ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2018) Volume 29, Issue 2
Study on soybean milk fermented by Lactobacillus plantarum YS-1 reduced the H2O2-induced oxidative damage in Caco-2 cells
1Beijing Advanced Innovation Center for Food Nutrition and Human Health, Beijing Technology and Business University (BTBU), Beijing, PR China
2Chongqing Collaborative Innovation Center for Functional Food, Chongqing University of Education, Chongqing, PR China
3College of Biological and Chemical Engineering, Chongqing University of Education, Chongqing, PR China
4College of Food Science, Southwest University, Chongqing, PR China
5Department of Nutrition and Food Hygiene, School of Public Health, Guilin Medical University, Guilin, Guangxi, PR China
#These authors have contributed equally to this work
- *Corresponding Authors:
- Xin Zhao
Beijing Advanced Innovation Center for Food Nutrition and
Human Health
Beijing Technology and Business University (BTBU), PR China
Jia-Le Song
Department of Nutrition and Food Hygiene
School of Public Health
Guilin Medical University, PR China
Accepted on October 30, 2017
DOI: 10.4066/biomedicalresearch.29-17-3132
Visit for more related articles at Biomedical ResearchThis study was conducted to investigate the free radicals scavenging activity and cytoprotective effects of Lactobacillus plantarum YS-1 Fermented Soy Milk (FSM-LPYS1) in Caco-2 human colon adenocarcinoma cells damaged by hydrogen peroxide (H2O2)-induced oxidation. FSM-LPYS1 showed a remarkable in vitro activity against 2, 2-Diphenyl-1-Picrylhydrazyl (DPPH) and hydroxyl radical (•OH). FSM-LPYS1 significantly decreased the levels of intracellular Reactive Oxygen Species (ROS), lipid peroxidation, and enhanced the activities and expression levels of Catalase (CAT), Superoxide Dismutase (SOD) and glutathione peroxidase (GSH-Px) in H2O2-treated Caco-2 cells (p<0.05). FSM-LPYS1 also reduced the protein levels of IL-8 and NF-κB in Caco-2 cells. These results clearly indicated that FSMLPYS1 had impressively scavenged free radicals and were able to reduce the H2O2-induced oxidative damage in Caco-2 cells by reducing the intracellular ROS generation, restraining lipid peroxidation, and by raising the endogenous antioxidant enzyme activity. In addition, the FSM-LPYS1 could also reduce the H2O2-induced inflammation reaction. FSM-LPYS1 is a functional food which has antioxidant properties and it can be used as high quality soybean processing food for exploitation of health benefits.
Keywords
Lactobacillus plantarum, Caco-2 human colon adenocarcinoma cells, DPPH, H2O2, Inflammation.
Introduction
Soybean contain many health beneficial properties such as antioxidant, anti-diabetic, anticancer that can help lowering breast cancer risk, preventing heart diseases, lowering cholesterol and osteoporosis [1]. Soybean and other soy-related products mainly contain isoflavone glucosides and aglycones have been known as health-promoting food and these products are extensively consumed by China, Japan, South Korea and other Asian countries [2]. However, glucosides in soy are difficult to be utilized in the human body due to their structure [3], but in comparison aglycones are absorbed easily in the human body [4]. It has been observed that soy isoflavone aglycones such as daidzein, genistein, and glycitein which contain anti-carcinogenic, antioxidant, and anti-atherosclerotic properties are helpful in preventing chronic diseases [5,6]. Lactic Acid Bacteria (LAB), a well-known probiotic that exhibit many health beneficial properties such as antimicrobial, anti-tumor, reduction of serum cholesterol, and strengthening the immune response [7]. LAB fermentation improves the food quality, safety and also increases the biological activity and adds nutritional value to these fermented products. Lactobaccillus fermented soy milk has been reported to show remarkable activity to scavenge free radicals [8,9], and diminished the proliferation of human MCF-7 breast cancer cells [10] and Hep3B liver cancer cells [11], also protected against the 2-amino-1-methyl-6-phenylimidazo (4, 5-b) pyridine-induced rat mammary carcinogenesis [12] and protect CCl4-induced hepatic damage in rats [13].
Reactive Oxygen Species (ROS) induced oxidative stress is one of the main factors for serious chronic degenerative diseases, such as cancer, diabetes, cardiovascular disease, atherosclerosis, aging and Alzheimer's disease [14]. Gradual build-up of ROS results in lipid peroxidation, protein oxidation and DNA damage, and also causes cell damage in intestinal epithelial cells [15]. The intestinal epithelium is important in absorbing nutrients, and it is also a physical barrier between the host and the external environment, so it serves as a important defense against pathogens and xenobiotics mediated by the gut immune system [16]. The pathogenesis of human inflammatory bowel diseases (IBD) has been linked with intestinal epithelial cell oxidative damage induced by ROS [15]. The overgenerated ROS also decreased the activity of endogenous antioxidant enzymes, including CAT, SOD, and GSH-Px, and depletes the non-enzymatic glutathione (GSH) in the injured cells [17]. This study was planned to investigate the antioxidant activity of soy milk fermented by a strain of Lactobacillus plantarum YS-1 that was isolated from yak yoghurt of Tibetan habitats to protect against H2O2-induced oxidative damage in human Caco-2 cells, and investigated its potential protective mechanisms.
Materials and Methods
Chemical reagents
TRIzol, OligodT18 primer, Murine Maloney Leukemia Virus (MMLV) reverse transcriptase, RNase inhibitor, ethidium bromide (EtBr) and agarose were purchased from Invitrogen (Thermo Fisher Scientific, Inc., Waltham, MA, USA). All other chemical reagents were of analytical grade and purchased from Sigma-Aldrich (Merck Millipore, Darmstadt, Germany).
Microorganism strains
Lactobacillus plantarum YS2 (M2016747 preserved in China Center for Type Culture Collection (CCTCC, Wuhan, China), which was identified from yak yoghurt produced in the Yushu (Qinghai Province, China). Lactobacillus delbrueckii subsp. bulgaricus (AB200048), which is used in yogurt manufacture, was purchased from CCTCC and uses as a reference strain.
Fermented soybean milk preparation
Entire soybean were first washed and then soaked in fresh Distilled Water (DW) for 12 h and after that were removed from DW. The soaked soybeans with 10 times their weight of DW were blended for 3 min, and filtered using double-layer cheesecloth to get the soybean milk. The filtered soybean milk was autoclaved at 121°C for 15 min, then cooled at room temperature (37°C), and incubated with Lactobacillus plantarum YS-1 and L. delbrueckii subsp. bulgaricus (LB) respectively at an initial population of approximately 5 Log CFU/ml at 37°C for 12 h to prepare the fermented soybean milk. At the end of fermentation, samples were taken for testing of pH, total acidity and amino type nitrogen. The pH of samples were measured and determined by using a pH meter. Diluted fermented soybean milk samples (20 ml) were combined with a formalin solution (20 ml, pH 7.4), and the pH was altered to 8.4 by titrating with NaOH (0.1 M). The volume of NaOH utilization was recorded in order to determine the total amino type nitrogen content of the samples.
Methanol extraction of fermented soybean milk
Fermented soybean milk samples were preserved at -80°C and went through extraction for three times with a 20-fold volume of methanol (80%, v/v) at room temperature for 12 h. The methanol extracts were synthesized and filtered through a filter paper, and concentrated under a vacuum at 37°C in a rotary evaporator. AT last the soybean milk extracts were dissolved in Dimethyl Sulfoxide (DMSO) and stored at -20°C for further analysis.
High-performance liquid chromatography (HPLC) assay
Proportion of genistein and daizein in fermented soy milk methanol extracts were decided by using a HPLC system (Waters Co., Milford, MA, USA), which was composed of a Waters 2695 separations module (Milford, MA, USA) and a Waters 2487 dual wavelength detector (Milford, MA, USA) with the wavelength set at 260 nm. The separation of genistein and daizein was accomplished by using a Kromasil C18 column (460 × 25 mm, 25 μm) and the mobile phase was constituted by methanol and water (40:60, v/v) at a flow rate of 1.0 ml/min. Genistein and daizein amount were distinguished and established against a standard compound based on its retention time and peak areas.
In vitro free radical scavenging assay (DPPH radicals)
Fermented soybean milk methanol extracts (0.5, 1.0, and 2.0 mg/ml) were mixed and shaken vigorously with 0.1 ml of DPPH radicals (final concentration, 150 μM), allowed to stand at 37°C for 30 min. Then absorbance of the reaction mixture was measured at 517 nm using a UV-2401PC spectrophotometer (Shimadzu Corporation, Kyoto, Japan) [18].
• OH scavenging activity
The 0.2 ml of fermented soybean milk methanol extracts (0.5, 1.0, and 2.0 mg/ml) were mixed with 1.2 ml of reaction solutions which contained deoxyribose (6 mM), H2O2 (3 mM), KH2PO4-K2HPO4 buffer (20 mM, pH 7.4), FeCl3 (400 μM), EDTA (400 μM), and ascorbic acid (400 μM) were incubated at 37°C for 1 h. Then, 1 ml of Thiobarbituric Acid (TBA)- Trichloroacetic Acid (TCA) solution were added up to the mixture and heated at 90°C for 30 min. Then absorbance of the reaction mixture was measured at 532 nm using a UV-2401PC spectrophotometer (Shimadzu Corporation, Kyoto, Japan) [19].
Cell culture and cell viability assay
Caco-2 human colon adenocarcinoma cells, which were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA), were regularly maintained in DMEM medium supplemented with 10% FBS and 1% penicillinstreptomycin in a humidified CO2 incubator (model 3154; Forma Scientific, Inc., Marietta, OH, USA) with 5% CO2 at 37°C. Firstly the caco-2 cells were seeded into 96 well plates at a density of 1 × 104 cells/well, and the cell viability was determined by MTT assay. Following incubation period of 24 h, the cells were first treated with the fermented soybean milk methanol extracts (10 and 100 μg/ml) for 24 h, and then exposed to H2O2 (250 μM) for an additional 4 h. Next, 100 μL of MTT reagent (0.5 mg/ml) was added to each well and the cells were incubated in a humidified incubator at 37°C to allow the MTT to be metabolized. Four hours later, formazan crystals were dissolved with DMSO (100 μL) in each well. At last the absorbance rate of the samples was measured at a wavelength of 540 nm using a microplate reader (model 680; Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Lipid peroxidation assay
Cooled Phosphate-Buffered Saline (PBS) used to wash the treated caco-2 cells, scraped into TCA (2.8%, w/v), and sonicated 3 times for 10 second intervals at 40 V setting over ice. Using a Bicinchoninic Acid (BCA) protein assay kit according to the manufacturer's instructions, the concentration of the total cell proteins was determined. The suspension (200 μL) was mixed with 1 ml of TBA (0.67%, w/v)-TCA (25%, w/v) solution, heated (30 min at 95°C), and centrifuged (3,000 Xg for 10 min at 95°C), TBA reacted with the products of lipid oxidative degradation, yielding red complexes. Absorbance was measured at 532 nm using a UV-2401PC spectrophotometer (Shimadzu Corporation, Kyoto, Japan) [20].
Intracellular ROS generation assay
Calcium- and magnesium-free PBS used to wash the treated Caco-2 cells, and incubated with serum and phenol red-free DMEM containing 20 μM of 2', 7'-Dichlorodihydrofluorescein Diacetate (DCFH-DA) at 37°C for 30 min. Then the medium was removed and the cells were washed twice with PBS. A FLUOstar OPTIMA fluorescence plate reader (FLUOstar OPTIMA; BMG Labtech, Offenburg, Germany) was used for measuring fluorescence; excitation was detected at 485 nm and emission was detected at 535 nm [21].
Intracellular antioxidant enzyme activity assay
For 24 h the Caco-2 cells grown in a six-well cell culture dish were incubated with fermented soybean milk extracts, and then treated with H2O2 (250 μM) for 4 h at 37°C. The cells were washed with PBS, removed by scraping, and centrifuged (3,000 Xg for 10 min at 4°C). Again the cells were washed with PBS, removed by scraping, centrifuged at 485 nm and resuspended in 300 μL cold lysis buffer (containing 1 mM EDTA), homogenized, and centrifuged at 4°C (12,000 Xg, 10 min). A BCA protein assay kit (Bio-Rad) according to the manufacturer's instructions used to determine the total protein levels. Catalase (CAT) activity (U/mg protein) in the supernatant was assessed according to the disappearance of the H2O2 substrate was measured using a UV-2401PC spectrophotometer (Shimadzu Corporation, Kyoto, Japan) at 260 nm [22]. SOD activity (U/mg protein) was measured using a modified auto-oxidation pyrogallol method [23]. GSH-Px activity (U/mg protein) was assayed according to the method described by Hafeman et al. [24].
Reverse transcription polymerase chain reaction (RTPCR) assay
Following to the manufacture’s recommendations total RNA was isolated using Trizol reagent and centrifuged at 12,000 Xg for 15 min at 25°C following the addition of chloroform. Isopropanol was added to the supernatant at a 1: 1 ratio and the RNA was piloted by centrifugation (12,000 Xg for 15 min at 4°C). After washing with ethanol, the RNA was solubilized in diethyl pyrocarbonate-treated RNase-free water and quantified by measuring the absorbance at 260 nm using a UV-1750 spectrophotometer (Shimadzu Corporation, Kyoto, Japan). Equal amount of RNA (1 μg) was reverse transcribed in a master mix containing 1 × reverse transcriptase buffer, dNTPs (1 mM), oligodT18 primers (500 ng), MMLV reverse transcriptase (140 U), and RNase inhibitor (40 U) for 45 min at 42°C. PCR was then carried out in automatic 2720 thermocycler (Applied Biosystem, Foster City, California, USA) for 28 cycles (94°C for 30 s, 55°C for 30 s, and 72°C for 40 s) followed by an 8 min extension at 75°C. The PCR products were separated in 2% agarose gels and visualized by EtBr staining [25]. β-actin was used for normalization. Sequences of primers used to specifically amplify the genes of interest were as follows: 5’-CCA CGT CCA TGC CTT TGG-3’ (forward) and 5’-TCA GCT GCT GCA GTC ACG TT-3’ (reverse) for SOD; 5’-CCG ACC GTC CGT AAA TGC TA-3’ (forward) and 5’-GCT TTT CAG ATA GGC TCT TCA TGT AA-3’ (reverse) for CAT; 5’-GAT TCG TTC CAA ACT TCC TGC TA-3’ (forward) and 5’-GCT CCC AGA ACA GCC TGT TG-3’ (reverse) for GSH-Px; 5’-CCA AAG CCA ACA GGG AGA A-3’ (forward) and 5’-AGG GAC AAC ACT GCC TGG AT-3’ (reverse) for β-actin.
Western blotting assay
50 μg of cellular protein extracts were separated by Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDSPAGE) and then electrotransferred on a nitrocellulose membrane (Schleicher and Schuell Bioscience, Inc., Keene, NH, USA) for Western blot analysis. Then the blots were incubated with antibodies against CAT, SOD, GSH-Px, NF-κB, IL-8 and β-actin (Cell Signaling Technology, Inc., Danvers, MA, USA), and incubated with horseradish peroxidaseconjugated secondary antibody (Cell Signaling Technology, Inc.) for 1 h at room temperature. The blots were washed three times with PBS containing 0.05% Tween-20 (PBS-T) and antibody binding was visualized by Enhanced Chemiluminescence (ECL Western Blotting Detection kit; GE Healthcare Life Sciences, Little Chalfont, UK).
Statistical analysis
The mean ± SD represented the data. Differences between the mean values for individual groups were assessed by one-way Analysis of Variance (ANOVA) with the Student-Neumann- Keuls post-hoc test, and the data were subjected to two-tailed statistical testing. p<0.05 was considered a statistically significant difference. For statistical analyses, SPSS software 19.0 (IBM Software, Armonk, NY) was used.
Results
Characterization of fermented soybean milk
As shown in Table 1, Lactobacillus plantarum YS-1 fermented soybean milk (FSM-LPYS1) exhibited the higher pH value (5.59) and total acidity (1.92%) than that in Lactobacillus bulgaricus fermented soybean milk (FSM-LB, 5.02 in pH, and 1.32% in total acidity). At the final fermented products, FSMLPYS1 contained higher levels of amino type nitrogen compared with that in FSM-LB (Table 1).
| Samples | pH | Total acidity (%) | Amino acid nitrogen (mg%) | Genistein (µg/ml) | Daidzein (µg/ml) |
|---|---|---|---|---|---|
| FSM-LPYS1 | 5.59 ± 0.05* | 1.32 ± 0.03 | 2141.5 ± 61.5 | 17.2 ± 2.1 | 20.3 ± 1.9 |
| FSM-LB | 5.02 ± 0.04 | 1.92 ± 0.04 | 1799.5 ± 59.5 | 9.0 ± 1.4 | 13.5 ± 2.3 |
Table 1. The changes of pH, total acidity, amino acid nitrogen, genistein and daidzein in different strains fermented soybean milk. *Data are presented as mean ± SD. FSM-LPYS1: soybean milk fermented with Lactobacillus plantarum YS-1; FSM-LB: soybean milk fermented with Lactobacillus bulgaricus.
In vitro free radical scavenging activity
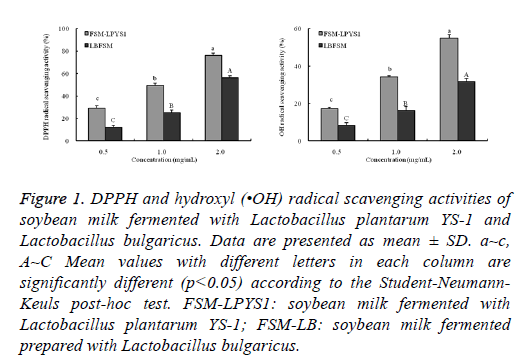
In free radical scavenging assay, At the concentrations of 2.0 mg/ml, FSM-LPYS1 showed the strongest free radical scavenging activity of 80.3% and 60.2% against DPPH and •OH radical, respectively, which was more than that for FSMLB (50.4% against DPPH and 30.9% against •OH) (Figure 1).
Figure 1: DPPH and hydroxyl (•OH) radical scavenging activities of soybean milk fermented with Lactobacillus plantarum YS-1 and Lactobacillus bulgaricus. Data are presented as mean ± SD. a~c, A~C Mean values with different letters in each column are significantly different (p<0.05) according to the Student-Neumann- Keuls post-hoc test. FSM-LPYS1: soybean milk fermented with Lactobacillus plantarum YS-1; FSM-LB: soybean milk fermented prepared with Lactobacillus bulgaricus.
Cell protective activity
When treated with 10 and 100 μg/ml of FSM-LPYS1 and FSM-LB for 24 h, the cells viability was more than 90% (data not shown). The concentrations ranging from 10 and 100 μg/ml were used for further studies. As shown in Table 2, 100 μg/ml of FSM-LPYS1 showed a great activity to increase the cell viability to 75.4% than that in FSM-LB treated Caco-2 cells (67.0%), as well as reduced the H2O2 induced Caco-2 cell death (p<0.05).
| Groups | Concentration (μg/ml) | Cell viability (%) | ROS (%) | MDA (nM/mg protein) |
|---|---|---|---|---|
| Normal | - | 100.00a1) ± 0.00 | 100.00d ± 0.00 | 0.22d ± 0.03 |
| Control | - | 49.71d ± 3.98 | 247.54a ± 15.82 | 1.56a ± 0.13 |
| FSM-LPYS1 | 10 | 53.89d ± 6.76 | 220.90b ± 12.35 | 1.38ab ± 0.22 |
| 100 | 75.40b ± 3.26 | 176.68c ± 11.29 | 1.01c ± 0.18 | |
| FSM-LB | 100 | 66.98c ± 1.90 | 191.25c ± 13.66 | 1.10bc ± 0.26 |
Table 2. Effect of soybean milk fermented with Lactobacillus plantarum YS-1 on cell viability, ROS and MDA levels in H2O2 (250 μM, 4 h)-treated Caco-2 cells. 1)Data are presented as mean ± SD. a~dMean values with different letters in the each column are significantly different (p<0.05) according to the Student-Neumann-Keuls post-hoc test. FSM-LPYS1: soybean milk fermented with Lactobacillus plantarum YS-1; FSM-LB: soybean milk fermented prepared with Lactobacillus bulgaricus.
Intracellular ROS levels and lipid peroxidation levels
FSM-LPYS1 treatment was potent for reducing the ROS levels and generation of MDA in H2O2-treated Caco-2 cells (p<0.05). H2O2 significantly increased the intracellular ROS levels to 247.5% (Table 2). Both FSM-LPYS1 and FSM-LB effectively reduced the H2O2 induced ROS generation (p<0.05). At the concentration of 100 μg/ml, FSM-LPYS1 was more effective in reducing ROS generation (176.7%) compared to FSM-LB treated cells (191.3%). H2O2 treatment also significantly induced the generation of MDA (to 1.56 nM/mg protein) in H2O2-treated Caco-2 cells. Caco-2 cells treated with 100 μg/ml of FSM-LPYS1 noticeably decreased MDA levels (1.01 nM/mg protein) while cells exposed to the same concentration of FSM-LB also decreased MDA generation (1.10 nM/mg protein).
Antioxidant enzyme activities
H2O2 treatment significantly decreased the activity of CAT (0.43 U/mg protein), SOD (2.24 U/mg protein) and GSH-Px (1.61 U/mg protein) than those in normal cells (Table 3). FSMLPYS1 treatment significantly increased the CAT and SOD activity in H2O2-treated Caco-2 cells (p<0.05). FSM-LPYS1 (100 μg/ml concentration) and FSM-LB treated cells increased the CAT activity (1.08 and 1.01 U/mg protein) and SOD activity (3.67 and 3.14 U/mg protein), respectively. In addition, FSM-LPYS1 also showed a great activity to enhance the GSHPx activity to 2.50 U/mg protein than that in FSM-LB (2.28 U/mg protein) at a concentration of 100 μg/ml.
| Groups | Concentration (μg/mL) | CAT (U/mg protein) | SOD (U/mg protein) | GSH-Px |
|---|---|---|---|---|
| Normal | - | 1.76a ± 0.11 | 5.89a ± 0.65 | 3.80a ± 0.11 |
| Control | - | 0.43d ± 0.10 | 2.24c ± 0.13 | 1.61d ± 0.13 |
| FSM-LPYS1 | 10 | 0.74b ± 0.19 | 3.00bc ± 0.28 | 2.01c ± 0.15 |
| 100 | 1.08b ± 0.12 | 3.67b ± 0.40 | 2.50b ± 0.20 | |
| FSM-LB | 100 | 1.01c ± 0.10 | 3.14b ± 0.70 | 2.28bc ± 0.27 |
Table 3. Effect of soybean milk fermented with Lactobacillus plantarum YS-1 on Catalase (CAT), Superoxide Dismutase (SOD) and glutathione peroxidase (GSH-Px) levels in H2O2 (250 μM, 4 h)-treated Caco-2 cells. 1)Data are presented as mean ± SD. a~dMean values with different letters in the each column are significantly different (p<0.05) according to the Student-Neumann-Keuls post-hoc test. FSM-LPYS1: soybean milk fermented with Lactobacillus plantarum YS-1; FSM-LB: soybean milk fermented prepared with Lactobacillus bulgaricus.
mRNA and protein expression of intracellular antioxidant enzymes
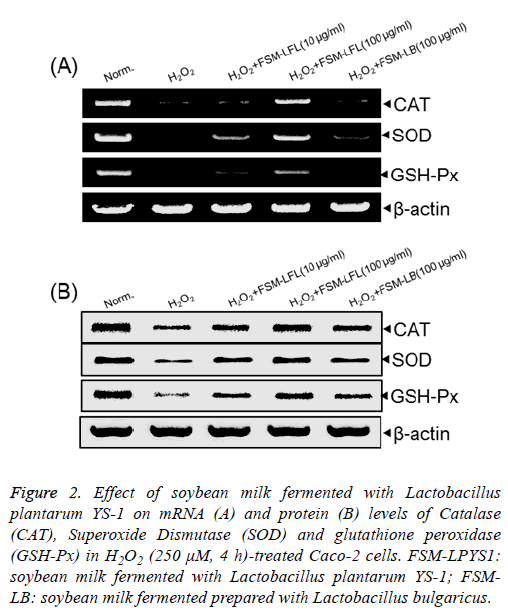
H2O2 treatment significantly decreased both mRNA and protein expression of CAT, SOD and GSH-Px compared to that found in normal Caco-2 cells (Figure 2). As demonstrated earlier as well that at the concentration of 100 μg/ml, FSMLPYS1 significantly increased the mRNA and protein levels of CAT, SOD and GSH-Px in comparison with that in FSM-LB treated Caco-2 cells.
Figure 2: Effect of soybean milk fermented with Lactobacillus plantarum YS-1 on mRNA (A) and protein (B) levels of Catalase (CAT), Superoxide Dismutase (SOD) and glutathione peroxidase (GSH-Px) in H2O2 (250 μM, 4 h)-treated Caco-2 cells. FSM-LPYS1: soybean milk fermented with Lactobacillus plantarum YS-1; FSMLB: soybean milk fermented prepared with Lactobacillus bulgaricus.
Protein expression of IL-8 and NF-κB
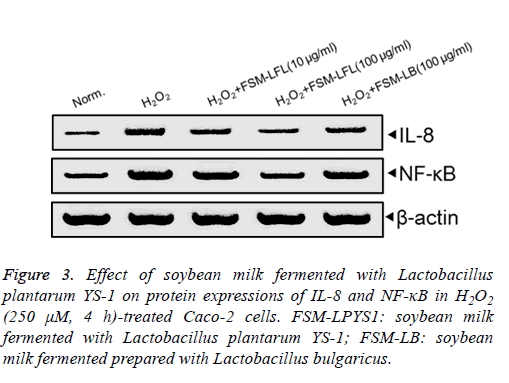
The protein levels of IL-8 were remarkably increased in response to treatment with H2O2 (250 μM) for 4 h than that in control cells (Figure 3). FSM-LPYS1 significantly diminished the H2O2-induced IL-8 expression in Caco-2 cells. H2O2 treatment significantly induced the over-expressions of NF-κB than in normal Caco-2 cells. However, following treated with 100 μg/ml of FSM-LPYS1 significantly decreased the protein levels of NF-κB which compared with that in FSM-LB treated Caco-2 cells. This result also suggested the FSM-LPYS1 showed an anti-inflammatory activity in H2O2-treated Caco-2 cells.
Figure 3: Effect of soybean milk fermented with Lactobacillus plantarum YS-1 on protein expressions of IL-8 and NF-κB in H2O2 (250 μM, 4 h)-treated Caco-2 cells. FSM-LPYS1: soybean milk fermented with Lactobacillus plantarum YS-1; FSM-LB: soybean milk fermented prepared with Lactobacillus bulgaricus.
Discussion
Higher levels of amino type nitrogen suggested a higher degree of protein hydrolysis and higher amounts of amino acids and peptides in the fermented products [10]. Soy isoflavone glucoside genistin and daidzin were hydrolysed by β- glucosidase into genistein and daidzein during the LAB fermentation [8]. So FSM-LPYS1 contained higher levels of genistein and daizein (17.2 and 20.3 μg/mL) than that in FSMLB (9.0 and 13.5 μg/ml). In this study, we have observed that both Lactobacillus plantarum YS-1 and Lactobacillus bulgaricus impressively decreased the pH with a rapid increase in total acidity during the 12 h of soy milk fermentation. LAB fermentation was also increased the productions of soybean aglycone in soy milk fermentation [26-28]. We also found the FSM-LPYS1 contained high levels of genistin and daidzin than those in FSM-LB. These results were identical to that in soybean milk fermented by Lactobacillus fermentum and Lactobacillus bulgaricus plus Saccharomyces boulardii [9]. This is due to the Lactobacillus fermentum showed better β- glucosidase activity than Lactobacillus bulgaricus at 37°C [9].
The highly reactive ROS which including hydroxyl (•OH) and superoxide (O2•) anions are both by-products during the aerobic respiration in living organisms. These ROS were in position to attack biological macromolecules such as DNA, protein, and lipids, promoting the lipid peroxidation, and subsequently caused serious oxidative damage in cell and tissue [29]. Some studies has reported that the living Lactobacillus fermentum and its fermented milk showed a great activity to against some free radicals (such as DPPH, O2• and •OH) [26,30], and effectively reduced lipid peroxidation in vitro [26]. And the isoflavone aglycone genestein and daidzein also showed a enhanced DPPH radical scavenging activity than their glucosides [5]. In free radical scavenging assay, FSMLPYS1 was effectively reduced the free radicals LSM-LB. The highly free radical scavenging activities of FSM-LPYS1 also associate with its contained high levels of amino type nitrogen. As reported by Je et al. and Rajapakse et al. that the high antioxidant activity of some fermented products was linked with their high levels of certain amino acids (such as lysine, glycine, valine and histidine) and histidine-containing peptides [27,28]. Increasing amino type nitrogen levels was also linked with the in vitro antioxidant activity during the milk LAB fermentation [31].
Enzyme-catalyzed or spontaneous mutation of superoxide anions formed a reactive oxygen metabolite, H2O2 and it can easily cross the cell membranes and react with Fe2+ or Cu2+ to form highly reactive •OH radicals by the Fenton reaction, so it increases the ROS generation, lipid peroxidation of polyunsaturated fatty acids, cell membrane leakage and DNA damage in colon Caco-2 cells [32].
In normal physiological condition, endogenous antioxidant enzyme system mainly consists of the enzyme CAT, SOD and GSH-Px which are both protective against ROS induced oxidative damage in living organisms [17].
Endogenous SOD catalyses the conversion of superoxide into H2O2, and these H2O2 is further reduced to H2O by CAT and GSH-Px [19]. So the enhancement of endogenous antioxidant enzyme activity was able to reduce the H2O2 induced oxidative damage in Caco-2 cells [32]. In addition, only Lactobacillus treatment was able to increase the total antioxidant activity and SOD activity in H2O2 induced oxidative damage of Caco-2 cell [33].
ROS induced oxidative stress activated the NF-κB signal pathway, and resulted in the generations of some inflammatory related cytokines (such as TNF-α, IL-1β, IL-6) and chemokine IL-8 generations [13,34]. In this study, FSM-LPYS1 showed a better activity to reduce the NF-κB and IL-8 protein expression in H2O2-treated cells. Inhibition of the NF-κB pathway was able to reduce the ROS induced cellular oxidative damage, and reduced the human IBD [34]. In addition, soybean milk fermented by mixed LAB starter that including Lactobacillus plantarum, Lactobacillus fermentum, and Lactobacillus rhamnosus were also markedly inhibited the chemokine IL-8 production in IL-1β treated Caco-2 cells [35].
Conclusion
From this study we can conclude that soybean milk fermented with Lactobacillus plantarum YS-1 showed a significant in vitro free radical scavenging activity and an effective protection against H2O2-induced oxidative damage in Caco-2 cells. And this was possible because of increased activity of endogenous antioxidant enzymes (CAT, SOD and GSH-Px) as well as reduced H2O2-induced ROS and MDA generation. In addition, FSM-LPYS1 also significantly reduced the IL-8 and NF-κB protein expression. Our results clearly indicate that the soybean milk fermented with Lactobacillus plantarum YS-1 can be used as a functional food to protect colon Caco-2 cells from the damage caused by H2O2-induced oxidation.
Acknowledgements
The present research was supported by Chongqing Research Program of Basic Research and Frontier Technology (cstc2016jcyjA0339) and Open fund of Beijing Advanced Innovation Center for Food Nutrition and Human Health, (20161001), China.
Conflict of Interest
There is no conflict of interest.
References
- Mateos-Aparicio I, Redondo Cuenca A, Villanueva-Suárez MJ, Zapata-Revilla MA. Soybean, a promising health source. Nutr Hosp 2008; 23: 305-312.
- Brouns F. Soya isoflavones: a new and promising ingredient for the health foods sector. Food Res Int 2002; 35: 187-193.
- Toda T, Sakamoto A, Takayanagi T, Yokotsuka K. Changes in isoflavone compositions of soybean foods during cooking process. Food Sci Technol Res 2000; 6: 314-319.
- Chien HL, Huang HY, Chou CC. Transformation of isoflavone phytoestrogens during the fermentation of soymilk with lactic acid bacteria and bifidobacteria. Food Microbiol 2006; 23: 772-778.
- Lee CH, Yang L, Xu JZ, Yeung SY, Huang Y, Chen ZY. Relative antioxidant activity of soybean isoflavones and their glycosides. Food Chem 2005; 90: 735-741.
- Nielsen IL, Williamson G. Review of the factors affecting bioavailability of soy isoflavones in humans. Nutr Cancer 2007; 57: 1-10.
- Masood MI, Qadir MI, Shirazi JH, Khan IU. Beneficial effects of lactic acid bacteria on human beings. Crit Rev Microbiol 2011; 37: 91-98.
- Wang YC, Yu RC, Chou CC. Antioxidative activities of soymilk fermented with lactic acid bacteria and bifidobacteria. Food Microbiol 2006; 23: 128-135.
- Rekha C, Vijayalakshmi G. Bioconversion of isoflavone glycosides to aglycones, mineral bioavailability and vitamin B complex in fermented soymilk by probiotic bacteria and yeast. J Appl Microbiol 2010; 109: 1198-1208.
- Chang WH, Liu JJ, Chen CH, Huang TS, Lu FJ. Growth inhibition and induction of apoptosis in MCF-7 breast cancer cells by fermented soy milk. Nutr Cancer 2002; 43: 214-226.
- Chen YF, Lee SL, Chou CC. Fermentation with Aspergillus awamori enhanced contents of amino nitrogen and total phenolics as well as the low-density lipoprotein oxidation inhibitory activity of black soybeans. J Agri Food Chem 2011; 59: 3974-3979.
- Ohta T, Nakatsugi S, Watanabe K, Kawamori T, Ishikawa F, Morotomi M, Sugie S, Toda T, Sugimura T, Wakabayashi K. Inhibitory effects of Bifidobacterium-fermented soy milk on 2-amino-1-methyl-6-phenylimidazo (4,5-b) pyridine-induced rat mammary carcinogenesis, with a partial contribution of its component isoflavones. Carcinogenesis 2000; 21: 937-941.
- Bai B, Yamamoto K, Sato H, Sugiura H, Tanaka T. Combined effect of 25-hydroxycholesterol and IL-1beta on IL-8 production in human colon carcinoma cell line (Caco-2). Inflammation 2005; 29: 141-146.
- Kadenbach B, Ramzan R, Vogt S. Degenerative diseases, oxidative stress and cytochrome c oxidase function. Trends Mol Med 2009; 15: 139-147.
- Rezaie A, Parker RD, Abdollahi M. Oxidative stress and pathogenesis of inflammatory bowel disease: an epiphenomenon or the cause? Dig Dis Sci 2007; 52: 2015-2021.
- Bourlioux P, Koletzko B, Guarner F, Braesco V. The intestine and its microflora are partners for the protection of the host: report on the Danone Symposium The Intelligent Intestine, held in Paris, June 14, 2002. Am J Clin Nutr 2003; 78: 675-683.
- Halliwell B. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol 2006; 141: 312-322.
- Hatano T, Kagawa H, Yasuhara T, Okuda T. Two new flavonoids and other constituents in licorice root: their relative astringency and radical scavenging effect. Chem Pharm Bull 1988; 36: 2090-2097.
- Halliwell B, Gutteridge JM, Aruoma OI. The deoxyribose method: a simple test-tube assay for determination of rate constants for reactions of hydroxyl radicals. Anal Biochem 1987; 165: 215-219.
- Fraga CG, Leibovitz BE, Tappel AL. Lipid peroxidation measured as thiobarbituric acid-reactive substances in tissue slices: characterization and comparison with homogenates and microsomes. Free Radic Biol Med 1988; 4: 155-161.
- Song JL, Choi JH, Seo JH, Kil JH, Park KY. Antioxidative effects of fermented sesame sauce against hydrogen peroxide-induced oxidative damage in LLC-PK1 porcine renal tuble cells. Nutr Res Pract 2014; 8: 138-145.
- Nelson D, Kies-Lfow L. Enthalpy of decomposition of hydrogen peroxide by catalase at 25 degrees C (with molar extinction coefficients of H2O2 solutions in the UV). Anal Biochem 1972; 49: 474-478.
- Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 1974; 47: 469-474.
- Hafeman DG, Sunde RA, Hoekstra WG. Effect of dietary selenium on erythrocyte and liver glutathione peroxidase in the rat. J Nutr 1974; 104: 580-587.
- Song JL, Gao Y. Effects of methanolic extract form Fuzhuan brick-tea on hydrogen peroxide-induced oxidative stress in human intestinal epithelial adenocarcinoma Caco-2 cells. Mol Med Rep 2014; 9: 1061-1067.
- Osuntoki A, Korie I. Antioxidant activity of whey from milk fermented with Lactobacillus species isolated from Nigerian fermented foods. Food Technol Biotechnol 2010; 48: 505-511.
- Je JY, Park PJ, Kim SK. Antioxidant activity of peptide isolated from Alaska Pollack (Theragra chalcogramma) frame protein hydrolysate. Food Res Int 2005; 38: 45-50.
- Rajapakse N, Mendis E, Jung WK, Je JY, Kim SK. Purification of a radical scavenging peptide from fermented mussel sauce and its antioxidant properties. Food Res Int 2005; 38: 175-182.
- Pacifici RE, Davies K. Protein, lipid and DNA repair systems in oxidative stress: the free-radical theory of aging revisited. Gerontology 1991; 37: 166-180.
- Wang AN, Yi XW, Yu HF, Dong B, Qiao SY. Free radical scavenging activity of Lactobacillus fermentum in vitro and its antioxidative effect on growing-finishing pigs. J Appl Microbiol 2009; 107: 1140-1148.
- Virtanen T, Pihlanto A, Akkanen S, Korhonen H. Development of antioxidant activity in milk whey during fermentation with lactic acid bacteria. J Appl Microbiol 2007; 102: 106-115.
- Wijeratne SS, Cuppett SL, Schlegel V. Hydrogen peroxide induced oxidative stress damage and antioxidant enzyme response in Caco-2 human colon cells. J Agri Food Chem 2005; 53: 8768-8774.
- Wu D, Zhong S, Zhang C, Xin Y. Antioxidant properties of lactobacillus and its protecting effects to oxidative stress Caco-2 cells. J Animal Plant Sci 2014; 24: 1766-1771.
- Morgan MJ, Liu ZG. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res 2011; 21: 103-115.
- Di Cagno R, Mazzacane F, Rizzello CG, Vincentini O, Silano M, Giuliani G, De Angelis M, Gobbetti M. Synthesis of isoflavone aglycones and equol in soy milks fermented by food-related lactic acid bacteria and their effect on human intestinal Caco-2 cells. J Agri Food Chem 2010; 58: 10338-10346.