ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 12
The effect of Baicalein on the NF-κB/P65 expression in the peripheral blood of patients with diabetic nephropathy and in vitro
Mingzheng Yang1*, Lin Kan2, Yingchun Zhu1, Lianye Wu1, Shoujun Bai1, Fangfang Cha1 and Qing Wang1
1Department of Nephrology, Qingpu Branch of Zhongshan Hospital Affiliated to Fudan University, Shanghai, PR China
2Department of Clinical Laboratory, Qingpu Branch of Zhongshan Hospital Affiliated to Fuan University, Shanghai, PR China
- *Corresponding Author:
- Mingzheng Yang
Department of Nephrology
Qingpu Branch of Zhongshan Hospital Affiliated to Fudan University, PR China
Accepted on May 04, 2017
To investigated the effect of Baicalein on NF-κB/P65 expression in diabetic nephropathy (DN) patients and in vitro. 98 DN patients were randomly divided into two major groups, control group and treatment group. In treatment group, on the basis of basic treatment plus valsartan, and then administered orally baicalein aluminum capsule for 3 months. The serum levels of NF-κB/P65, TNF-α, urinary albumin excretion rate (UAER) and IL-8 were determined. Then the molecular mechanism of Baicalein on the expression of NF-κB/P65, TNF-α and IL-8 were studied. Human tubular epithelial HK2 cells were first exposed in high concentration of glucose for three weeks. Then the HK2 cells were treatment with various concentrations of Baicalein and NF-κB/P65 siRNA. Early apoptosis rate, gene expression and protein expression levels were determined via flow cytometry, RT-PCR and Western blotting, respectively. Gene interaction was detected by luciferase reporter gene assay. Our results indicated that Baicalein not only reduced the early apoptotic rate, but also inhibited the NF-κB/P65, TNF-α and IL-8 expression in the high glucose-damaged HK2 cells. Moreover, it up-regulated the miR-326 expression, which directly bind to the NF-κB/P65 3’UTR and played a negative regulatory role. This study provides a reference for the direction of the pharmacological mechanism of Baicalein in the treatment of DN patients.
Keywords
Baicalein, NF-κB/P65, Diabetic nephropathy, In vitro.
Introduction
It was reported that DN caused renal glomerulus and renal tubule interstitial fibrosis. The characteristic pathological changes include the glomerular sclerosis, thickening of renal tubular basement membrane and the accumulation of mesangial extracellular matrix (ECM) [1]. In the past decades, it has shown that activation of innate immune response and chronic inflammatory reactions were the factors of DN [2]. Nuclear Factor-Kappa B/P65 (NF-κB/P65) is normally inactive in the cytoplasm of most quiescent cells [3]. Diabetic hyperglycemia and advanced glycosylation end products (AGEs) trigger the inflammation response by activating NF-κB in the mesenteric cells. Then further induce the transcription of various factors, including cytokines such as tumor necrosis factor (TNF) and interleukins (i.e. IL-18, IL-2 and IL-6) [4]. It also plays an important role in the regulation of the expression of vascular endothelial growth factor (VEGF) and transforming growth factor beta-1 (TGF-1) [5].
Baicalein is the key active ingredient (5.41%) of the Chinese medicine Baical Skullcap Root, which has significant anti-inflammatory, anti-fibrosis and anti-oxidation effects [6].
Accumulating evidence showed that Baicalein inhibited the degradation of NF-κB inhibitor. Then it suppressed the formation of NF-κB-DNA complex and the expression of NF- κB dependent genes. Moreover, Baicalein improve hypopiesia and tachycardia caused by lipopolysaccharide, and has a strong anti- endotoxin effect [7]. In the present study, the serum levels of NF-κB/P65, TNF-α and IL-8 in the peripheral blood of patient with DN before and after Baicalein treatment were compared. The molecular mechanism involved was further investigated in vitro by treating the high glucose-damaged HK2 cells with Baicalein. It will provide new insights for the prevention and treatment of DN.
Materials and Methods
Patients and experimental treatment
A total of 98 DN patients were recruited to our hospital during September 2012 to September 2014 in this study, with 48 males and 50 females, aging from 18 to 75 years. The average age of these patients was 60.5 years, with the history of diabetes ranging from 8 to 24 years. Then patients were randomly divided into two major groups: 56 in the treatment group and 42 in the control group. All procedures were in accordance with the Helsinki Declaration of 1964. Qingpu Branch of Zhongshan Hospital Affiliated to Fudan University reviewed and approved the study protocol. The informed consent was obtained from each patient. All patients were diagnosed with type 2 diabetes. Urinary albumin excretion rate (UAER) in the urine sample was measured by radioimmunoassay. According to the Mogensen DN staging scale, if two measures of UAER were between 20 μg/min-200 μg/min and there was no sever heart or liver disease involved, the patient was diagnosed as early DN.
Patients were obligated to control their protein intake for 4 weeks before the experimental treatment, so that their fasting blood glucose level was under 7.0 mmol/L and postprandial blood glucose levels were under 10 mmol/L. Valsartan (Novartis, Switzerland) was added to the basic treatment for both groups. In the treatment group, patients were orally administrated with Baicalein capsule (80 mg, Xiuzheng Pharmaceutical Group, PR China) 1.2 g each time and 3 times a day for 3 months. Then the serum levels of NF-κB/P65, TNF-α and IL-8, as well as the UAER in the urine sample from the two groups were compared.
Experimental reagents
Opti-MEM medium and Lipofectamine 2000 were purchased from Invitrogen (Carlsbad, CA, USA); Trizol, hig-glucose DMEM medium and Fetal Bovine Serum (FBS) were obtained from Sijiqing (Hangzhou, PR China); MMVL reverse transcriptase, TaqDNA polymerase, dNTP and PCR primers were purchased from QIGAEN (Hilden, Germany). The NF- κB/P65, TNF-α and IL-8 monoclonal antibodies were from Beyotime Biotechnology (Shanghai, P.R. China). The target sequence for NF-κB/P65-specific siRNA: 5’- AAGAGCATCATGAA GAAGAGT-3’, and the control siRNA: 5’-CCGAACTAAGCAT GCACAGT-3’ were synthesized by Saibaisheng Biotechnology (Beijin, P.R.China) and stored at -20C° before further manipulation [8].
Cell culture
The human tubular epithelial HK2 cell line was obtained from ATCC (USA). HK2 cells were grown in RPMI1640 medium with 10% FBS in a 37°C incubator with a humidified, 5% CO2 atmosphere. When the cells reached 80% confluency, divided into 5 groups: (1) Control group, with glucose concentration of 5 mmol/L; (2) High-glucose group, with glucose concentration of 30 mmol/L; (3) Baicalein+High-glucose group, with glucose concentration of 30 mmol/L and Baicalein concentration of 100 ng/mL; (4) NF-κB/P65 siRNA + High-glucose group, with glucose concentration of 30 mmol/L and cells transfected with 20 nmol/L NF-κB/P65 siRNA); (5) NC + High-glucose group, with glucose concentration of 30 mmol/L and cells transfected with 20 nmol/L control siRNA.
ELISA
The serum of the peripheral blood samples from two groups were collected, and the ELISA assays were performed according to the manufacturer’s instruction. OD values were measured at the wavelength of 450 nm, and the protein concentration of NF-κB/P65, TNF-α and IL-8 were calculated according to the standard curve.
Construction of a hsa-miR-326-expressing vector
The sequence for hsa-miR-326 (5′- gggggcagggccuuugugaaggc-3′) was obtained from the miRNA registry (MIMAT0017027). To avoid early termination, TTGGCCACTGACT was selected to be part of miR expression vector template. TGCT was added at the 5′ end of the positive-sense strand of the miR expression vector, and GTCC was added to the 5′ end of its antisense strand. Additionally, a nonspecific sequence was designed and sent to Shanghai Gene Pharma. The eukaryotic expression vector plasmid targeting hsa-miR-326 was designated pmiR-326 [9].
Flow cytometry assay
HK2 cells were seeded at 1.0 × 106 cells/mL in 12-well plates (Costar) and transfected in 500 μL media/well. After transfection, the cell suspensions were centrifuged at 2000 rpm for 5 min, and the supernatant was discarded. The cells were washed twice in PBS and suspended in 5 μL Annexin V-FITC, gently mixed, and incubated for 15 min at 2-8°C in the dark. 10 μL PI was added to the cells, and incubated for 5 min at 2-8°C in the dark. Flow cytometry was then conducted.
Quantitative real-time RT-PCR
RNA samples were extracted using Trizol (Invitrogen, Camarillo, CA, USA). Quantitative real-time RT-PCR was performed using an Applied Biosystems 7900 Real-Time PCR System (Foster City, CA, USA). U6 expression was used as an internal control for miRNA, while GAPDH expression was used as an internal control for mRNA. The primers used in quantitative real-time PCR analysis were as follows: GAPDH F: 5′-AGTGCCAGCCTCGTCTCATA-3′, R: 5′- TTGAACTTGCCGTGGGTAGA-3′; NF-ΚBp65 F: 5′- GGAAAGGAACTCTGTCAGAT-3′; R: 5′- TAGGCTGAGGGTACTCAATCA-3′; miR-326 RT: CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGC TGGAGGA F: ACACTCCAGCTGGGCCTCTGGGCCCTTC; R: 5′- CAGTGCTGGGTCCGAGTGA-3′; TNF-α F: 5′- GGATCTCAAAGACAACCAAC-3′; R: 5′- ACAGAGCAATGACTCCAAAG-3′; IL-8 F: 5′- ATGACTTCCAAGCTGGCCGTG; R: 5′- TTATGAATTCTCAGCCCTCTTCAAAAACTTCTC-3’; U6 F: 5′-CTCGCTTCGGCAGCACA3′; R: 5′- AACGCTTCACGAATTTGCGT-3′.
Western blot analysis
The cells from each group were collected, washed 3 times in PBS, treated with 100 μL of protein lysate for 30 min at 4°C, and centrifuged at 12000 rpm for 15 min. The supernatant was collected and examined using a protein quantification kit (BCA protein quantification). Then the supernatant was subjected to 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) electrophoresis separation and the proteins were electrotransferred to nitrocellulose membranes. The membranes were treated with 50 g/L skim milk for 1 h and incubated with primary antibodies against NF- ΚBp65, TNF-α, and IL-8 (rabbit polyclonal antibodies; Cell Biotech, Tianjin, China) at 4°C overnight. The membranes were incubated with secondary antibodies conjugated to horseradish peroxidase (1:1000; Pharmingen, Becton Dickinson, San Diego, CA, USA) at room temperature for 1 h after washed 3 times with TBST.
Luciferase reporter assay
Plasmid carriers of wild-type or mutant NF-κB/P65-3′UTR luciferase reporter were designed and synthesized by Shanghai Gene Chem Co., Ltd. HK2 cells were seeded in 96 well plates for 24 h to reach 80% confluency. Then cells were transfected with mimic miR-326(5′- GGGGGCAGGGCCUUUGUGAAGGC-3′). miRNA inhibitor (5′-UcACCGAUgggUUaaaACUAACCUA-3′), negative control miRNA (NC: 5′- CCUCCCUAGAAaaCUGccAGGUU-3′), a negative control inhibitor (NCI: 5′-AACCAAAAGGGUUCUAGGGAGG-3′), mimic, miRNA inhibitor, control miRNA, a miRNA inhibitor, or empty plasmid. Luciferase activity was measured after 24 h transfection.
Statistical analysis
All measurement data are expressed as mean ± SD. Data were first processed using the variance homogeneity test with the SPSS 20.0 software package. Statistical analysis was conducted using one-way analysis of variance (ANOVA). P<0.05 was considered to be statistically significant.
Results
Baicalein suppressed the expression of NF-κB/P65, TNF-α and IL-8 in the peripheral blood and reduced the UAER of DN patient
To investigate effect of Baicalein on DN patients, we first detected the serum levels of NF-κB/P65, TNF-α and IL-8, as well as UAER prior to any treatment. Valsartan was then added to their basic treatment. For the treatment group, patients were also orally administrated with Baicalein capsule. The result indicated that the level of NF-κB/P65, TNF-α, IL-8 and UAER were significantly decreased in treatment group compared to control group after 3 months (P<0.05) (Table 1).
| Group | NF-KB/P65 (ng/L) | TNF-α (ng/L) | IL-8 (ng/L) | UAER (ug/min) | |
|---|---|---|---|---|---|
| Control group | Before treatment | 30.96 ± 3.55 | 28.96 ± 5.64 | 23.18 ± 2.13 | 125 ± 18 |
| After treatment | 11.34 ± 2.53 | 15.69 ± 3.28 | 13.11 ± 1.98 | 54 ± 28 | |
| Treatment group | Before treatment | 29.77 ± 3.69 | 25.47 ± 4.93 | 21.39 ± 3.69 | 136 ± 30 |
| After treatment | 7.94 ± 1.03* | 8.90 ± 1.77* | 6.35 ± 0.86* | 32 ± 21* | |
| *P<0.05, compared to the control group. | |||||
Table 1. Comparison of NF-kB/P65, TNF-α, IL-8 serum levels and UAER (mean ± SD).
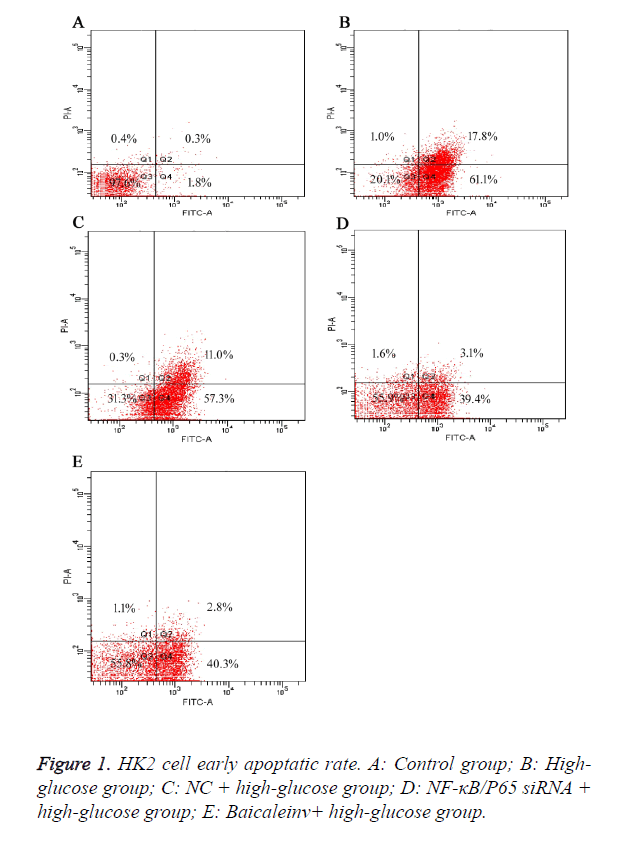
Baicalein decreased the early apoptotic rate of high-glucose damaged HK2 cells
To further elucidate the effect of Baicalein on the early apoptosis of high-glucose damaged human tubular epithelial HK2 cells. The result showed that the apoptotic rates of cells in group B (high-glucose group) were higher than group A (control group) after 48 h. It suggested that high concentration of glucose induced the early apoptosis of HK2 cells. Compared to group B (high-glucose group) and C (NC+high-glucose group), the apoptotic rates of cells in group D (NF-κB/P65 siRNA+high-glucose group) and E (Baicaleinv+high-glucose group) were decreased, but still higher than group A (control group). It suggested Baicalein could suppress the early apoptosis of HK2 cells induced by high concentration of glucose. Moreover, the early apoptotic rates of HK2 cells with NF-κB/P65 knocked-down were obviously decreased, implying that NF-κB/P65 might play a key role in the early apoptosis of HK2 cells (Figure 1).
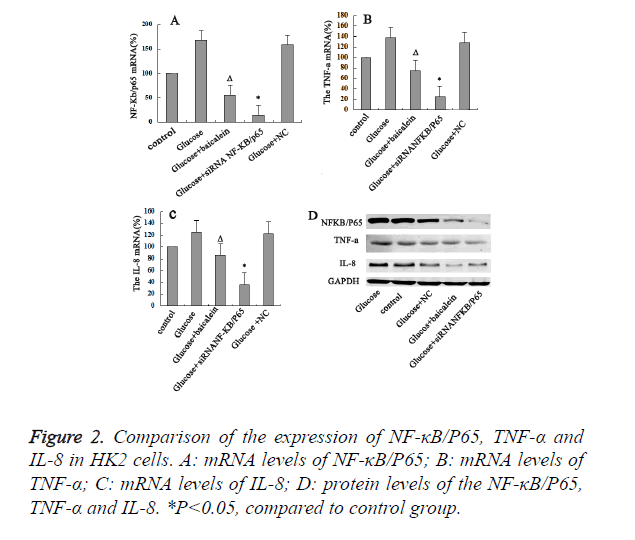
Baicalein inhibited the expression of NF-κB/P65 in HK2 cells
Our results indicated that levels of mRNA, protein levels of NF-κB/P65, TNF-α and IL-8 for the high-glucose group were significant higher than control group. It indicated that NF-κB/ P65, TNF-α and IL-8 might be the important regulators in the development of DN. In addition, the levels of mRNA, protein levels of NF-κB/P65, TNF-α and IL-8 for the Baicalein-treated group were obviously lower than those of the control and high-glucose group (P<0.05). Moreover, when NF-κB/P65 was further knocked-down with siRNA in the high-glucose damaged HK2 cells, the expression levels of both TNF-α and IL-8 were also decreased. It suggested that NF-κB/P65 expression could affect the expression of both TNF-α and IL-8. Hence, high concentration of glucose could activate NF- κB/P65 in the HK2 cells, which would further activate TNF-α and IL-8. Then Baicalein inhibit the expression of NF-κB/P65 in HK2 cells (Figure 2).
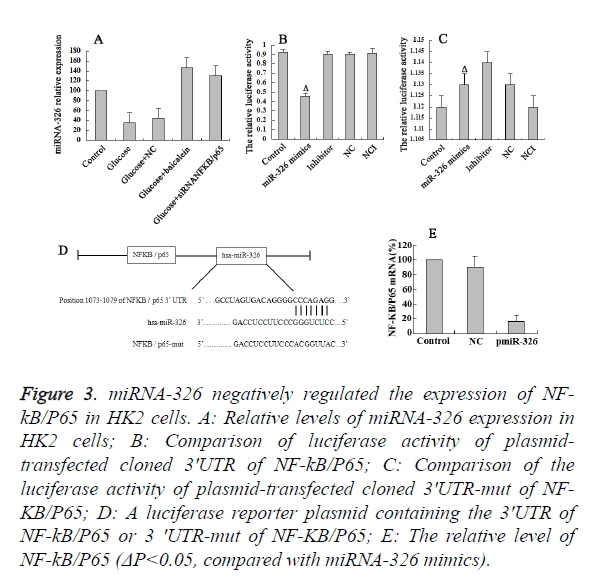
Baicalein up-regulated the miR-326 expression
The results of RT-PCR revealed the high expression of miR-326 in the Bacailin and NF-κB/P65 siRNA group, and low expression of miR-326 in high-glucose and NC group (Figure 3A). It suggested that down-regulation of NF-κB/P65 trigger the up-regulation of miR-326 expression. To determine whether or not the observed effects depend on the regulation of the 3’ untranslated region (3’-UTR) of NF-κB/P65, a luciferase reporter plasmid containing the 3’-UTR of NF-κB/P65 was constructed (Figure 3D). Co-transfection of Hk2 cells with miR-326 mimics was performed to detect the binding site on the NF-κB/P65 3' UTR (1073-1079bp). The co-transfection of HK2 cells with miR-326 mimics and NF-KB/P65 3' UTR significantly inhibited the luciferase activity (P<0.05) (Figure 3B). However, co-transfection of HK2cells with miR-326 mimics and psiCHECK-2-mut- NF-κB/P65 3' UTR had little effect on the activity of luciferase (P>0.05) (Figure 3C). The above results demonstrated that miR-326 has a regulatory effect by binding to 3’ UTR of NF-κB/P65. Moreover, it was shown that the expression of miR-326 in the glucose-damaged HK2 cells was relatively low. The RT-PCR results indicated that the levels of NF-κB/P65 were significantly down-regulated in the high-glucose damaged HK2 cells on miR-326 over expression. Hence, Baicalein up-regulate the miR-326 expression, which could directly bind to the NF-κB/P65 3’UTR and played a negative regulatory role.
Figure 3. miRNA-326 negatively regulated the expression of NF-kB/ P65 in HK2 cells. A: Relative levels of miRNA-326 expression in HK2 cells; B: Comparison of luciferase activity of plasmidtransfected cloned 3'UTR of NF-kB/P65; C: Comparison of the luciferase activity of plasmid-transfected cloned 3'UTR-mut of NF-KB/ P65; D: A luciferase reporter plasmid containing the 3'UTR of NF-kB/P65 or 3 'UTR-mut of NF-KB/P65; E: The relative level of NF-kB/P65 (ΔP<0.05, compared with miRNA-326 mimics).
Discussion
The incidence of Diabetic Nephropathy (DN) is approximately 20%-40% among diabetic patients, and it has become the main cause of End-Stage Renal Disease (ESRD) [10]. For most DN patients, the disease progressed rapidly even though their diet and blood pressure were strictly controlled [11]. Therefore, it is of great significance to explore its pathogenesis and develop new treatment strategy. Nuclear Factor-Kappa B/P65 (NF-κB/ P65) is the key regulator for the transcription and expression of inflammation-related genes [12]. Activation of NF-κB in the mesenteric cells induce the transcription of various factors, including cytokines such as TNF-α and interleukins (i.e. IL-18, IL-2 and IL-6), which are important factors in the inflammation response [3]. In the present study, upon treatment with Valsartan and Baicalein for 3 months, the levels of NF- κB/P65, TNF-α, IL-8, and the UAER of the DN patients were significantly decreased. Numerous studies have shown that Baicalein suppressed the degradation of NF-κB/P65 inhibitors. Then it inhibited the formation of NF-κB-DNA complex, the expression of NF-κB dependent genes, as well as the elevation of serum TNF-α level. Therefore, it has a strong anti-endotoxin effect [7]. In view of regulation of NF-κB/P65 in TNF-α and IL-8 expression, it might be the new therapeutic target in the future treatment with Baicalein for DN.
In order to investigate the molecular mechanism, we first induced the HK2 cells with high concentration of glucose for a week and then treated the cells with certain concentration of Baicalein and/or transfected with NF-κB/P65 siRNA for 48 hours. The results showed that NF-κB/P65 played an important role in the regulation of the early apoptosis of HK2 cells, and Baicalein lower the early apoptosis rate of HK2 cells induced by high concentration of glucose. High concentration of glucose could activate NF-κB/P65 in the HK2 cells, which further activated the expression of TNF-α and IL-8. These results consistent with Yang et al. [13]. Moreover, downregulation of NF-κB/P65 expression suppress the expression of TNF-α and IL-8, and Baicalein suppress the expression of NF- κB/P65 in the HK2 cells. It has shown that Baicalein inhibited the expression of type IV collagen in the proximal tubule cells (Coll IV), Fibronectin (FN) and TGF-β1 induced by high concentration of glucose [14]. In vivo study, suppression of the Coll IV, FN and TGF-β1 expression could reduce the ECM accumulation, which protected the kidney from damage [15]. For the first time, we have demonstrated that NF-κB/P65 was the molecular target for Baicalein. As Baicalein has marked anti-inflammatory, anti-fibrosis and anti-oxidation effects, it has the potential therapeutic value in the future treatment of DN [16].
miRNA is a non-coding RNA with the length of 21-24 nucleotides, which widely exists in plants and animals. By binding to the nucleotides at the 3’ non-coding region of miRNA, the mRNA translation of the target genes is suppressed, thereby regulates the target gene expression at the transcription level [17]. miRNA targeting in the DN regulatory role may become a new DN markers and therapeutic targets in recent studies [18]. In the present study, miR-326 has a negative regulatory effect by binding to 3' UTR of NF-κB/P65. High miR-326 expression prolonged survival likely via the decreasing invasive potential of PDAC cell. At the same time, miR-326 played a key role in regulating TGF-1 expression and other profibrotic genes [19,20]. Therefore, miR-326 was essential in the progression of both lung fibrosis and DN. Moreover, we demonstrated that Baicalein up-regulated the miR-326 expression in high-glucose damaged HK2 cells.
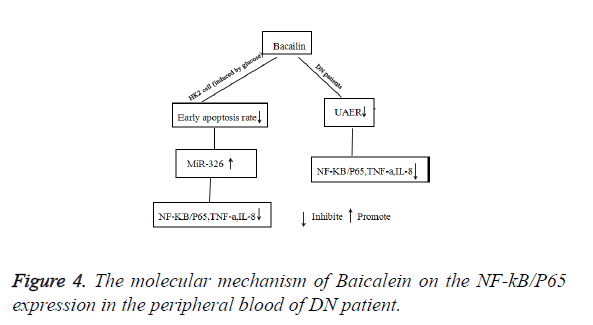
In conclusion, our study demonstrated that Baicalein significantly reduced the levels of NF-κB/P65, TNF-α, IL-8 and UAER of DN patients. The possible molecular mechanism was that Baicalein not only could reduce the early apoptosis of HK2 cells, but also promote the expression of miR-326. Then suppressed the expression of NF-κB/P65 and decreased the levels of TNF-α, and IL-8 (Figure 4).
Acknowledgement
This work was supported by the Shanghai Health and Family Planning Commission research funding (NO: 201440304).
References
- Edna L, Ronny G, Marianna R. Short and long term neuro-behavioral alterations in type 1 diabetes mellitus pediatric population. World J Diabetes 2015; 6: 259-270.
- Miralem M, Amela D, Esad P, Orhan L, Almir F, Belma AB, Enes T. Metabolic syndrome and serum liver enzymes level at patients with type 2 diabetes mellitus. Med Arch 2015; 69: 251-255.
- Mara C, Wei KL, Adina G, Subhadra V. Nandula MB, Shen Q. Mutations of multiple genes cause deregulation of NF-kB in diffuse large B-cell lymphoma. Nature 2009; 459: 717-721.
- Simone VD, Franzè E, Ronchetti G, Colantoni A, Fantini MC, Fusco DD, Sica GS, Sileri P, Macdonald TT, Pallone F, Monteleone G, Stolfi C. Th17-type cytokines, IL-6 and TNF-a synergistically activate STAT3 and NF-kB to promote colorectal cancer cell growth. Oncogene 2015; 34: 3493-3503.
- Alessandra F, Rita C, Anna C, Maria FP, Chiara O, Elisa C, Michaël S, Anna TP, Mauro M. Molecules altering the intracellular thiol content modulate NF-kB and STAT-1/IRF-1 signalling pathways and IL-12 p40 and IL-27 p28 production in murine macrophages. PLoS One 2013; 8: e57866.
- Zhang K, Lu J, Mori T, Smith-Powell L, Synold TW, Chen S, Wen W. Baicalin increases VEGF expression and angiogenesis by activating the ERR{alpha}/PGC-1{alpha} pathway. Cardiovasc Res 2011; 89: 426-435.
- Luo W, Wang CY, Jin LJ. Baicalin downregulates porphyromonas gingivalis lipopolysaccharide-upregulated IL-6 and IL-8 expression in human oral keratinocytes by negative regulation of TLR signaling. PLoS One 2012; 7: e51008.
- Seino Y, Nanjo K, Tajima N, Kadowaki T, Kashiwagi A, Araki E, Ito C, Inagaki N, Iwamoto Y, Kasuga M, Toshiaki H, Haneda M, Kohjiro U. Report of the committee on the classification and diagnostic criteria of diabetes mellitus, The committee of the Japan diabetes society on the diagnostic criteria of diabetes mellitus. Diabetes Investig 2010; 1: 212-228.
- He JH, Li YM, Li YG, Xie XY, Wang L, Chun SY, Cheng WJ. hsa-miR-203 enhances the sensitivity of leukemia cells to arsenic trioxide. Exp Ther Med 2013; 5: 1315-1321.
- Yamagishi S, Matsui T. Advanced glycation end products, oxidative stress and diabetic nephropathy. Oxid Med Cell Longev 2010; 3: 101-108.
- Kanwar YS, Sun L, Xie P, Liu FY, Chen S. A glimpse of various pathogenetic mechanisms of diabetic nephropathy. Annu Rev Pathol 2011; 6: 395-423.
- Kim DH, Park MH, Choi YJ, Chung KW, Park CH, Jang EJ, An HJ, Yu BP, Chung HY. Molecular study of dietary heptadecane for the anti-inflammatory modulation of NF-kB in the aged kidney. PLoS One 2013; 8: e59316.
- Yang B, Hadgkinson A, Oates PJ, Millward, BA, Demaine, AG. High glucose induction of DNA-binding activity of the transcription factor NF-KB in patients with diabetic nephropathy. Biochim Biophys Acta 2008; 1782: 295-302.
- Lianxu C, Hongti J, Changlong Y. NF-Kbp65-specific siRNAinhibits expression of genes of COX-2, NOS-2 and MMP-9 in rat IL-l B-induced and TNF-a-induced chondrocytes. Osteoarthr Cartilage 2006; 14: 367-376.
- Wu ZC, Liu SW, Dong JW. Effect and mechanism of baicalein on extracellular matrix of kidney in type 2 diabetic rats. Chinese J Integrated Traditional Western Nephrol 2009; 10: 116-119.
- Cheng PY, Lee YM, Wu YS, Chang TW, Jin JS, Yen MH. Protective effect of baicalein against endotoxic shock in rats in vivo and in vitro. Biochem Pharmacol 2007; 73: 793.
- Liu X, Chen X, Yu X, Tao Y, Bode AM, Dong Z, Cao Y. Regulation of microRNAs by epigenetics and their interplay involved in cancer. J Exp Clin Cancer Res 2013; 32: 96.
- Kato M, Natarajan R. MicroRNAs in diabetic nephropathy: functions, biomarkers, and therapeutic targets. Ann N Y Acad Sci 2015; 1353: 72-88.
- Zhang ZL, Bai ZH, Wang XB, Bai L, Miao F, Pei HH. miR-186 and 326 predict the prognosis of pancreatic ductal adenocarcinoma and affect the proliferation and migration of cancer cells. PloS one 2015; 10: e0118814.
- Sudipta D, Manish K, Vinny N, Pattnaik B, Prakash YS, Anurag Agrawal, Balaram G. MicroRNA-326 regulates profibrotic functions of transforming growth factor-in pulmonary fibrosis. Am J Resp Cell Mol 2014; 50: 882-892.