ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2018) Volume 29, Issue 19
Synthesis and characterization of AC-CdO-TiO2 nanocomposite recyclable and multi-application study
Marimuthu G1*, Prithivirajan B2 and Jebastin Sonia Jas M2,3
1Department of Chemistry, Swami Dayananda College of Arts and Science, Manjakkudi, Tiruvarur District, Tamil Nadu, India
2Research and Development Centre, Bharathiar University, Coimbatore, India
3Department of Chemistry, IFET College of Engineering, Villupuram, India
- *Corresponding Author:
- Marimuthu G
Department of Chemistry
Swami Dayananda College of Arts and Science
Tamil Nadu, India
Accepted date: August 9, 2018
DOI: 10.4066/biomedicalresearch.29-18-910
Visit for more related articles at Biomedical ResearchThe synthesis of AC-CdO-TiO2 nanocomposite material has been successfully achieved by precipitation method and sonication technique. High photocatalytic activity of AC-CdO-TiO2 nanocomposite material used Reactive Black 5 (RB 5) under UV-light irradiation and that of TiO2; the nanocomposite material was characterized by High-Resolution Scanning Electron Microscopy (HR-SEM) with Elementary Dispersive X-ray (EDX), High-Resolution Transmission Electron Microscopy (HR-TEM), XRD analysis, Photoluminescence spectroscopy (PL) and UV-Vis DRS analysis. This nanocomposite material obtained by photodegradation of RB 5 dye at various parameters is reported. The reaction of this nanocomposite material was found to be stable and reusable. The antibacterial activity of prepared material was investigated against Gram negative and positive bacterial strains and through their electrochemical activity.
Keywords
RB 5 dye, Catalyst, Antibacterial activity, Electrochemical activity
Introduction
Dye molecules are used for textile industry in manufacturing production, clothing, wastewater from printing and photodegradation materials [1-5]. Semiconductor oxide (CdO, TiO2, ZnO and SnO2), is an exceptional photocatalyst, medicine and sensing element for optoelectronic applications [6-10].
Titania semiconductors are used in photocatalyst, paint industries, paper, and plastics industries due to the presence of excellent semiconductor oxide [11]. Two or more semiconductors with appropriate band energy levels is an improved way to increase the photocatalytic activity of the semiconductor because of the useful separation of photogenerated electron-hole pairs and interfacial charge transfer efficiency [12-14]. Such semiconductor oxides have been considered to hold an effective chemical, physical and photocatalytic properties [15,16]. Recently complex semiconductor oxides have been reported to show similar properties [17-22]. Activated carbon is a brilliant adsorbent of waste water pollutants, due to the following reasons (i) activated carbon adsorb the dye and release it on surface of the photocatalyst (ii) degradation by oxidation [23-25]. Activated carbon-loaded semiconductor oxide undergoes high photodegradation [26-28].
The nanocomposite material was characterized by highresolution scanning electron microscopy (HR-SEM) with elementary dispersive X-ray (EDX), high-resolution transmission electron microscopy (HR-TEM), XRD analysis; photoluminescence spectroscopy (PL) and UV-Vis DRS. This nanocomposite material can photodegrade RB 5 dye at various parameters under the UV-light irradiation at 365 nm.
The mineralization of dyes is confirmed by chemical oxygen demand measurements. An achievable mechanism is proposed for higher activity of AC-CdO-TiO2 that of TiO2 nanocomposite material. Reaction of this nanocomposite material was found to be stable and reusable. AC-CdO-TiO2 nanocomposite material showed highest antibacterial activity and electrochemical activity compared to that of TiO2 nanocomposite material.
Experimental Section
Materials
Cadmium acetate dehydrates, tetra isopropyl orthotitanate (C12H28O4Ti), reactive black 5 (RB 5) dye and activated carbon (AC), citric acid, NH4OH were used as received. Coumarin (1 mM of 4-hydroxycoumarin) is a chemical of Sigma Aldrich and used as such. The ethanol and aqueous solution was obtained using double distilled water. All glasswares were cleaned with acid followed by thorough washing with distilled water.
Preparation of AC-CdO-TiO2 nanocomposite material by precipitation method
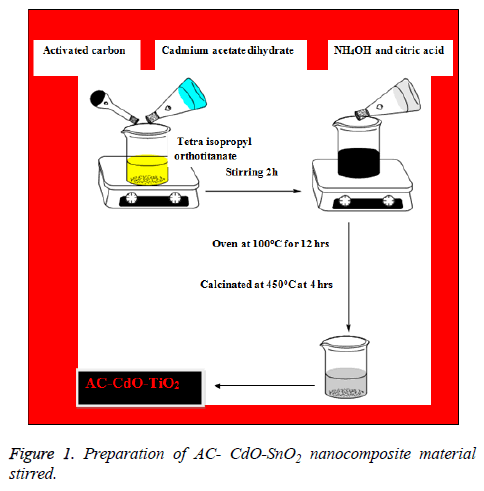
AC-CdO-SnO2 nanocomposite material was synthesized by precipitation method. Initially cadmium acetate dihydrate (0.4 M) was dissolved in anhydrous ethanol solution in beaker A. 0.4 m citric acid and tetra isopropyl orthotitanate in ethanol is taken as another solution in beaker B and activated carbon (AC) was dissolved in anhydrous ethanol solution in beaker C, the solution in A and solution in C are added to solution in beaker B and stirred well. To these 2 drops of NH4OH is added at room temperature under vigorous stirring until the precipitate was formed. The obtained precipitate was washed with water and ethanol. Then the precipitate was collected and dried in oven at 100°C for 12 h. The resulting powder was finally calcinated at 500°C at 4 h (Figure 1).
Characterization
Ultraviolet and visible (UV-vis) absorbance spectra were measured over a range of 800-200 nm with a Shimadzu UV-1650PC recording spectrometer using a quartz cell with 10 mm optical path length. High resolution scanning electron microscopy (HR-SEM) as well as elementary dispersive X-ray (EDX) evaluation experiments was performed on a FEI Quanta FEG 200 instrument with EDX analyzer facility at 25°C. The nanoparticles’ size and structure verifications were done by transmission electron microscopy (HR-TEM) making use of PHILIPS CM200. Each spectrum was recorded with an acquisition time of 18 s. XRD spectrum was recorded on the X'PERT PRO model X-ray diffractometer from pan analytical instruments operated at a voltage of 40 kV and also a current of 30 mA with Cu Kα radiation. Photoluminescence (PL) spectra at a room temperature were recorded using a Perkin-Elmer LS 55 fluorescence spectrometer. UV spectral measurements were done using a Hitachi-U-2001 spectrometer. Ultraviolet and visible (UV-vis) absorbance spectra were measured over a range of 800-200 nm with a Shimadzu UV-1650PC recording spectrometer using a quartz cell with 10 mm of optical path length. The antibacterial activity was studied by disc diffusion method; the test compound was dissolved in DMSO (200 mg/ml) for about half an hour. Commercially available drug disc, ciprofloxacin (10 mg/disc) was used as positive reference standard and cyclic voltammetry (CV) measurements were carried out using CHI 60 AC electrochemical analyzer (CHI Instruments Inc. USA). The Photovoltaic properties of the material was characterized by recording the photo current voltage (I-V) curve under illumination of A.M.1.5 (100 MW/cm2).
Photocatalysis
The photocatalytic activities of the photocatalysts AC-CdO-SnO2 nanocomposite material were evaluated by the photodegradation of dye. The light source was UV lamp at 365 nm. The reaction was carried out at ambient temperature (303 K). Aqueous suspensions of dye (40 ml, 1 × 10-4 M) and 0.080 g of photocatalyst were loaded in reaction tube of 50 ml capacity with a prior 40 min in the irradiation, the suspension was magnetically stirred in dark to ensure the establishment of an adsorption or desorption equilibrium. The suspension was kept under constant air-equilibrated condition. At the intervals of given irradiation time. The suspension was measured spectrophotometrically at max=595 nm (RB 5) within the theory to Beer-Lambert law limit.
Results and Discussion
HR-SEM with EDX analysis
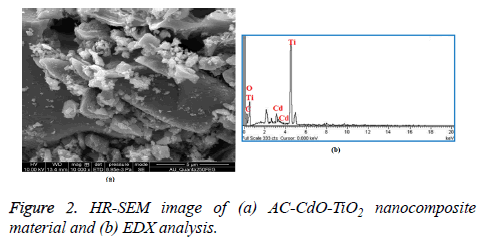
The HR-SEM image of AC-CdO-TiO2 nanocomposite material is shown in Figure 2a. The particles are in the form of aggregates, uniformly distributed in various sizes and have a morphological structure of nanoflakes. The AC-CdO-TiO2 nanocomposite material maintained at a high calcination temperature for 4 h and presented in RB 5 dye was shown to have highly photocatalytic activity. Figure 2b shows EDX analysis which confirms that only Ti, Cd, C and O are present in AC-CdO-TiO2 nanocomposite material.
HR-TEM analysis
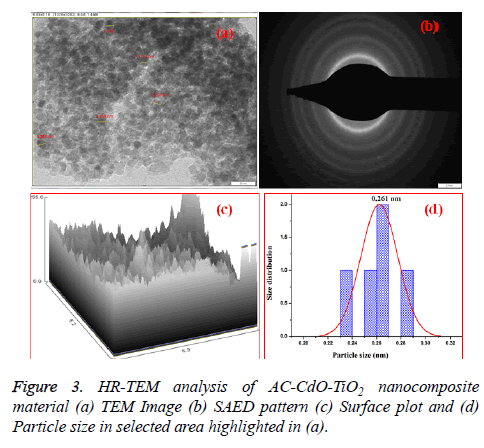
The high-resolution transmission electron microscope (HRTEM) measurements of AC-CdO-TiO2 nanocomposite material are shown in Figure 3a. It is established that the presence of particles are depicted from the HR-TEM micrographs of the mixed nanoparticles at 20 nm as nanospherical chain shaped structure. In Figure 3b, the selected area of electron diffraction (SAED) pattern shows bright diffraction rings corresponding to polycrystalline nature.
This revealed that the high resolution TEM images (HR-TEM) and selected area diffraction pattern (SADP) of AC-CdO-TiO2 nanocomposite material. It was seen that the crystal grain size, crystalline volume, and crystallinity reduced as the doping ratio increased in accordance with the XRD results. The tiny spherical particles with a high surface area make superior contact area with microbe. Figure 3c represented an image profile and Figure 3d shows the particle size distribution by selected particle area (0.261 nm) as highlighted in Figure 3a.
XRD analysis
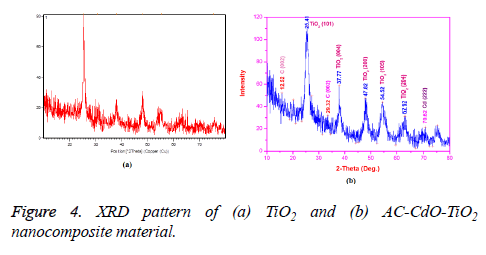
The XRD spectra of TiO2 and AC-Cd-TiO2 nanocomposite material were shown in Figures 4a and 4b respectively. According to the crystal structure spectra, the well-defined peaks typical of TiO2 were clearly noticed. This is in compliance with the reports of the norms of the Joint Committee on Powder Diffraction Standard JCPDS card no. 21-1272. Samples gave six distinctive TiO2 peaks at 25, 29.91, 38.0, 47.87, 56.92, 64.5 and 74.74 as shown in Figure 4a with the diffractions of the TiO2 (1 0 1), (0 0 4), (2 0 0), (1 0 5) and (2 0 4) crystal planes (anatase TiO2).
The XRD spectrum of AC-CdO-TiO2 nanocomposite material is shown in Figure 4. In the Figure 4b the distinctive peaks at 25, 29.32, 34.5, 47.87, 56.94 64 and 70.62, 78 were represented respectively. X-ray diffraction pattern was calculated using the Debye-Scherrer formula, L=0.89 λ/β Cos θ where L is the crystalline size (nm), λ is the wavelength (nm), β is the full width at half maximum intensity (FWHM-in radian), and θ is the Bragg diffraction angle (º). The average crystalline sizes of the AC-CdO-TiO2 nanocomposite material products were calculated to be about 21 nm.
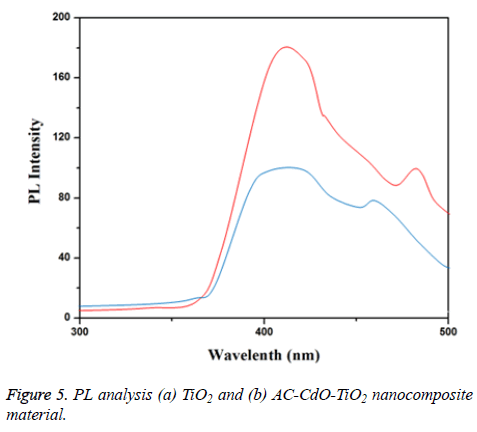
PL analysis
PL spectra of prepared TiO2 and AC-CdO-TiO2 nanocomposite material were shown in Figures 5a and 5b respectively. As the photoluminescence occurs due to electron-hole recombination, its intensity is directly proportional to the rate of electron-hole recombination and is directly proportional to the rate of electron-hole recombination. The prepared TiO2 gave two emissions at 425 and 480 nm. The prepared AC-CdO with TiO2 does not shift the emission of TiO2 but the intensity of PL emission is less when compared to TiO2 nanocomposite material. The improved near band edge emission (NBE) of pure TiO2 shows better crystallinity, whereas the slight decrease of NBE in the AC-CdO with TiO2 indicates the increase of intrinsic defect and is the reason for the decrease of near band edge emission. The decrease of near band edge emission present increases photocatalytic activity, this is because of suppression of recombination of electron-hole pairs by which better photocatalytic activity.
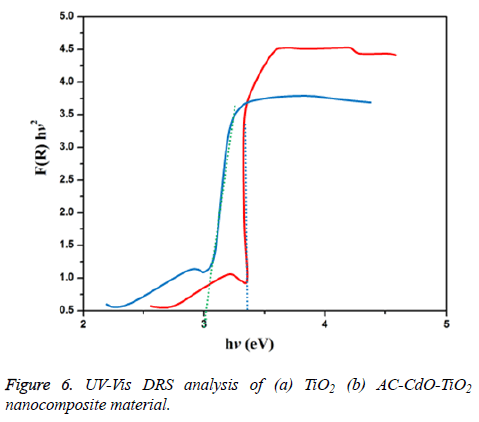
UV-vis DRS spectrum
UV-Vis-DRS (diffuse reflectance spectra) analysis of TiO2 and AC-CdO-TiO2 nanocomposite material was displayed in Figure 6. The bio-synthesis AC-CdO-TiO2 nanocomposite material shows increased absorption in both UV and visible region than that of TiO2 nanocomposite material and can be used as a UV and visible light active semiconductor photocatalytic nanocomposite material. The UV-Vis spectra were transformed to the Kubelka-Munk function F(R) to separate the extent of light absorption from scattering. The band gap energy is obtained from the plot of the modified Kubelka-Munk function (F (R) E)1/2 versus the energy of the absorbed light E by the Equation 1.
(1-R)1/2
(F(R) E)1/2=(X h v) → (1)
2R
The final result indicates band gab energy of 3.4 eV and 3.0 eV corresponding to the chemical synthesized TiO2 and AC-CdO-TiO2 nanocomposite material. The lower band gab energy supports for superior photocatalytic activity [29].
Photodegradation of RB 5 dye
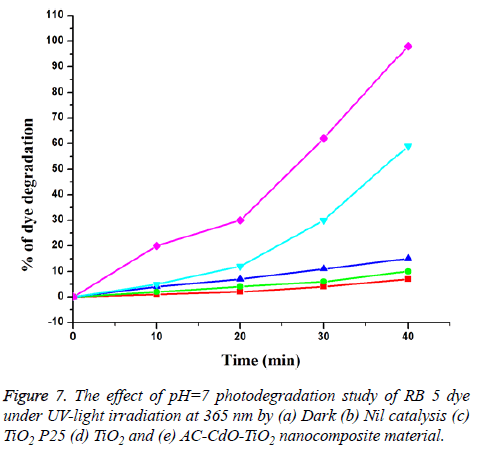
Primary analysis (Photodegradation of RB 5 dye with artificial UV-light): The pH analysis in photodegradation of RB 5 dye compared to fast dye was investigated in the pH range 3, 7, 9 and 11 and the results were reported. It was observed that the degradation increases with an increase in pH up to 7 and then decreases.
The photodegradation of RB 5 dye in aqueous medium in the presence of catalyst and the atmospheric air were studied using multi lamp photoreactor with mercury UV lamps at wavelength 365 nm. The initial dye concentration was 1 × 10-4 M and the pH of dye is neutral (pH=7). It was shown to be in dark colour. After photodegradation, the colour changes at irradiation time 40 min as shown in Figure 7. The reaction time affords the photodegratation of RB 5 dye. Thus AC-CdO-TiO2 exhibited very superior photocatalytic activity when compared to that of TiO2, dark and nil catalyst (Figure 7). The RB 5 dye was resistant to photolysis with AC-CdO-TiO2 nanocomposite material in the dark with a dye concentration of 1 × 10-4 M. RB 5 dye undergoes % degradation of 0, 20, 30, 62 and 98 and TiO2 undergoes % degradation of 0, 5, 12, 30 and 59. The result indicates that AC-CdO-TiO2 nanocomposite material has greater photocatalytic activity.
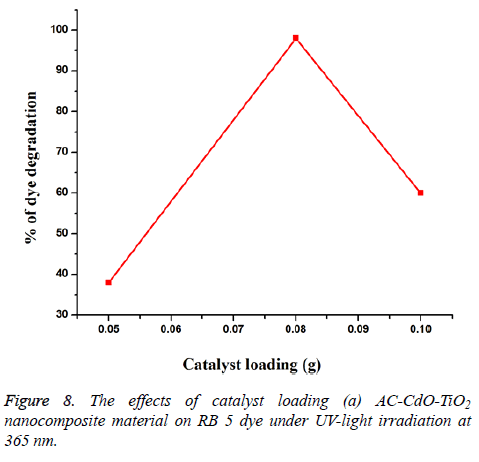
Effect of catalyst loading: Experiments performed with different amounts of AC-CdO-TiO2 nanocomposite material showed that the photodegradation efficiency increased with an increase in amount up to 0.08 g/50 ml and then then slightly decreased as observed in catalyst loadings of 0.05, 0.08 and 0.1 g/50 ml. AC-CdO-TiO2 on RB 5 dye undergoes % of degradation 38, 98 and 60% (Figure 8). Among the catalyst loadings 0.08 g showed optimal photocatalytic activity.
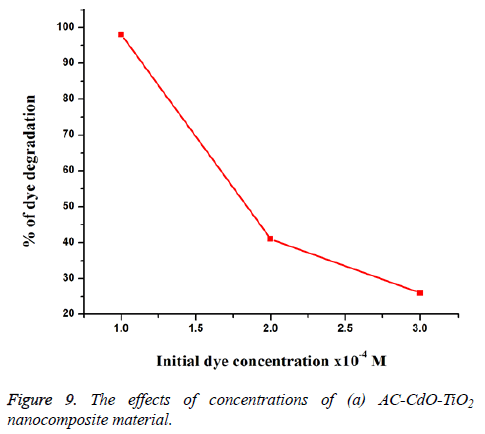
Effect of different concentrations of RB 5 dye: The effect of dye concentration for the photodegradation of RB 5 dye on AC-CdO-TiO2 nanocomposite material at 1 × 10-4 M concentration showed high photocatalytic activity than at 2 × 10-4 M and 3 × 10-4 M concentration (Figure 9). The result shows, high concentration of dye molecule decreases degradation range and catalyst surface area. So high concentration showed slow photocatalytic activity whereas low concentration of dye molecules showed fast activity of dye.
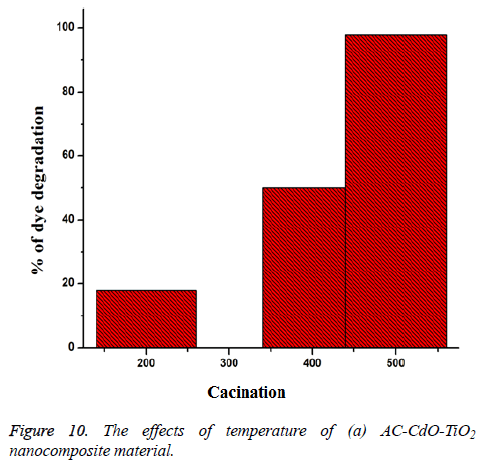
Effect of different temperature: The photodegradation of AC-CdO-TiO2 nanocomposite material uses different calcination temperatures (200°C (18%), 400°C (50%) and 5000°C (98%)) under UV-light absorption at time 60 min. High calcination temperature showed optimum photocatalytic activity than low calcination temperature. High activity was shown corresponding to its polycrystalline nature (Figure 10).
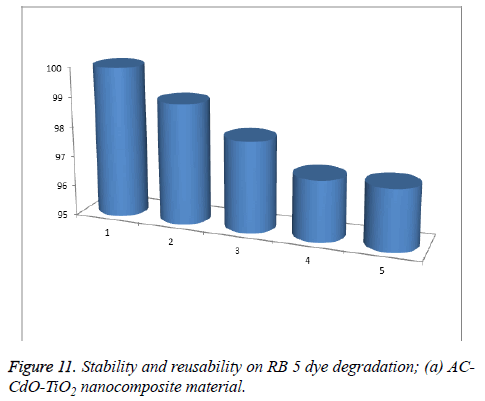
Stability of the reusable photo catalyst: The reusability of AC-CdO-TiO2 nanocomposite material was investigated by the photocatalytic degradation properties of the photocatalyst by repeating RB 5 dye photocatalytic degradation experiments five times. After each cycle, the photocatalysts were washed thoroughly with water, and a fresh solution of RB 5 dye was made before each photocatalytic run in the photoreactor under UV-light and the results are shown in Figure 11.
The complete degradation of the AC-CdO-TiO2 nanocomposite material occurred in 1st-5th cycles with 97% degradation. After the fifth cycle, the efficiency of catalysts decreased in comparison to the total degradation of RB 5 dye. There is no significant change in reaction, indicating the stability of photocatalyst. This is due to the loss of catalysts (0.3%), during the water washing of catalysts, which was not observed in the naked eye in this nanocomposite material (Figure 12).
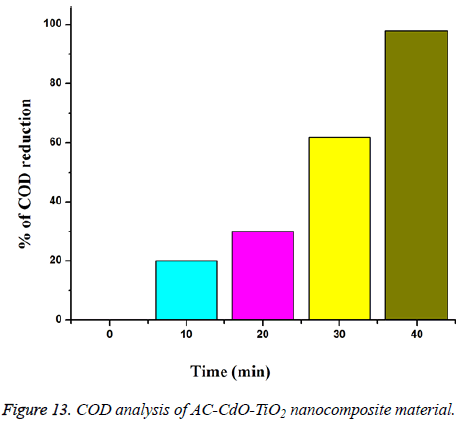
Chemical oxygen demand analysis (COD): Chemical oxygen demand analysis of RB 5 dye on mineralization of AC-CdO-TiO2 nanocomposite material photocatalyst, loading amount of 0.080 g on dye concentration (1 × 10-4 M) suspension in 40 ml of pH=7 solution and air passing with UV-light at 365 nm (Figure 13).
AC-CdO-TiO2 nanocomposite % of chemical oxygen demand reduction of dye at 10 min (20%), 20 min (29%), 30 min (61%) and 40 min (98%) is obtained [29].
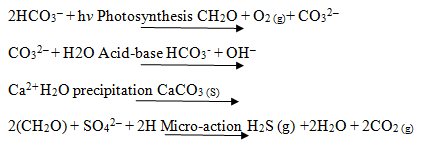
The mineralization is also specific by formation of calcium carbonate when the evolved gas (carbondioxide) through photodegradation is accepted and calcium hydroxide is obtained. This result indicates almost complete photodegradation of dye and this reaction is as follows:
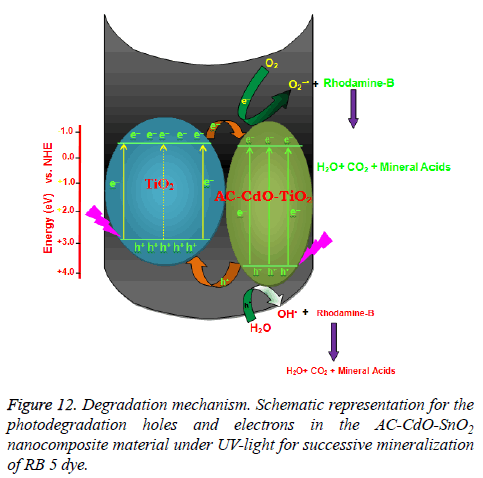
Mechanism for photocatalytic effect of AC-CdO-TiO2: On the basis of these observations, a tentative mechanism for photocatalytic degradation of RB 5 dye is proposed as follows:
1(RB 5)0+hv → (RB 5)1 → (1)
1(RB 5)1+ISC → 3(RB 5)1 → (2)
AC-CdO-TiO2 (SC)+hv → e- (CB)+h+(VB) → (3)
-OH+h+ → •OH → (4)
•OH + 3(RB 5)1 → Leuco (RB 5) → (5)
Leuco (RB 5) → Products → (6)
(RB 5) dye absorbs radiation of desired wavelength and it forms excited singlet state. Further, it undergoes intersystem crossing (ISC) to give its more stable triplet state. Along with this, the AC-CdO-TiO2 nanocomposite material (SC) also utilizes this energy to excite its electron from valence band to the conduction band. An electron can be abstracted from hydroxyl ion by hole (h+) present in the valence band of semiconductor generating •OH radical. This hydroxyl radical will oxidize methyl green to its leuco form, which may be ultimately degraded to products. It was confirmed that the •OH radical participates as an active oxidizing species in the degradation of RB 5 dye as the rate of degradation was appreciably reduced in presence of hydroxyl radical scavenger (2-propanol) (Figure 12) [30].
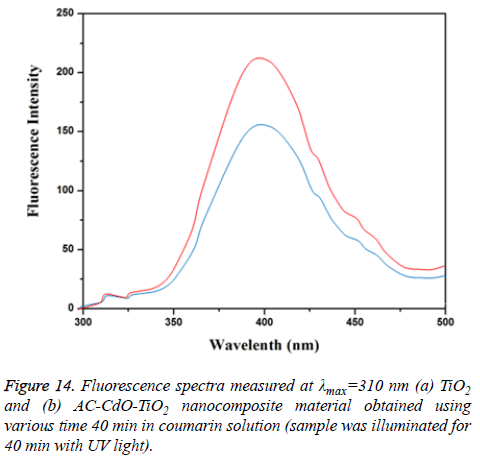
•OH analysis: The detection of •OH by the changes in the fluorescence spectra of a coumarin solution under visible-light irradiation as a function of irradiation time 40 min. Fluorescence intensity arises due to hydroxyl radicals as the active species. Further the formed hydroxyl radicals on the surface of AC-CdO-TiO2 nanocomposite material illuminated by visible-light were detected by fluorescence technique same as that of TiO2 nanocomposite material. The formation of •OH is directly related to the photocatalytic mechanism of the ACCdO- TiO2 nanocomposite material (Figure 14) [31].
Electrochemical application
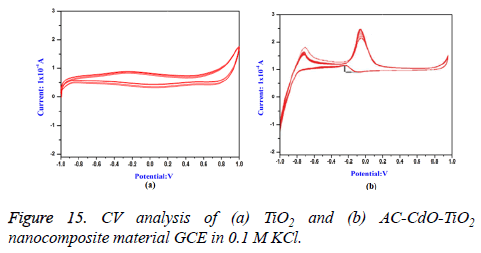
Cyclic voltammogram (CV) analysis: Cyclic voltammogram (CV) of methanol was recorded in alkaline medium (0.5 M NaOH) on glassy carbon electrode (GCE) and coating of catalysts in TiO2 and AC-CdO-TiO2 nanocomposite material was done. The forward peak current density for methanol oxidation on AC-CdO-TiO2 nanocomposite material showed higher electrocatalytic activity (2.5 mA cm-2) than TiO2 (1 mA cm-2) indicating that AC-CdO-TiO2 nanocomposite material is superior to TiO2 (Figures 15a and 15b).
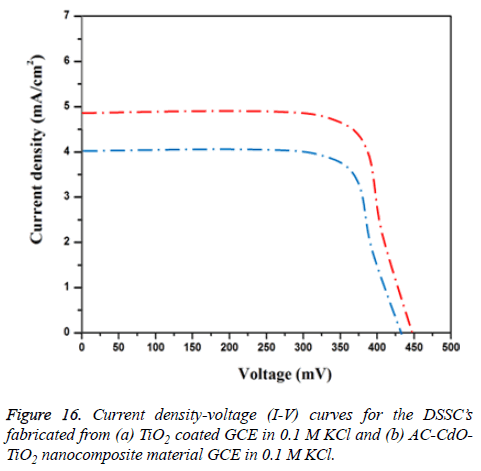
Photovoltaic properties: Figure 16 shows the photo current voltage (I-V) behavior of fabrication in the dye sensitized solar cell (DSSCs). Prepared TiO2 and AC-CdO-TiO2 nanocomposite material act as photoelectrode coated on fluorine doped Tin oxide (FTO-plate) glass substrate. The routine solar cell is fabricated with prepared TiO2 and ACCdO-TiO2 nanocomposite material with ruthenium dye (535- bisTBA, N719). From the data, it is clear that (N719) ACCdO- TiO2 nanocomposite material based cell gives brilliant activity with the use of dye since the sensitizer reunites the maximum value of short-circuit current density, Jsc (4.9 mA/cm2) than TiO2 (4 mA/cm2), open-circuit voltage, Voc (500 mV), fill-factor, FF (0.94) and efficiency, η (1.7%). The result indicating AC-CdO-TiO2 nanocomposite material superior electrocatalytic activity [32,33].
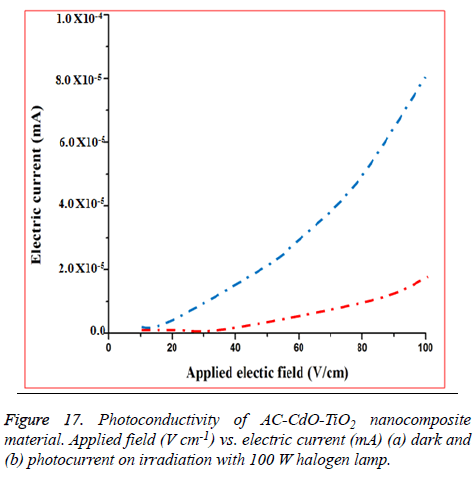
Conductivity studies: Photoconductivity result of AC-CdO-TiO2 nanocomposite material represent increased current and increased charge carriers in photocurrent compared to dark current. This nanocomposite material exhibited (+ve) photoconductivity as well as solar cell application (Figure 17) [34,35].
Antibacterial activity
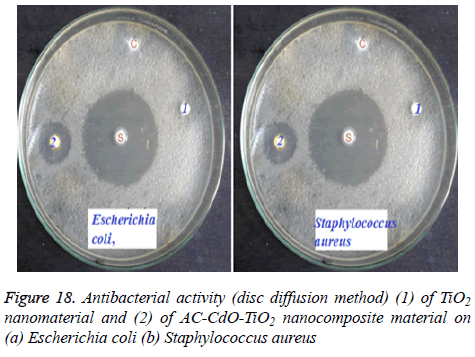
Antibacterial activity of TiO2 and AC-CdO-TiO2 nanocomposite material are shown in Figure 18. CdO-SnO2 nanocomposite material showed activity against both Gram positive strains and Gram negative strains. 15 mm and 11 mm inhibition zone was developed against Escherichia coli and Staphylococcus aureus. The AC-CdO-TiO2 nanocomposite material showed high antibacterial activity against Gram positive bacteria rather than Gram negative bacteria. This was due to the differences in the cell wall composition of these two bacteria. AC-CdO-TiO2 nanocomposite material increases the value of the electron-holes charge separation by decreasing the band gap energy which leads to an impediment in the recombination rate for high antibacterial activity, hence used in biomedical applications [36,37].
Conclusion
The mild reaction conditions, easy work-up, clean reaction profiles render this approach as an interesting alternative to the existing methods. The HR-SEM image of AC-CdO-TiO2 nanocomposite material showed the morphological structure as nanoflakes which were further confirmed by EDX analysis. HR-TEM measurements indicated nanospherical chain shaped structures corresponding to its polycrystalline nature. The photodegradation efficiency of AC-CdO-TiO2 nanocomposite material on RB 5 showed a high photocatalytic activity by employing various experimental parameters namely high photocatalytic effect of pH=7 whereas the nanocomposite material with the catalyst (0.080 g) loading showed optimum photocatalytic activity when compared to TiO2, furthermore, nanocomposite material at concentration of 1 × 10-4 M showed high photocatalytic activity with the catalysis was stable and recyclable. Finally, AC-CdO-TiO2 nanocomposite material was found to show high antibacterial activity on (a) Escherichia coli, (b) Staphylococcus aureus and a high current produced effective short circuit than that of TiO2 nanocomposite material.
Conflict of Interest
The authors declare no competing financial interest.
References
- Balakrishnan M, Arul Antony S, Gunasekaran S, Natarajan RK. Impact of dyeing industrial effluents on the groundwater quality in Kancheepuram (India). Ind J Sci Technol 2008; 1: 1.
- Vijaykumar MH, Vaishampayan PA, Shouche YS, Karegoudar TB. Decolouri-zation of naphthalene-containing sulfonated azo dyes by Kerstersia sp strain VKY1. Enzym Microb Tech 2007; 204-211.
- Asad S, Amoozegar MA, Pourbabaee AA, Sarbolouki MN, Dastgheib SM. Decolorization of textile azo dyes by newly isolated halophilic and halotolerant bacteria. Bioresour Technol 2007; 98: 2082-2088.
- Liu W, Chao Y, Yang X, Bao H, Qian S. Biodecolorization of azo, anthraquinonic and triphenylmethane dyes by white-rot fungi and a laccase-secreting engineered strain. J Ind Microbiol Biotechnol 2004; 31: 127-132.
- OMahony T, Guibal E, Tobin JM. Reactive dye biosorption by Rhizopus arrhizus biomass. Enzym Microb Tech 2002; 31: 456-463.
- Balachandran S, Praveen SG, Velmurugan R, Swaminathan M. Facile fabrication of highly efficient, reusable heterostructured Ag-ZnO-CdO and its twin applications of dye degradation under natural sunlight and self-cleaning. RSC Adv 2014; 4: 4353-4362.
- Kalele S, Gosavi SW, Urban J, Kulkarni SK. Nanoshell particles: synthesis, properties and applications. Curr Sci 2006; 91: 1038-1052.
- Manikandan A, Judith Vijaya J, John Kennedy L. Comparative investigation of structural, optical properties and dyesensitized solar cell applications of ZnO nanostructures. J Nanosci Nanotechnol 2013; 13: 3068-3073.
- Loganathan B, Chandraboss VL, Murugavelu M, Senthilvelan S, Karthikeyan B. Synthesis and characterization of multimetallic-core and siliceousshell Au/Pt/Ag@SiO2 sol-gel derived nanocomposites. J Sol-Gel Sci Tech 2014.
- Murugavelu M, Karthikeyan B. Synthesis, characterization and evaluation of green catalytic activity of nano Ag-Pt doped silicate. J Alloys Comp 2013; 547: 68-75.
- Shon HK, Phuntsho S, Vigneswaran S. A study on the infl uence of ionic strength on the elution behaviour of membrane organic foulant using advanced separation tools. Desaline Water Treat 2008; 225: 235-248.
- Yiamsawas D, Boonpavanitchakul K, Kangwansupamonkon W. Preparation of ZnO nanostructures by solvothermal method. Microsc J Soc Thailan 2009; 23: 75-78.
- Balachandran S, Prakash N, Thirumalai K, Muruganandham M, Sillanpaa M, Swaminathan M. Facile construction of heterostructured BiVO4-ZnO and its dual application of greater solar photocatalytic activity and self-cleaning property. Ind Eng Chem Res 2014; 53: 8346-8356.
- Balachandran S, Swaminathan M. The simple, template free synthesis of a Bi2S3-ZnO heterostructure and its superior photocatalytic activity under UV-A light. Dalton Trans 2013; 42: 5338-5347.
- Papp J, Soled S, Dwight K, Wold A. Surface acidity and photocatalytic activity of TiO2, WO3/TiO2, and MoO3/TiO2 photocatalysts. Chem Mater 1994; 6: 496-500.
- Fu X, Clark LA, Yang Q, Anderson MA. Enhanced photocatalytic performance of titania-based binary metal oxides: TiO2/ SiO2 and TiO2/ZrO2. Environ Sci Technol 1996; 30: 647-653.
- Chanchal M, Mainak G, Arun KS, Jaya P, Ramkrishna S, Tarasankar P. Robust cubooctahedron Zn3V2O8 in gram quantity: a material for photocatalytic dye degradation in water. Cryst Eng Comm 2013; 15: 6745-6751.
- Tanmay KG, Niladri B. Photodegradation of rhodamine 6G in aqueous solution via SrCrO4 and TiO2 nano-sphere mixed oxides J Mater Res Technol 2013; 2: 10-17.
- Rui S, Yajun W, Feng Z, Yongf Z. Zn3V2O7(OH)2(H2O)2 and Zn3V2O8 nanostructures: controlled fabrication and photocatalytic performance. J Mater Chem 2011; 21: 6313.
- Kamalakkannan J, Chandraboss VL, Prabha S, Senthilvelan S. Advanced construction of heterostructured InCrO4-TiO2 and its dual properties of greater UV-photocatalytic and antibacterial activity. RSC Adv 2015; 5: 77000-77013.
- Kamalakkannan J, Chandraboss VL, Loganathan B, Prabha S, Karthikeyan B, Senthilvelan S. TiInCrO6-nanomaterial synthesis, characterization and multiapplications. Appl Nanosci 2015.
- Kamalakkannan J, Chandraboss VL, Prabha S, Karthikeyan B, Senthilvelan S. High photocatalytic activity of heterostrutured InMoO3-TiO2 nanocomposite material under natural sun light irradiation. Can Chem Trans 2015; 3; 327-339.
- Tsumura T, Kojitani N, Umemura H, Toyoda M, Inagaki M. Composites between photoactive anatase-type TiO2 and adsorptive carbon. Appl Surf Sci 2002; 196: 429-436.
- Velasco LF, Parra JB, Ania CO. Role of activated carbon features on the photocatalytic degradation of phenol. Appl Surf Sci 2010; 256: 5254-5258.
- Mezohegyi G, van der Zee FP, Font J, Fortuny A, Fabregat A. Towards advanced aqueous dye removal processes: a short review on the versatile role of activated carbon. J Environ Manage 2012; 102: 148-164.
- Kamalakkannan J, Chandraboss VL, Prabha S, Senthilvelan S. Activated carbon loaded N, S co-doped TiO2 nanomaterial and its dye wastewater treatment. Inter Lett Chem Phy Astron 2015; 8: 147-164.
- Prabha S, Chandraboss VL, Kamalakannan J, Senthilvelan S. UV-light assisted photodegradation and antibacterial activity of activated charcoal supported cadmium doped ZnO nanocomposite material. Inter J Modern Res Rev 2015; 3: 587-594.
- Chandraboss VL, Kamalakkannan J, Senthilvelan S. Characterization of activated charcoal supported bismuth doped SiO2 (ac-Bi@SiO2) nanocomposite sphere for photocatalytic application. Can Chem Trans 2015; 3: 410-429.
- Subash B, Krishnakumar B, Swaminathan M, Shanthi M. Highly efficient, solar active, and reusable photocatalyst: Zr-loaded Ag-ZnO for reactive red 120 dye degradation with synergistic effect and dye-sensitized mechanism. Langmuir 2013; 29: 939-949.
- Kamalakkannan J, Senthilvelan S. Morphology convenient flower like nanostructures of CdO-SiO2 nanomaterial and its photocatalytic application. WSN 2017; 62: 46-63.
- Chandraboss VL, Kamalakkannan J, Prabha S, Senthilvelan S. An efficient removal of methyl violet from aqueous solution by an AC-Bi/ZnO nanocomposite material. RSC Adv 2015; 5: 25857-25869.
- Zhang Y, Wang L, Liu B, Zhai J, Fan H, Wang D, Lin Y, Xie T. Synthesis of Zn-doped TiO2 microspheres with enhanced photovoltaic performance and application for dye-sensitized solar cells. Elctrochimica Acta 2011; 56: 6517-6523.
- Kamalakkannan J, Chandraboss VL, Prabha S, Karthikeyan B, Senthilvelan S. Preparation and characterization of TiInVO6-nanomaterial using precipitation method and its multi applications. J Mater Sci Mater Electron 2015.
- Balachandran S, Selvam K, Babub B, Swaminathan M. The simple hydrothermal synthesis of Ag-ZnO SnO2nanochain and its multiple application. Dalton Trans 2013; 42: 16365-16374.
- Balachandran S, Thirumalai K, Swaminathan M. Facile hydrothermal synthesis of a highly efficient solar active Pr6O11-ZnO photocatalyst and its multiple applications. RSC Adv 2014; 1-12.
- Banerjee S, Gopal J, Muraleedharan P, Tyagi AK, Raj B. Physical and chemistry of photocatalytic titanium dioxide visualization of bactericidal activity using atomic force microscopy. Curr Sci 2006; 90: 1378-1383.
- Kiran G, Singh RP, Ashutosh P, Anjana P. Photocatalytic antibacterial performance of TiO2 and Ag- doped TiO2 against S. aureus. P. aeruginosa and E. coli. Beilstein J Nanotechnol 2013; 4: 345-351.