ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
- Biomedical Research (2015) Volume 26, Issue 2
Studies on the chemical constituents of Radix astragali and their inhibitory effect on HepG2 proliferation.
The First Affiliated Hospital of Dalian Medical University, Dalian 116044, China
- *Corresponding Author:
- Ying Ba
The First Affiliated Hospital
Dalian Medical University
Dalian 116044
China
Accepted date: September 04 2014
To analyze the chemical constituents of ethanol extract of Radix Astragali, and to study their inhibitory effect on HepG2 cell line proliferation. Column chromatography, thin-layer chromatography and preparative liquid chromatography were used to extract and isolate compounds, and NMR spectroscopy was used to analyze the structure of the compounds; MTT assay and flow cytometry were used to determine the anticancer effect of the ethanol extract of Radix Astragali. Four compounds were isolated from the ethyl acetate and n-butanol fractions of Radix Astragali ethanol extract, which were structurally identified as astragaloside, uridine, 7,2'-dihydroxy-3',4'- dimethoxyisoflavan- 7,2'-dioxo-β-D-glucoside and (3R)-8,2'-dihydroxy-7,4'- dimethoxyisoflavan. MTT assay results showed that HepG2 cell growth was inhibited to varying degrees in each experimental group, and the inhibitory effects exhibited apparent dose- and time-effect relationships, the longer the drug action, the stronger the inhibitory effect; flow cytometry found that 48 h after the action of different concentrations of Radix Astragali ethanol extracts on HepG2 cells, the apoptosis rate of HepG2 cells significantly increased. Within the experimental dose range, Radix Astragali ethanol extract has proliferation inhibitory effect on HepG2 cells.
Keywords
Radix Astragali, Cell line HepG2, Astragaloside
Introduction
Chinese herbal medicine Radix Astragali is the root of herbaceous plants Astragalus membranaceus (Fisch) Bge. var. mongholicus (Bge.) Hsiao or Astragalus membrane aceus (Fisch) of family Leguminosae. As an important qi-tonifying traditional Chinese medicine, Radix Astragali is warm in nature, sweet in taste, and has the effects of invigorating qi and strengthening exterior, inducing diuresis and dispelling toxin, astringing wound and promoting skin regeneration, replenishing qi and strengthening middle warmer. It is mainly used for collapse of middle qi, hematochezia, metrorrhagia, chronic diarrhea, rectocele, exterior deficiency, spontaneous perspiration, qi deficiency, edema, uterine prolapse, etc. [1-2].
Modern studies have shown that Radix Astragali contains a variety of substances such as glycosides, polysaccharides, flavonoids, amino acids and trace elements, and has anti-tumor, immunomodulatory, anti-viral, anti-aging, anti-oxidation, anti-radiation and anti-stress pharmacological effects [3-5].
In this paper, crude extract of Radix Astragali was obtained, isolated, and its compounds were identified. Meanwhile, anti-cancer effect of ethanol extract of Radix Astragali was explored in order to lay the foundation for the clinical application of Radix Astragali.
Materials and Methods
Instruments and reagents
NMR spectrometer (Bruker Avance 500); mass spectrometer (YG-20 250 (EI-MS); melting point apparatus (XT4A micro melting point apparatus); rotary evaporator (Eyela, Japan); CO2 incubator (CO-150, NBS, USA); SW-CJ-SF clean bench (Suzhou Purification Equipment Factory, Sujing Group); flow cytometry (Beckman Coulter, USA); reagents used for extraction and isolation were all of analytical grade.
Drugs
Radix Astragali was purchased from the medicinal material market, which was identified as Astragalus membranaceus (Fisch) Bge. var. mongholicus (Bge.) Hsiao; 1640 medium and fetal bovine serum were purchased from Gibco; MTT, Annixin-v and PI were purchased from Sigma.
Cell lines
Hepatoma cell line HepG2 was purchased from China Medical University.
Extraction and isolation of Radix Astragali
10 kg of Radix Astragali crude drug was taken, ground into coarse powder, and extracted under reflux three times with 70% ethanol, the extracted solutions were combined, and ethanol was removed from the extract. The extract was then suspended in 5-fold amount of water, and slightly heated. After complete dissolution, the product was successively extracted with chloroform, ethyl acetate and n-butanol. The solvent was then removed, and extracts of each fraction were taken.
The ethyl acetate fraction was isolated by silica gel column chromatography (200 mesh), and gradient-eluted with different proportions of chloroform-methanol, 500 ml was taken as one fraction, after solvent removal, the fractions were subjected to thin layer chromatography, and identical fractions were combined. The combined fractions were subjected to preparative thin layer chromatography or preparative liquid chromatography for isolation of compounds, and compound 1 was obtained.
N-butanol fraction was passed through macroporous adsorption resin for rough isolation, and then isolated with water, as well as 20%, 40%, 60%, 80% and 95% ethanol, respectively. 40% ethanol fraction was taken and isolated by column chromatography. The method was basically consistent with the "ethyl acetate fraction", and compounds 2-4 were obtained.
Cell culture
HepG2 Cell lines were cultured with DMEM containing 10% fetal bovine serum, and routinely subcultured in a 37°C, 5% CO2 incubator, the cells in logarithmic growth phase were used for the experiment.
Preparation of test solution and experimental grouping
In accordance with the method in "2.1 Extraction and isolation of Radix Astragali", the ethyl acetate fraction was taken, and prepared into 50, 100 and 150 mg/L test solutions and set aside. The experiment had 4 groups, namely 3 test groups, and a control group, which used an equal volume of phosphate buffered saline (PBS) instead of the drug.
Cell proliferation inhibition experiment
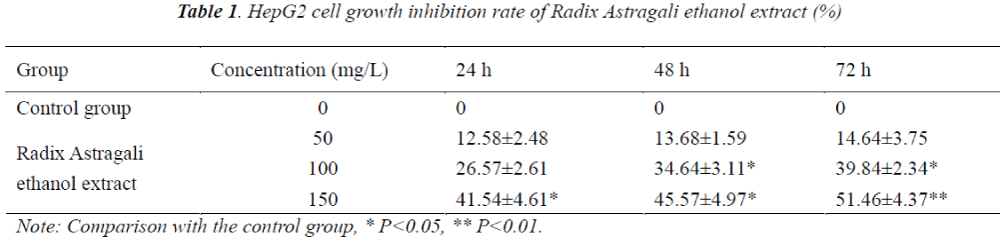
MTT assay was used to determine the inhibitory effect of Radix Astragali ethanol extract on HepG2 cells. A 4×106 cells/mL HepG2 single cell suspension was added to 96-well culture plates, then 20 μL of culture medium was added to each well, the plates were incubated in a 37°C, 5% CO2 incubator for 24 h, then 50, 100 and 150 mg/L Radix Astragali ethanol extracts were added to act on HepG2 cells, six replicate wells were set up for each group, and the control group was added with an equal volume of PBS, and routinely cultured, the groups were tested at 24 h, 48 h and 72 h, respectively. 20 μL of MTT was added to each well, after the incubation was continued for an additional 4 h, the supernatant was removed, and each well was added with 150 μL of dimethyl sulfoxide (DMSO), the plates were then placed on a micro shaker and shaken for 5 min, and absorbance (A value) of each well was measured at 570 nm wavelength using automatic microplate reader. Inhibition rate was calculated.
Cell inhibition rate = (1 - mean A value of treatment group / mean A value of control group) × 100%.
Flow cytometric determination of the effect of Radix Astragali ethanol extract on HepG2 cell cycle
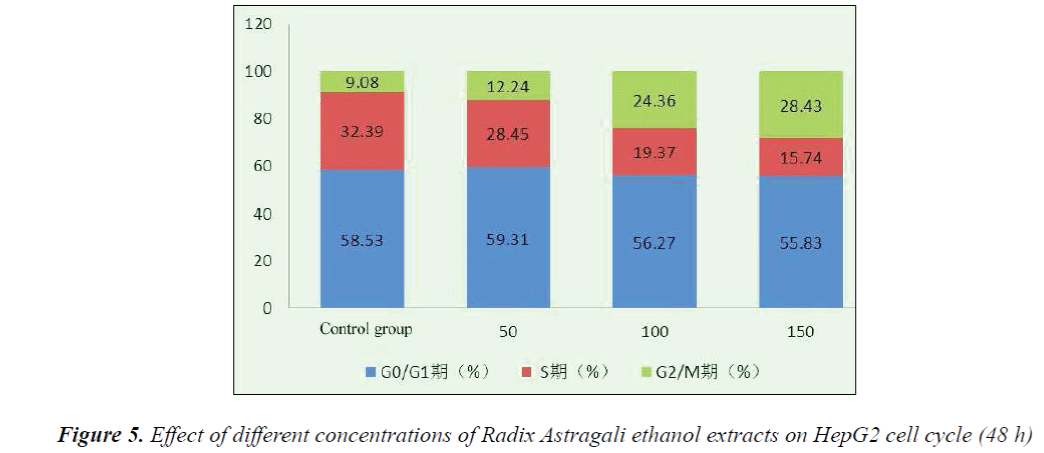
The HepG2 cells were seeded in culture flasks at 1×105 cells/mL, and cultured at 37°C with 5% CO2 for 24 h, then medium was replaced with fresh one, 50, 100 and 150 mg/L Radix Astragali ethanol extracts were added, and culture was continued for another 48 h. After digestion with 0.25% trypsin, the cells were collected, centrifuged for 5 min, and washed twice with PBS to produce a single cell suspension, which was stored at 4°C overnight. The cell suspension was then centrifuged, washed twice with PBS, PI stained, and incubated at 37° under dark conditions for 30 min, followed by determination of cell cycle distribution by flow cytometry.
Flow cytometric determination of apoptosis rate
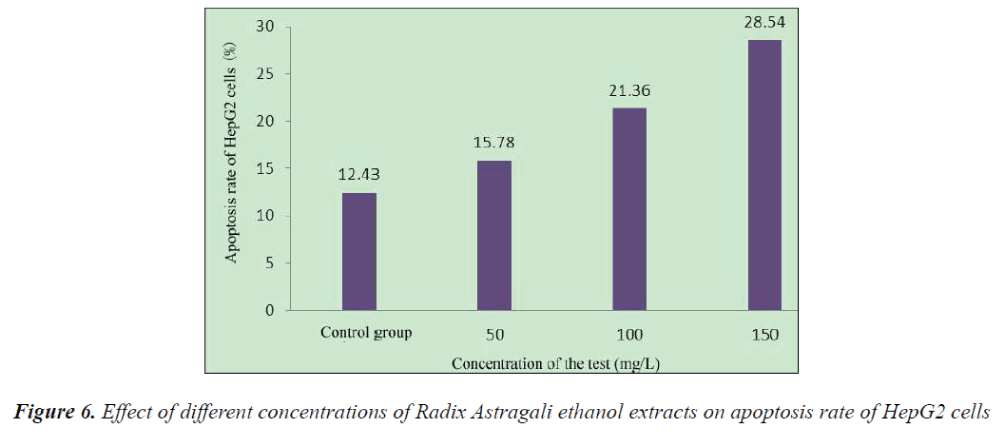
Logarithmic growth phase HepG2 cells were taken and cultured for 24 h, then Radix Astragali ethanol extracts with final concentrations of 50, 100 and 150 mg/L were added, respectively, after culturing for another 24 h, the cells were collected, centrifuged, supernatant removed, PBS washed three times, and fixed with 70% ice-cold ethanol at -20°C overnight, then washed with PBS, stained with PI, and incubated under dark conditions for 20 min, followed by addition of 400 μl of ice-cold buffer. Apoptosis rate was determined by flow cytometry.
Results
Structural identification of compounds
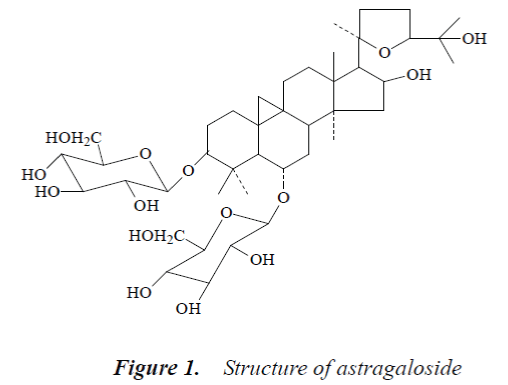
Compound 1: white powder, mp. 299-300°C. 1H-NMR (500MHz, DMSO-d6) δ:0.20 (1H d, J=4.1 Hz, 19-αH), 0.52 (1H, d, J=4.1 Hz, 19-βH), 0.96 (6H, s, 21-CH3, 28-CH3), 1.09 (3H, s,18-CH3), 1.15 (3H, s, 26-CH3), 1.16 (3H, s, 30-CH3), 1.20 (3H, s, 27-CH3), 1.20 (3H, s, 29-CH3), 4.81 (1H, br.s, 16-αH), 4.91 (1H, d, J=7.2 Hz, Glc-H-1’), 5.13 (1 H, d, J=7.2 Hz, Xyl-H-1); 13C-NMR (500MHz, DMSO-d6) δ: 33.2 (C-1), 32.5 (C-2), 86.9 (C-3), 40.9 (C-4), 51.0 (C-5), 77.0 (C-6), 35.3 (C-7), 44.5 (C-8), 21.0 (C-9), 30.0 (C-10), 25.8 (C-11), 32.1 (C-12), 45.7 (C-13), 46.2 (C-14), 5.6 (C-15), 72.9 (C-16), 56.8 (C-17), 21.5 (C-18), 30.4 (C-19), 87.4 (C-20), 28.1 (C-21), 33.9 (C-22), 25.5 (C-23), 81.2 (C-24), 70.5 (C-25), 28.6 (C-26), 28.3 (C-27), 19.0 (C-28), 29.0 (C-29), 15.9 (C-30), 3-O-β-D-Xyl: 106.6 (C-1’), 74.6(C-2'), 76.1(C-3’), 70.5 (C-4'), 65.4 (C-5'); 6-O-β-D-Glc: 103.2 (C-1”), 72.4 (C-2"), 77.7 (C-3”), 71.5 (C-4"), 75.8 (C-5"), 62.3 (C-6").
The above data were basically consistent with the literature [6], so the structure of compound 1 was astragaloside.
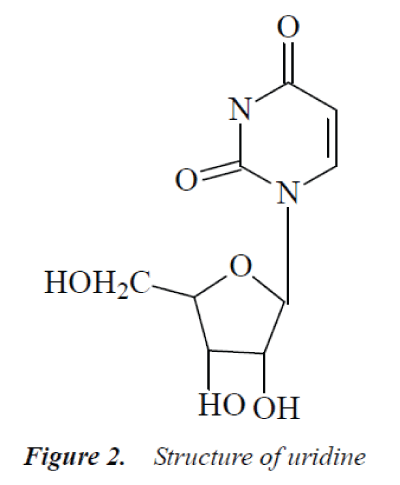
Compound 2: colorless needle crystals (methanol), easily soluble in water. 1H-NMR (500MHz, DMSO-d6) δ: 11.29 (1H, s, -NH), 7.92 (1H, d, J=8.2 Hz, H-6), 5.81 (1H, d, J=5.3 Hz, H-1’), 5.77 (1H, dd, J=8.4 Hz, 1.7 Hz, C5-OH), 5.43 (1H, d, J=5.2 Hz, C3’-OH), 5.11 (2H, m, C2’-OH, C5’-OH), 3.80-4.10 (3H, m, H-2', H-3', H-4'), 3.50-3.67 (2H, m, H-5’); 13C-NMR (500MHz, DMSO-d6) δ: 164.4 (C-4), 151.0 (C-2), 141.0 (C-6), 102.4 (C-s), 88.5 (C-1’), 85.6 (C-4'), 72.7 (C-3’), 71.4 (C-2'), 61.0 (C-5').
The above data were basically consistent with uridine reported in the literatures [7-8], so compound 2 was identified as uridine.
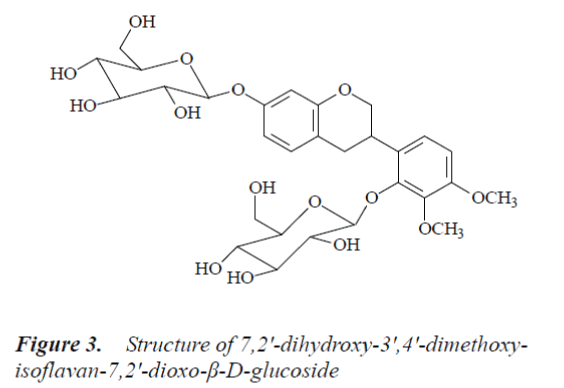
Compound 3: white powder, m.p.150-151°C, easily soluble in methanol. 1H-NMR (500MHz, DMSO-d6) δ: 2.75 (1H, dd, J=4.8 Hz, J=15.4 Hz, Ha-4), 2.82 (1H, dd, J=15.2 Hz, J=12.2 Hz, Hb-4), 3.70 (1H, m, H-3), 3.73 (3H, s, C3’-OMe), 3.80 (3H, s, C4’-OMe), 4.75, 4.86 (1H, d, J=7.5 Hz, anomeric H), 6.51 (1H, d, J=2.4 Hz, H-8), 6.63 (1H, dd, J=2.7 Hz, J=8.3 Hz, H-6), 6.79 (1H, d, J=9.3 Hz, H-5'), 7.02 (1H, d, J=9.2 Hz, H-6'), 6.96 (1H, d, J=8.7 Hz, H-5); 13C-NMR (500 MHz, DMSO-d6) δ: 69.5 (C-2), 30.1 (C-3), 31.5 (C-4), 117.8 (C-4a), 132.1 (C-5), 108.6 (C-6), 156.6 (C-7), 103.9 (C-8), 155.2 (C-8a), 129.0 (C-1’), 148.1 (C-2'), 140.9 (C-3'), 153.9 (C-4'), 109.2 (C-5'), 122.5 (C-6'), 105.2 (C-1”), 74.4 (C-2"), 78.1 (C-3"), 71.2 (C-4"), 77.4 (C-5"), 62.4 (C-6"), 102.3 (C-1’’’), 73.5 (C-2’’’), 77.1 (C-3"'), 70.2 (C-4"'), 76.7 (C-5"'), 62.1 (C-6"'), 56.1 (C4’-OMe), 60.9 (C3’-OMe).
By comparing the above data with the literature [9], compound 3 was identified as 7,2'-dihydroxy-3',4'-dimethoxyisoflavan-7,2'-dioxo-β-D-g lucoside.
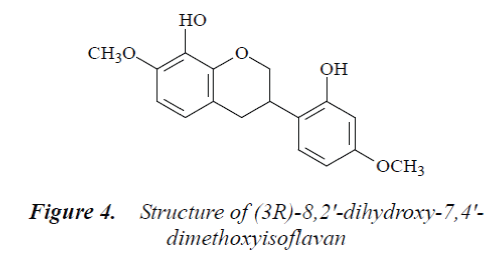
Compound 4: colorless needle crystals (methanol), m.p. 151°C, easily soluble in acetone and methanol. 1H-NMR (500 MHz, DMSO-d6) δ: 2.86 (1H, ddd, J=16.5Hz, J=5.2Hz, J=2.2Hz, H-4α), 2.92 (1H, dd, J=16.5 Hz, J=10.8 Hz, H-4β), 3.42 (1H, m, H-3), 3.91 (3H, s, C7-OMe), 3.88 (3H, s, C4’-OMe), 3.95 (1H, t, J=10.2 Hz, H-2α), 4.27 (1H, br d, J=10.5 Hz, H-2β), 6.50 (1H, d, J=2.1 Hz, H-3'), 6.44 (1H, dd, J=8.6 Hz, J=2.6 Hz, H-5'), 6.55 (1H, d, J=9.5 Hz, H-6), 6.89 (1H, d, J=9.3 Hz, H-5), 6.81 (1H, d, J=8.1 Hz, H-6'); 13C-NMR (500 MHz, DMSO-d6) δ: 71.0 (C-2), 31.9 (C-3), 31.5 (C-4), 115.9 (C-4a), 123.6 (C-5), 103.9 (C-6), 155.9 (C-7), 135.5 (C-8), 152.9 (C-8a), 121.6 (C-1'), 148.4 (C-2'), 103.5 (C-3'), 156.6 (C-4'), 109.1 (C-5'), 131.8 (C-6'), 57.2 (7-OMe), 61.2 (4'-OMe).
By comparing the above data with the literature [10], compound 4 was identified as (3R)-8,2'-dihydroxy-7,4'- dimethoxyisoflavan.
Inhibition of cell proliferation
Compared with the control group, HepG2 cell growth was inhibited to varying degrees in each experimental group, and the inhibitory effects exhibited apparent dose-effect and time-effect relationships, the longer the drug action, the stronger the inhibitory effect.
Cell cycle analysis
Flow cytometry with PI staining results indicated that 48 h after the action of different concentrations of Radix Astragali ethanol extracts on HepG2 cells, the number of S phase cells significantly reduced, resulting in the promotion of cell differentiation to G0-G1 and G2-M phases, however, with the increase of dose, the tumor cells mainly remained in the G2-M phase. After 48 h action of 150 mg/L Radix Astragali ethanol extract, the proportion of G2-M phase cells reached 28.43%.
Determination of apoptosis rate
Compared with the control group, 48 h after the action of different concentrations of Radix Astragali ethanol extracts on HepG2 cells, the apoptosis rate of HepG2 cells was significantly increased.
Discussion
Cell proliferation is an important life characteristic of living organisms; multicellular organisms produce new cells by cells division for replenishing the aged and dead cells in the body to maintain relative homeostasis of organisms. The balance of various regulatory factors in the cell cycle regulates the normal proliferation and differentiation of human cells. Functional and structural changes in certain regulatory factors can lead to cell cycle regulation disorder. Modern medicine has proved that an important cause for the genesis and development of tumors is abnormal gene regulation, which can lead to over-proliferation of cells or reduced apoptosis [11].
At present, cancers are treated mainly by surgery, radiotherapy and chemotherapy, of which surgical treatment is the basic approach, while chemotherapy is an important approach for clinical treatment of cancers, which is used throughout the entire treatment course, especially in the comprehensive treatment emphasized in recent years, chemotherapy is used both before and after surgery. However, most chemotherapeutic drugs do not have specificity, which cannot selectively kill tumor cells, or distinguish between normal cells and tumor cells, resulting in extensive and serious adverse reactions during chemotherapy [12].
In recent years, with the gradual deepening of knowledge and research on traditional Chinese medicine, anti-cancer effect of traditional Chinese medicines has become a hot topic of research. Due to the advantages of traditional Chinese medicine such as multi-targets, selectivity and low toxicity, it plays an increasingly important role in the field of antineoplastic drugs. With the extension of research, the anti-tumor mechanisms of traditional Chinese medicine are also becoming increasingly clear. One mechanism is by direct killing of tumor cells; the other is by improving immunity and enhancing resistance to tumor invasion through tonic effect.
Radix Astragali has the effects of tonifying qi and lifting yang, consolidating superficies and arresting sweating, dispelling toxins and promoting skin regeneration, which is generally used in the study of treatment of malignancies such as liver cancer, stomach cancer and leukemia [13-16].
In this paper, four compounds were isolated from the ethyl acetate and n-butanol fractions of Radix Astragali ethanol extract, which were structurally identified as astragaloside, uridine, 7,2'-dihydroxy-3',4'-dimethoxyisoflavan- 7,2'-dioxo-β-D- glucoside and (3R)-8,2'-dihydroxy- 7,4'-dimethoxyisoflavan, respectively. Meanwhile, the proliferation inhibitory effect of ethyl acetate fraction of Radix Astragali ethanol extract on HepG2 cells was studied. MTT assay was used to determine the inhibitory effect of Radix Astragali ethanol extract on HepG2 cells, and the effects of Radix Astragali ethanol extract on cell cycle and apoptosis rate of HepG2 cells were determined using flow cytometry.
The results showed that compared with the control group, HepG2 cell growth was inhibited to varying degrees in each experimental group, and the inhibitory effects exhibited apparent dose- and time-effect relationships, the longer the drug action, the stronger the inhibitory effect. 48 h after the action of different concentrations of Radix Astragali ethanol extracts on HepG2 cells, the number of S phase cells significantly reduced, resulting in the promotion of cell differentiation to G0-G1 and G2-M phases, however, with the increase of dose, the tumor cells mainly remained in the G2-M phase, after 48 h action of 150 mg/L Radix Astragali ethanol extract, the proportion of G2-M phase cells reached 28.43%. Flow cytometry found that compared with the control group, 48 h after the action of different concentrations of Radix Astragali ethanol extracts on HepG2 cells, the apoptosis rate of HepG2 cells could be significantly increased.
References
- Hand book of Flora Medicine Effeetive Constituents. Beijing: press of People’s Health 1986: 586-589.
- William BS, Siram C. The nuelear magnetic resonance spectra of some l,4-disubstituted naphthalenes. J. Phys. Chem 1996; 70: 3505-3509.
- Duan P, Wang ZM. Clinical Study on Effect of Astragalus in Efficacy Enhancing and Toxicity Reducing of Chemotherapy in Patients of Malignant Tumor. Chinese Journal of Integrated Traditional and Western Medicine 2002; 22: 515-517.
- Zhu Y, Liu XX, Mao YL. Effects of Astragalus injection on NK cells and T lymphocyte subsets of patients with middle or advanced primary hepatocellular carcinoma. Guangxi Medical Journal 2002; 2: 252-253.
- Yang Y, Song SG, Chen MZ. Effects of the total extract of astragalosides on the growth, AFP secretion and γ-GT activity of human hepatoma cells. Chinese Journal of Clinical Pharmacology and Therapeutics 2001; 6: 18-20.
- Hirotam M. Astragalosides from hairy root cultures of Astragalus membra- naceus. Phytochem 1994; 36: 665-670.
- Jones AJ, Grant DM, Winkley MW, Robins RK. Carbon- 13 magnetic resonance. XVII. Pyrimidine and purine nucleosides. Journal of the American Chemical Society 1970; 92: 4079-4087.
- Gatlin L, Davis JC. Comparison of ribose and deoxyribose nucleosides by NMR and deductions regarding ribose and deoxyribose nucleic acids. I. Tautomeric form. J. Am. Chem. Soc 1962; 84: 4464-4470.
- Subarnas A, Oshima Y, Hikino H. Isoflavans and pterocarpan from Astragalus mongholicus. Phytochem 1962; 30: 1777-1780.
- Song CQ, Zheng ZR, Liu D. Antimicrobial isoflavans from Astragalus membranaceus (Fisch.) Bunge. Acta Botanica Sinica 1997; 39: 486-488.
- Zheng DX. Apoptosis (Programmed Cell Death). New Developments in Cell Biology. Shanghai: Shanghai Scientific & Technical Publishers 1994: 81-82.
- TutoroDiaz DM, Ramirez M. Loss of heterozygoslty of P16 correltes with minimal residual disease at the end of the induction therapy in non-high riske childhood B-cell precursor acute lymphoblastic leukemia. LeukRes 2002; 26: 817-820.
- Xie SR, Shen H, Zhao S, Zhang GX. Growth inhibiting and promoting apoptosis of astragalus polysaccharides on gastric cancer cell. Hebei Journal of Traditional Chinese Medicine 2009; 31: 1373-1378.
- Yang Y, Hou JS, Chi HJ, Fan CY, Han M. Experimental study on the intervention effect of Astragalus membranaceus on airway inflammation in rats with chronic obstructive pulmonary disease. Shandong Medical Journal 2010; 50: 49-51.
- Liu XL, Wang ZY. The effect of huangqi injection on cell growth and NF-kB protein expression of hepatocellular carcinoma cells HepG2. Chinese Journal of General Surgery 2011; 20:. 775-778.
- Jiang Y, Li DM, Chen M. Effects of Astragalus polysaccharides on airway inflammation and interleukin-3 expression in asthmatic rats. China Journal of Modern Medicine 2009, 19: 1779-1781.