were carried out. Then, synthetic cDNA was used for PCR. The primer sequences are listed in Table 1.
| Primer sequences and product size | |||
|---|---|---|---|
| Gene | Accession number | Primer sequence (5'→3') |
Product size (bp) |
| Caspase 3 | NM_004346 | TCTCATGCTGCAGAGGGTAC | 100 |
| TAATTCAATTGCAACTACCTGAC | |||
| Caspase 9 | NM_001229 | CTGCTTAGGGTCGCTAATGC | 113 |
| GGGCCCTGGCCTTATGATG | |||
| GAPDH | NM_002046 | GCTGGGGCTCATTTGCAGG ------------------------------------------------ CGGAGGGGCCATCCACAGT |
258 |
Table 1. Primer sequences and lengths of PCR products of Caspase 3 and Caspase 9 genes.
Statistical analysis
Data were analyzed by SPSS 23 software, using one-way ANOVA or Paired-sample t-test. Tukey’s post hoc test was used for analyzing the data within groups. The results are indicated as mean ± Standard Error of the Mean (SEM) and considered as meaningful at P ≤ 0.05.
Results
Morphological study of cultured MCF7 and hADSCs
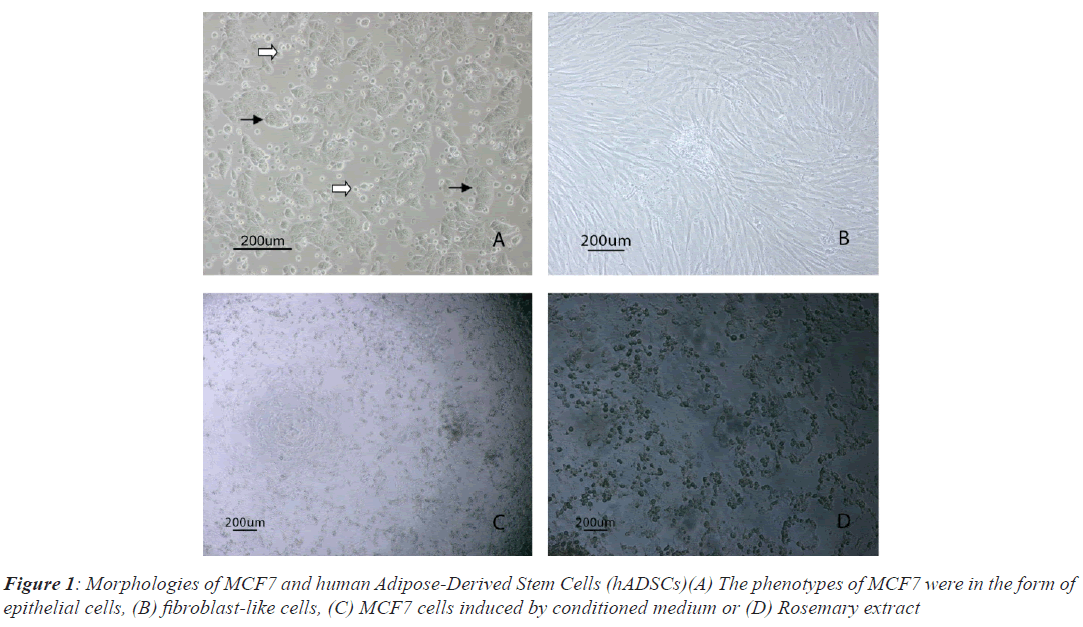
MCF7 cells had very high proliferation rate and grew colonies with high density in vitro culture conditions, so that the nuclei of dividing cells in different cell cycles observed easily. MCF7 cells were attached to the bottom of the flask (black arrows) in the form of epithelial shape, while floating dead cells were observed in Figure 1A (white arrows). Isolated hADSCs possess typical spindlelike morphology at passage 3, as shown in Figure 1B. Morphological changes of MCF-7 cells post induced by CM and RE were observed in Figures1C and 1D.
Evaluation of doubling time by MTT assay
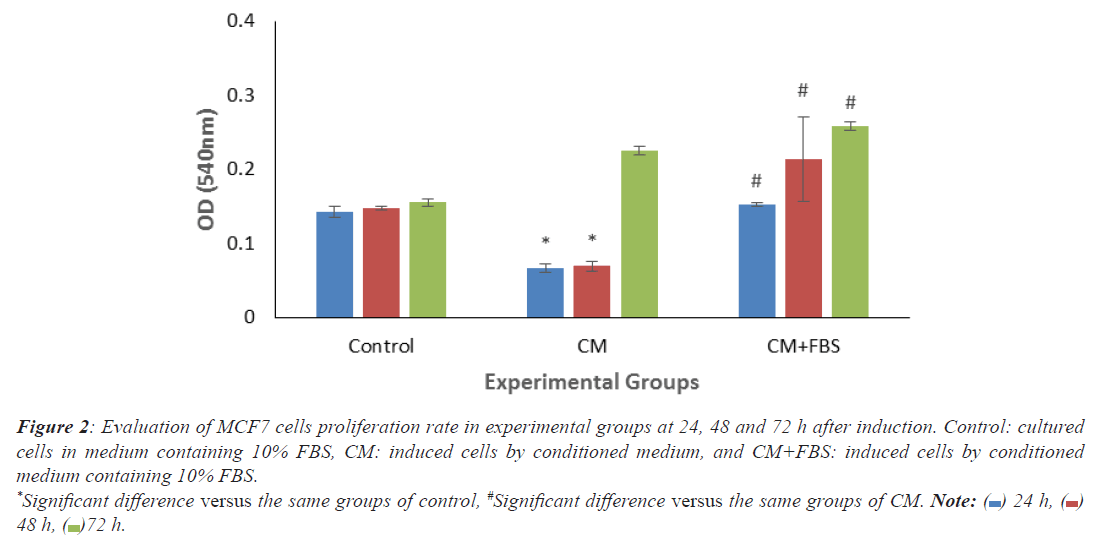
As shown in histogram 1, significant decrease of OD levels was observed in treated MCF7 cells by CM (24 and 48 h), compared to the same groups in control. By adding serum, all treated groups showed significant increase compared to the same groups of CM (Figure 2).
Figure 2: Evaluation of MCF7 cells proliferation rate in experimental groups at 24, 48 and 72 h after induction. Control: cultured
cells in medium containing 10% FBS, CM: induced cells by conditioned medium, and CM+FBS: induced cells by conditioned
medium containing 10% FBS.
*Significant difference versus the same groups of control, #Significant difference versus the same groups of CM. Note:  24 h,
24 h,  48 h,
48 h,  72 h.
72 h.
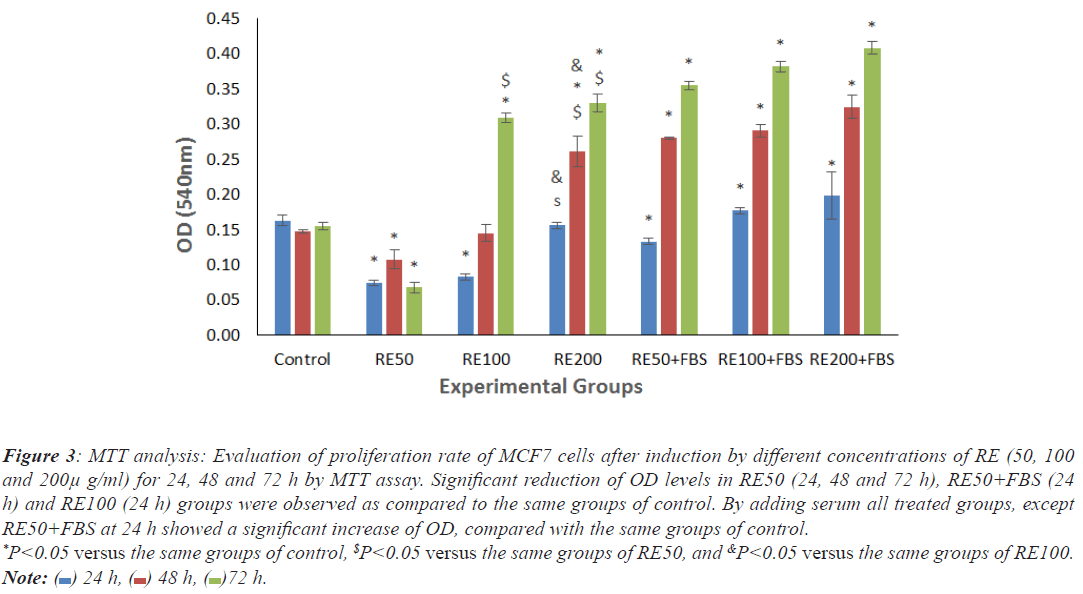
There were significant decrease of OD levels in cells exposed to RE50 (24, 48 and 72 h), RE50+FBS (24 h) and RE100 (24 h) as compared to the same groups of control. By adding serum, all treated groups except RE50+FBS at 24 h showed significant increase of proliferation rate in comparison with the same groups of control (Figure 3).
Figure 3: MTT analysis: Evaluation of proliferation rate of MCF7 cells after induction by different concentrations of RE (50, 100
and 200μ g/ml) for 24, 48 and 72 h by MTT assay. Significant reduction of OD levels in RE50 (24, 48 and 72 h), RE50+FBS (24
h) and RE100 (24 h) groups were observed as compared to the same groups of control. By adding serum all treated groups, except
RE50+FBS at 24 h showed a significant increase of OD, compared with the same groups of control. *P<0.05 versus the same groups of control, $P<0.05 versus the same groups of RE50, and &P<0.05 versus the same groups of RE100. Note:  24 h,
24 h,  48 h,
48 h,  72 h.
72 h.
The expression of Caspase 3 and 9 genes using RT-PCR technique
Based on the results of two previous tests, all experimental groups except RE 200 were selected for RT-PCR study. The PCR products analyzed by 1.5% agarose gel electrophoresis and finally, the intensity of gene bands were checked by the Image J software.
Comparison of Caspase-3 gene expression in cells induced by CM and RE at different doses after 24, 48 and 72 h
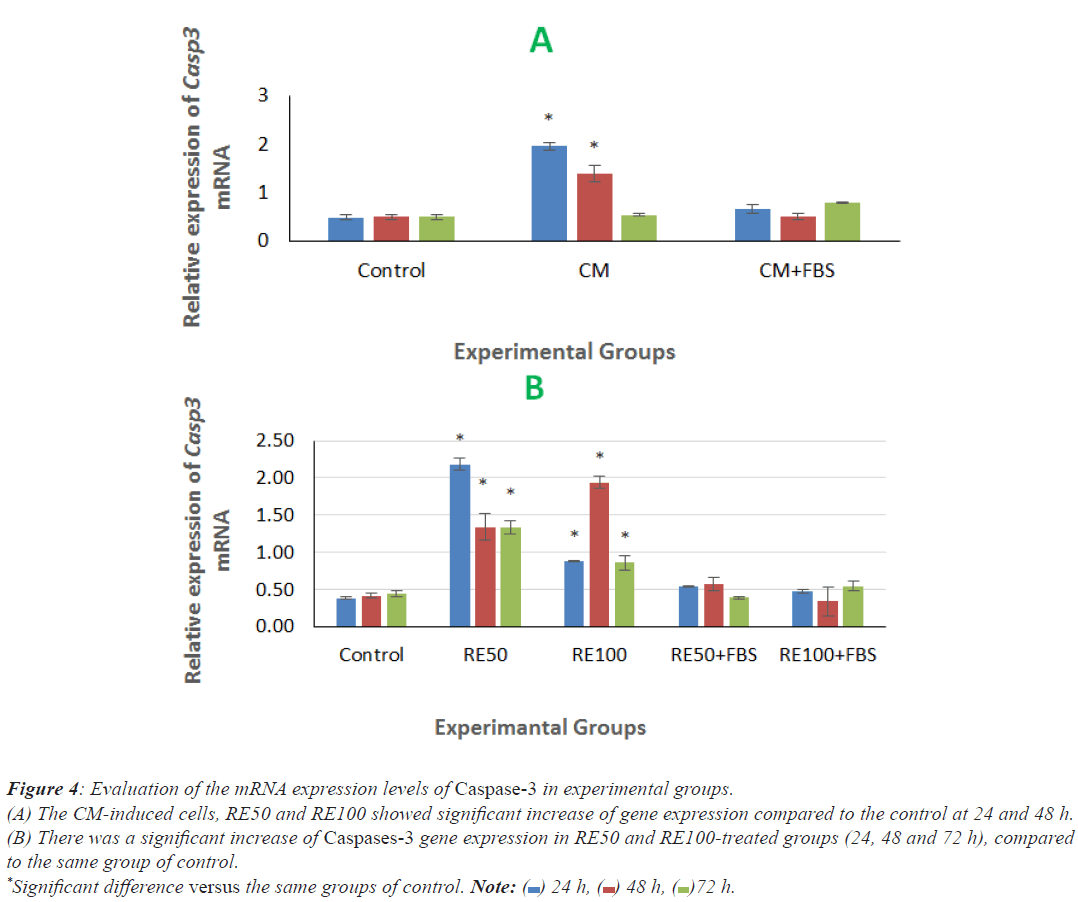
There was a significant increase of Caspases-3 gene expression in CM-induced cells at 24 and 48 h post induction, compared to the same groups of control (Figure 4A). In addition, RE50 and RE100-treated groups (24, 48 and 72 h) showed significant increase of this gene expression in comparison with the same group of control (Figure 4B).
Figure 4: Evaluation of the mRNA expression levels of Caspase-3 in experimental groups.
(A) The CM-induced cells, RE50 and RE100 showed significant increase of gene expression compared to the control at 24 and 48 h.
(B) There was a significant increase of Caspases-3 gene expression in RE50 and RE100-treated groups (24, 48 and 72 h), compared
to the same group of control.
*Significant difference versus the same groups of control. Note:  24 h,
24 h,  48 h,
48 h, 72 h.
72 h.
Comparison of Caspase-9 gene expression in cells induced by CM and RE at different doses after 24, 48 and 72 h
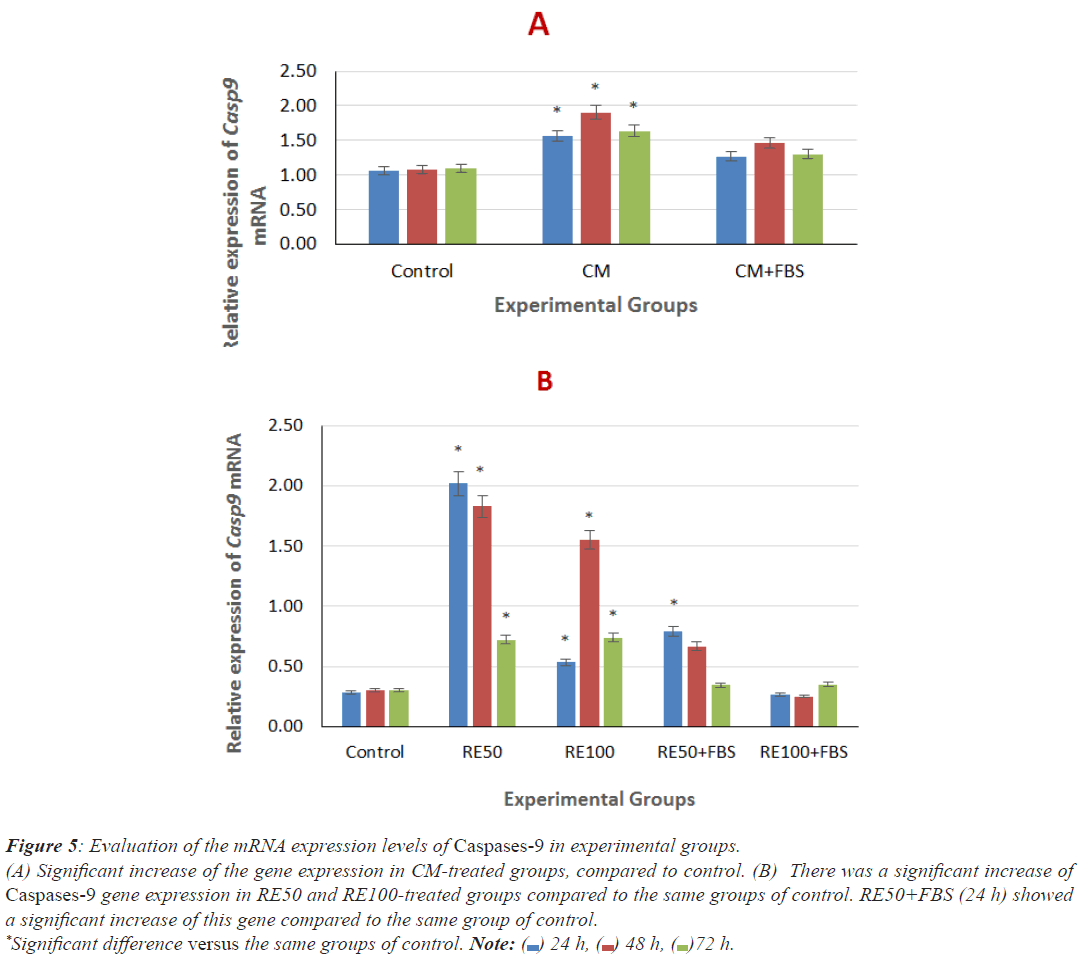
As shown in Figures 5A and 5B, there was a significant increase of Caspase-9 mRNA level in CM groups (24, 48 and 72 h), compared to the same groups of control. There were a significant increase of gene expression in RE50 and RE100- treated groups (24, 48 and 72 h) and RE50+FBS (24 h), compared to the same groups of control. No significant difference between the other RE-treated groups with serum compared to the same group of control was observed.
Figure 5: Evaluation of the mRNA expression levels of Caspases-9 in experimental groups.
(A) Significant increase of the gene expression in CM-treated groups, compared to control. (B) There was a significant increase of
Caspases-9 gene expression in RE50 and RE100-treated groups compared to the same groups of control. RE50+FBS (24 h) showed
a significant increase of this gene compared to the same group of control.
*Significant difference versus the same groups of control. Note:  24 h,
24 h,  48 h,
48 h,  72 h.
72 h.
Discussion
Up to now, according to evidence, cancer has become a major health concern in many countries [42]. Breast cancer is the most common cancer among women and as the most important cause of mortality worldwide in women [43]. The cause of breast cancer is not yet fully understood, but various factors such as genetic, environmental and hormonal factors have been implicated in cancer [42]. Breast cancer has many cell types, including MCF7, BT20, T47D, MDA-MB-231 [44]. MCF7 stands for the Michigan Cancer Foundation 7 and first extracted from a 69-year-old woman in 1970 [39,45]. These cells have shown coherent structure and strong cell adhesion in vitro [46]. These cells have estrogen and progesterone receptors, so that, the proliferation rate in the breast cell line is very high. Cell proliferation can be inhibited by treatment with anti-estrogens [46,47]. The expression of estrogen receptors on the surface of MCF7 cells has caused these cells to respond to chemotherapy frequently [46]. Cancer is treated with a combination of methods including chemotherapy, radiotherapy, immunotherapy, and surgery. However, these therapies have their own limitations and cannot specifically target cancer cells; therefore the mortality rate is still high in cancer patients, indicating the ineffectiveness of these therapies [48]. Nowadays, after the removal of breast, patient's own adipose tissue is transplanted to the surgical site in order to preserve the organ anatomy [3]. Stem cells provide autologous transplantation [49]. The microenvirenment secreted by stem cells of transplanted tissue can prevent the malignancy of cancer cells [3]. Mesenchymal stem cells as multipotent somatic stem cells are used in regenerative medicine [50]. In the tumor microenvironment, MSCs appear with two roles of pre-tumor cells and antitumor cells. MSCs role depend on many factors, including the type of MSCs, the type of experimental cancer cell, in vivo or in vitro conditions, the factors secreted by MSCs, the interactions between MSCs, host immune cells and cancer cells [48]. MSCs can also induce anti-proliferative and apoptotic activities in cancer cells [51]. Although cell therapy improves the patient's clinical symptoms partially, but the loss of large number of cells at the site of transplantation and the potential risk of post-transplantation cancer have convinced many researchers to focus on the use of CM [13]. Kinnaird et al. in 2004 and Lee et al. in 2009 reported the presence of cytokines in the CM collected from MSCs under hypoxic conditions. Studies have shown that there are many factors in CM, including VEGF, bFGF, EGF, TGF-b, HGF, HGF, PDGF, KGF, TNF and IL-6, etc [14]. CM has several advantages over stem cells and can be easily prepared, frozen, packaged and transported. In addition, because CM is cell-free, there is no need for donor-recipient matching to prevent transplant rejection. CM contains diverse cytokines, growth factors and tissue repair factors which secreted by stem cells. Recently, CM has used in preclinical studies as an alternative therapy for wound healing [14].
In this study, the average number of cultured MCF7 cells exposed to CM for 24 and 48 h significantly decreased as compared to cultured cells in medium containing serum (control). Therefore, CM can cause cancer cells death. The proliferation rate of MCF7 cells at 72 h increased significantly in comparison to that in the control group and CM induction failed to reduce the viable cells.
In a study CM was used to treat melanoma, a malignant skin tumor and the proliferation rate of tumor cells decreased and cells stopped in G1 phase [8]. Glioma is a common brain tumor and very invasive. Current treatments for this disease are surgery, radiation, and chemotherapy, but these treatments rarely have therapeutic features [20]. Using CM can increase caspases 3 and 9 and consequently increase apoptosis of cancer cells. The number of cells in G0/G1 phase was increased by CM treatment. CM treatment also reduced cell proliferation and migration in lung and rectal cancer [20] and caused high apoptosis in bladder, prostate, colon, pancreatic and liver cancer cells [18,19,52]. Apoptosis begins with initiator caspases which activates downstream executioner caspases [53]. It has been reported that apoptosis plays an important role in the elimination of primary tumor cells and thus prevents disease progression [54].
In this study the results showed that reducing the number of cancer cells after induction with CM of hADSC for 24 and 48 h is likely due to the expression of caspase3 and caspase9 genes.
CM contains factors that increase the chemical sensitivity of breast cancer cells to chemotherapy. CM containing Interferon which is a multifunctional cytokine and acts as a tumor growth inhibitor, apoptosis inducer and causes cells to remain in the G0/G1 phase [55], so that the tendency to use anti-cancer food supplements have also increased in recent years. Compounds originated from plants have attracted scientific attention for their use as agents for the prevention and treatment of cancer [26]. Medicinal herbs are as rich as effective medicines. Up to now, herbs are still found in 40 percent of prescription drugs. Rosemary is a popular culinary/medicinal plant [33]. Rosemary is rich in antioxidant compounds including Carnosic Acid (CA), carnosol and Rosmarinic Acid (RA) [22]. It was reported CA have medicinal activity [34]. Among the various components of Rosemary Extract (RE), CA is the most active compound and plays an important role in the anticancer effects of rosemary. Many studies have shown the low doses of CA with pre-differentiation effect [26]. Recent studies have shown that RE and its polyphenols, including CA and RA, are good candidates for drug production and further research. They may be used either as chemicals to target specific pathways that induce apoptosis and decrease cell survival or be used to enhance the anticancer effects of chemotherapy. Overall, in vivo studies have reported using high doses of RE can manifest the absence of side effects and reduced toxicity [26]. It should be noted that the level of polyphenols, bioactive compounds and healing power in RE are affected by many factors, including plant growth conditions (soil, climate, exposure to stressors), extraction process (protocol) and storage of RE [26]. The pharmacological effects of rosemary components for cancer prevention and treatment have been investigated in vitro and in vivo [56]. CA is the major phenolic compound derived from rosemary. It has been reported that CA have antimicrobial, anti-obesity, antimutagenic, antioxidant, antitumor, anti-cancer, anti-inflammatory, neuroprotective and hepatoprotective activities while is non-toxic for human use [57,58]. Also Wijeraten and Cuppett reported that CA inhibits lipid peroxidation under oxidative stress conditions by 80%-100% [25]. Another study showed that CA induces cell cycle inhibition mainly at the G2/M stage [25]. It also increases apoptosis in numerous cancer cells but not in normal cells [36]. Research have shown that RE has inhibited advanced colon, breast (MDA-MB-231 and MCF7), liver, stomach, skin (melanoma), blood (leukemia), cervical (epithelioid), ovarian, prostate, mouth and lungs cancers and reduces the rate of proliferation of cancer cells [27,28,35]. RE blocked these cells in different phases of the cell cycle, leading to inhibition of cell proliferation and subsequent apoptosis [28,33]. CA also inhibits the proliferation of ovarian cancer cells line A2780 and increases the sensitivity of other ovarian cancer cells line A2780CP70 to the chemotherapeutic agent [26]. CA inhibits leukemia cells proliferation and increases apoptosis [36,59]. Other studies have shown that CA induces apoptosis in colon cancer cells [34]. CA can induce apoptosis through endoplasmic reticulum by ROS in renal cells carcinoma line. CA inhibited cell viability and increased apoptosis in liver cancer and small cells of lung cancer. It was reported that CA could inhibit proliferation rate and induce apoptosis in MCF-7, MDA-MB-231 and MDA-MB-468 cell lines. MCF7 cells are more sensitive to CA treatment [26].
In this study, average live MCF7 cells exposed to RE (50 μg/ml concentration) for 24 and 48 h showed a decline in cell viability compared to control. Only exposure to RE50+FBS and RE 100 μg/ml without serum at 24 h post induction decreased significantly cell viability, compared to control. Therefore, RE at a concentration of 50 μg/ ml for 24, 48 and 72 h incubation was the most effective for inducing MCF7 cell death. RT-PCR results showed the significantly increased expression of caspases 3 and 9 apoptotic genes in MCF7 cells exposed to RE at concentration of 50 μg/ml, in comparison with cultured cells in medium containing serum. The high expression of these genes were significantly correlated with RE apoptosis promoting effect on MCF-7 cells.
As the cells treated by 50 and 100 μg/ml of RE in the presence of serum (for 24 and 48 h), showed significant decrease of apoptotic genes, it was concluded that RE exhibit anti-apoptotic role.
Conclusion
Rosemary extract and conditioned medium without serum had apoptotic effect on in vitro cultured MCF7 cells, in the presence of serum, anti-apoptotic role could be appeared, like a double-edged sword. Understanding the effects of RE and CM on MCF7 cells, could help to design more effective treatments against cancer. Pre-exposure of MCF7 cancer cells with conditioned medium and rosemary extract could be a complementary treatment along with chemotherapy.
Acknowledgement
This study supported by Damghan University, Damghan, Iran. Authors declare no conflict of interest with any other research, studies or publications.
Authors’ Contributions
M. HGK designed the experiments, supervised, directed and managed the study; F. MM collected data and discussed the results.
Conflict of Interest
The authors declare that there are no conflicts of interest.