ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
- Biomedical Research (2014) Volume 25, Issue 4
Statins in Chronic Hepatitis C: Stage result.
“Lucian Blaga” University of Sibiu, Faculty of Medicine, Romania
- *Corresponding Author:
- Romeo-Gabriel Mihăilă
Universitatea “Lucian Blaga” din Sibiu
Facultatea de Medicină
Str Lucian Blaga, nr 2B, 550169, Sibiu
Romania
Accepted July 27 2014
There are interactions between lipid metabolism and hepatitis C virus (HCV) which are still in study. The response to standard double or triple drug combination in patients with chronic hepatitis C (CHC) is not ideal. This review focuses on the possibility of associating a statin to the treatment of these patients to improve outcomes. Statins inhibit not only 3- hydroxy-3-methyl-glutaryl-CoA reductase activity, but also the production of some farnesylated and geranyl-geranylated compounds, involved in signal transduction pathways and in HCV replication. Their effect is synergistic with that of interferon. Based on promising experimental studies, statins have been applied alone or in combination with standard therapy in several clinical trials. Their results range from no antivirus effect to a significant increase of the rate of sustained virological response (SVR). Statins are, in general, safe drugs. However, sometimes they can cause adverse effects, especially in liver, muscle or kidney. Only few patients develop an increase of aminotrasferases; this is believed to be related to lipid lowering, which is class characteristic, transient and often dose-related. The real liver toxicity of statins is rare. It is widely accepted today that statins are relatively safe drugs for the treatment of CHC, and as adjuvant, it is hoped that they will improve the treatment response.
Keywords
Chronic hepatitis C, fluvastatin, hepatitis C virus, statins, sustained virological response
Introduction
More than 10 years ago, administering a statin to a patient with liver cytolysis was forbidden because it was believed that statins may dangerously increase the serum level of aminotranferases. They inhibit not only 3-hydroxy-3- methyl-glutaryl-CoA reductase activity, and, finally, cholesterol synthesis, but also other products involved in intra-cellular signal transduction. In addition, they have antioxidant action. Due to these effects, subsequently they have been proved be beneficial in nonalcoholic steatohepatitis (NASH). Clinical trials with statins in NASH have shown a decrease in serum aminotransferases level and reducing fatty liver, a fact demonstrated imagistically [1]. Statins are the only lipid-lowering drugs able to reduce cardiovascular risk of patients with nonalcoholic fatty liver disease [2]. While in NASH there is a direct correlation between oxidative stress (measured by plasma oxysterol level) and low-density lipoprotein-cholesterol (LDL-cholesterol), in chronic hepatitis C (CHC) an inverse correlation between the high levels of this oxidative stress marker and low LDL-cholesterol was shown [3].
Interactions between hepatitis C virus (HCV) and hepatocyte lipid metabolism are still ongoing studies. It is believed that lipids play key roles in the HCV life cycle [4]. In a recent experimental study, HCV increased the level of reactive oxygen species and decreased the ratio between nicotinamide adenine dinucleotide (NAD+) and NADH. It also inhibited the silent information regulator 1 (SIRT1) and finally upregulated the genes involved in fatty acid synthesis and downregulated the genes involved in fatty acid β-oxidation. A SIRT1 activator was able to decrease cholesterol and triacylglycerol synthesis and HCV replication [5]. Low lipid levels seen in HCV infection correlate with resistance to interferon therapy [4]. The interaction between low serum cholesterol levels and IL-28B rs12979860 T/T genotype is a predictor of unfavorable evolution in chronic HCV patients with normal aminotransferases [6]. Previous experimental studies have found that some statins inhibit HCV replication. This effect is due to the inhibition of prenylation and geranylgeranylation processes [7,8]. and was synergistic in combination with interferon [7].
The purpose of this review is to summarize the results of experimental and clinical studies that used statins in infection with hepatitis C virus in order to draw conclusions on their effectiveness. Articles in PubMed March 06th, 2013 have been taken into consideration. The terms of search were: chronic hepatitis C + statin, atorvastatin, fluvastatin, lovastatin, pitavastatin, rosuvastatin, simvastatin. 41 articles were taken into consideration, 5 of them excluded because the summary was not available.
Experimental researches
A study made in a genome-length HCV RNA replication system (OR6) established that fluvastatin had the strongest anti-HCV activity and its combination with interferon was synergistic on HCV RNA replication. Atorvastatin and simvastatin had moderate inhibitory effects, while lovastatin was less efficient and pravastatin had no anti- HCV activity [7,8]. Another study made in OR6 system also showed that statins (atorvastatin, fluvastatin, lovastatin, pitavastatin, and simvastatin) in combination with interferon enhanced its anti-HCV effect [8].
The combination of fluvastatin or interferon-α or β with β-carotene, linoleic acid or vitamin D2 had an additive effect on the inhibition of HCV RNA replication, in the same OR6 system. Alone, vitamin E increased HCV RNA replication, but did not inhibit the antivirus effect of fluvastatin or interferon, unlike the effect of the other three nutrients [9].
Polyunsaturated endoplasmic reticulum liposomes (PERL) were able to reduce the cellular cholesterol load and thus had anti-HCV activity, with decreased viral secretion, in vitro. The lowering of plasma membrane cholesterol reduced the cellular infectivity of HCV. In addition, there was a competition between PERL and cholesterol for binding to cellular receptors [10].
Clinical trials
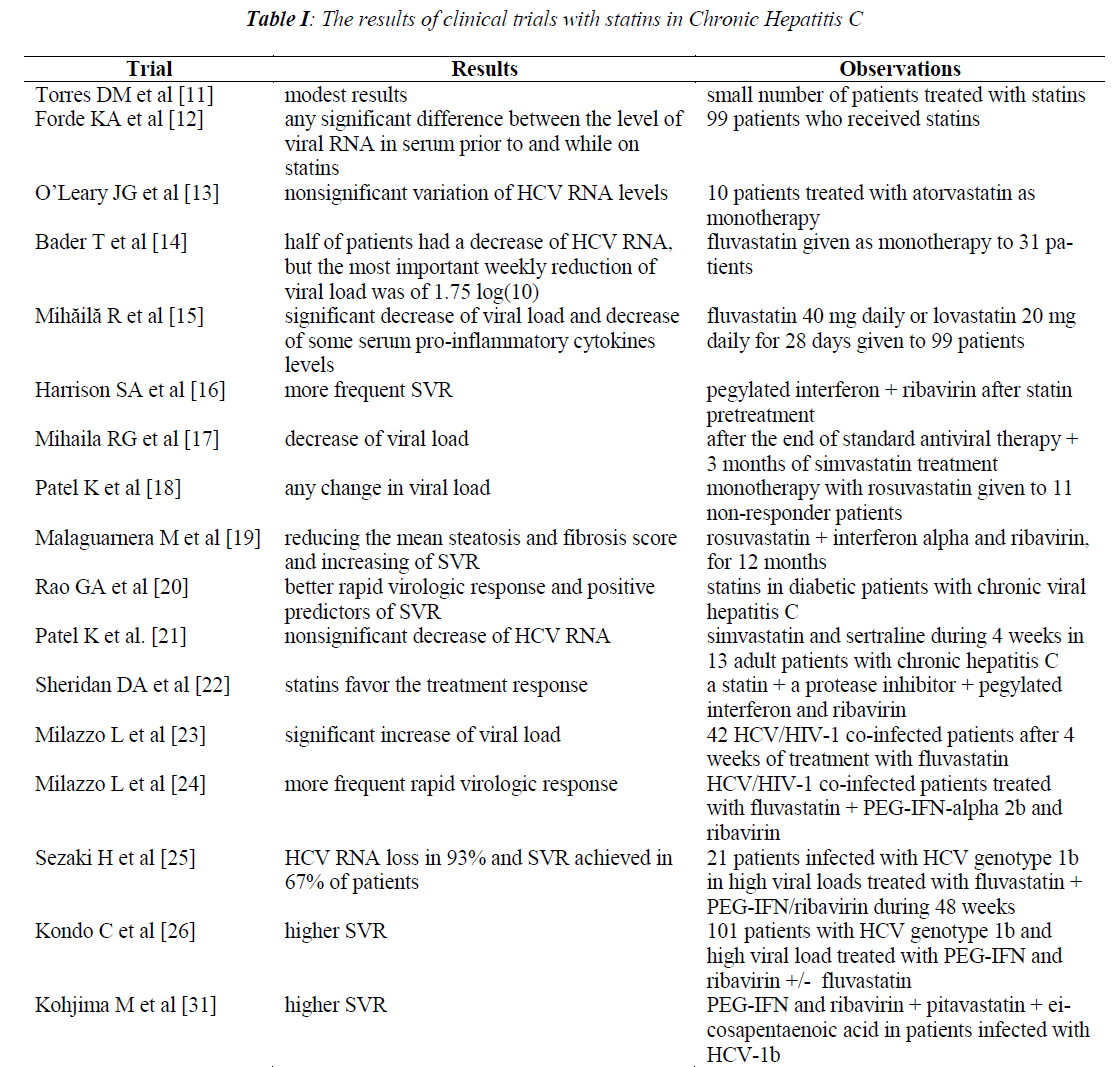
The first prospective trial which used statins for the treatment of a heterogenous and small number of patients with CHC had modest beneficial results, in 2007 [11].
In a study made in Philadelphia on dyslipidemic patients infected with HCV there was no significant difference between serum viral RNA level prior to and while on statins, but the number of patients was only 99 (excluding the witnesses) [12].
In a pilot study, 10 patients infected with HCV were treated with a daily dose of 20 mg atorvastatin, as monotherapy. The HCV RNA levels did not vary significantly after 4 and 12 weeks of treatment, comparing with baseline [13].
Fluvastatin was given as monotherapy to a group of 31 patients with viral hepatitis C while HCV RNA and liver tests were repeated weekly. Half of the patients had a decrease of HCV RNA under a daily dose of 80 mg or less. 82% of them showed the first drop within four weeks. Then, viremia remained constant for 2-5 weeks to the majority of those who responded. The most important weekly reduction of viral load was of 1.75 log [10]. Only 22% of the patients had a continuous decrease of viral load until the end of the study. This treatment was safe, but statins could not produce a marked and persistent decrease of viral load, so that they could be tested in future studies as adjunctive therapy along with antiviral medication [14].
In a multicenter clinical trial, 99 patients with CHC received fluvastatin 40 mg daily or lovastatin 20 mg daily for 28 days. There was a significant decrease of viral load after both drugs comparing with baseline, more important after the fluvastatin treatment (p=0.00092). Both drugs also induced a decrease of some serum pro-inflammatory cytokines levels (IL-6 and TNF-α) comparing with baseline. In addition, fluvastatin contributed to decreasing the serum IL-8 level. The serum level of IL-10, an antiinflammatory cytokine, was not influenced by any statin [15].
The sustained virologic response obtained after pegylated interferon + ribavirin treatment was significantly more frequent in CHC patients with baseline increased LDL levels, with low HDL levels or after statin pretreatment [16].
A significant decrease of viral load was observed in patients with CHC and liver cytolysis after the end of the standard antiviral therapy; these patients subsequently received 3 months of simvastatin treatment. The serum level of alkaline phosphatase and cholesterol also decreased after 1 and 2 months of simvastatin treatment. The viral load did not vary significantly in those without hepatic cytolysis treated or not treated with simvastatin [17]. Instead, monotherapy with rosuvastatin given to 11 non-responder patients with CHC did not produce any significant changes of viral load in serum or lipid fraction. The authors observed an inverse correlation between HCV RNA and HDL level, at baseline [18].
Rosuvastatin, in addition to interferon alpha and ribavirin, for 12 months, proved to be useful in a prospective, randomized trial with 65 CHC patients: it reduced the mean steatosis and fibrosis score and increased SVR (51% comparing to 18% of relapsers) [19].
A large clinical study was made on diabetic and nondiabetic patients with CHC in order to establish the predictors of sustained virological response (SVR) because these patients are likely to develop diabetes, which reduces their chances of getting the virus clearance (lowering the viremia). As expected, SVR was significantly less frequent in diabetic than in non-diabetic ones. The positive predictors of SVR in diabetic patients were: lower baseline viremia, higher level of low-density lipoprotein, and administration of statins which induced a better rapid virological response. Non-diabetic patients treated with statins also achieved better SVR. The negative predictors of SVR were insulin treatment and a high hemoglobin A1c level in patients treated with interferon, but not in those treated with metformin [20].
Because simvastatin and sertraline have synergistic activity against HCV replication in vitro, this combination was studied in adult patients with CHC who were not receiving simultaneous treatment with interferon. Simvastatin and sertraline in daily dose of 40 mg and, respectively 50 mg for 7 days, and then 80 mg and, respectively 100 mg for 3 weeks produced only a transient and small decrease of HCV RNA, which was not significant, although the combination was well tolerated. Only 13 patients completed this pilot study, so the findings should be viewed with caution [21].
The results of CHC treatment with pegylated interferon and ribavirin are not satisfactory, because the effectiveness is limited to about 55% of the patients. This mostly affects the patients with genotype 1 (only 40-50% of them responded). This is the reason why recently a protease inhibitor has been added to this combination. Unlike other host factors, the serum level of low-density lipoprotein cholesterol and statin use are important for the response to triple antiviral therapy. These findings are based on the interaction between HCV and hepatocyte lipid metabolism [22].
The serum LDL levels are considered to be a prognostic indicator for SVR in HCV patients treated with interferon. HCV-RNA was measured in 42 HCV/HIV-1 co-infected patients after 4 weeks of treatment with fluvastatin 80 mg/day or placebo. The viral load was significantly higher in fluvastatin treated patients comparing with baseline. As expected, the serum cholesterol and LDL levels the serum cholesterol and LDL levels decreased significantly. The authors explained this increase in viral load by the LDL receptor up-regulation induced by statins, required for HCV entry into hepatocytes, and a supposed increase of virus replication [23]. In another randomized study made on HCV/HIV-1 co-infected patients, fluvastatin in a daily dose of 80mg or no medication was added to PEGIFN- alpha 2b and ribavirin. Patients who received fluvastatin had a more frequent rapid virological response more frequent rapid virological response as compared to those treated only with standard therapy (p=0.02). Although SVR was more frequent in patients treated with fluvastatin compared to placebo, the difference was not significant (p=0.08). When we analyze the result, we must take into account the small number of patients who received fluvastatin (21 patients) or no medication (23 patients) [24].
In a pilot study made on 21 patients infected with HCV genotype 1b in high viral loads, the treatment with fluvastatin 20 mg/day given along with PEG-IFN/ribavirin during 48 weeks led to HCV RNA loss in 93% of the patients. SVR was achieved in 67% of those who were treated either 48 weeks or 72 weeks. [25] The results of a large trial made on 101 patients with HCV genotype 1b and high viral load who were treated with PEG-IFN and ribavirin +/- fluvastatin have been recently published. This statin did not influence a rapid virological response nor the end of treatment response, but the sustained virological response rate was significantly higher in patients who received fluvastatin (P=0.0422). The authors found that male and major genotype IL28B SNPs were only independent predictors of SVR in fluvastatin arm [26]. It has recently been showed that fluvastatin added to PEGIFN/ ribavirin therapy significantly reduced the relapse rate in patients with CHC infected with HCV genotype 1b and high viral load who obtained complete early virological response. The relapse of those who obtained late virological response was less frequent in fluvastatin arm, but the difference was not statistically significant. According to the multivariate analysis the absence of fluvastatin treatment and low total ribavirin dose were independent factors which contributed to relapse [27].
Statins in association with PEG-IFN and ribavirin may be useful for the treatment of non-genotype 1 patients, because no direct acting antiviral drug is available for them. Statins in combination with PEG-IFN, ribavirin and a protease or polymerase inhibitor could also contribute to improve SVR in genotype 1 patients [28].
An active new drug against HCV is telaprevir, a substratum for P-glycoprotein and cytochrome P450 3A4 and an inhibitor of cytochrome P450 3A4, that can increase serum atorvastatin concentration [29]. Its co-administration with atorvastatin or other substrate of renal or hepatic transporters should be avoided or carefully monitored [30].
Because eicosapentaenoic acid can suppress the expression of low-density lipoprotein receptor, that is necessary for the HCV entry in hepatocyte, this drug in combination with ribavirin and pitavastatin was added to PEG-IFN in patients infected with HCV-1b. This association induced a higher SVR as compared to patients who received only PEG-IFN and ribavirin therapy. In both treatment groups, patients with genotype TT of IL-28B had a higher SVR. The quadruple drug association contributed to the achievement of a higher SVR rate also in non-TT genotype patients. This result is a proof of the effectiveness of the combination between a statin and an inhibitor of the HCV penetration into hepatocytes, added to standard therapy [31].
In Table I there is a summary of the results of clinical trials with statins.
Safety
It is widely accepted that in general statins are safe drugs. However, sometimes they can cause adverse effects, especially in liver, muscle or kidney. Some researchers suspect that clinical trials did not include patients with potential risk of developing hepatotoxicity and thus their results are not superimposable with those in daily practice. An aged woman who was given fluvastatin in a daily dose of 80 mg for 6 weeks had a large increase of ALT, which disappeared 10 weeks after the drug interruption. The resumption of fluvastatin at a daily dose of 40 mg, then 80 mg for 2 months produced only a slight increase in ALT [32]. As the severity of adverse effects is dosedependent, in such situations re-treatment with the same drug in a lower dose may be a solution for some patients. For others the change of statin is effective. Statin is not always solely responsible for the observed adverse effects, as in the above case.
Studies on the safety of statin use have not shown any increase in transaminases or hepatotoxicity in patients with CHC who had been given statins for cardiovascular disease therapy as compared to those patients who had not been given statins, so those with stable CHC can use these drugs also for prophylaxis and treatment of cardiovascular suffering [33].
Statin administration for 1 year in dyslipidemic patients produced a moderate-severe increase in liver biochemistry, with an incidence comparable to dyslipidemic patients infected with HCV treated with statins. Severe adverse effects were rare [34].
A group of 17 patients with CHC currently treated with statins had no significant liver enzyme elevation. This observation dates back to 2005 [35]. The USA study of drug-induced liver injury reported in 2007 showed that statins given to patients with chronic liver diseases were safe [36].
A patient with hypobetalipoproteinaemia induced by chronic HCV infection, who had also untreated familial hypercholesterolaemia, was reported [37]. This adverse effect was not induced by statins, but, considering the interaction between HCV and lipid metabolism, it is an interesting case.
An adverse effect appeared during statin therapy and persisted after drug interruption was a hyaline inclusion myopathy. The patient had a history of increased alcohol intake, was treated for hepatitis C, and had a vigorous physical activity [38].
In a multicenter randomized study involving 326 hypercholesterolemic patients with compensated chronic liver disease (of which 23% had CHC), the treatment with pravastatin (80 mg/day) produced no increase in ALT greater than 2 times more frequently than those observed in placebo arm, so that the treatment was considered safe and well tolerated [39].
All lipid-lowering drugs, including statins, can produce a mild increase of aminotransferases, which is class characteristic, transient and often dose-related. Less than 3% of patients treated with lipid-lowering drugs develop an increase of serum ALT level of 3 times the upper limit of the normal value. It is believed that this effect is related to lipid lowering and it is not an expression of liver toxicity. Real liver toxicity is rare. In the literature there are 5 cases of liver failure related of lovastatin administration. There is no proof that monitoring of liver enzymes can reduce the adverse reactions that are the expression of liver toxicity [40].
Conclusions
The myth that statins are contraindicated in chronic liver disease belongs to the past.
Numerous studies have reported that statins given to patients with stable chronic liver diseases are generally well tolerated. Aminotransferases increase is mostly mild, probably due to lipid lowering and it is not an expression of liver toxicity, which occurs rarely.
The response to statin therapy is extremely diverse and the virological response to treatment of patients with CHC depends on many factors related to the host and the virus, that the various responses to different treatments (which include only statins or also statins) of small groups of patients is explicable. Many clinical trials included only a small number of patients, thus their conclusions must be interpreted with caution, for example those who tested the HCV/HIV-1 co-infected patients.
No all statins have anti-HCV activity. The best virological response in monotherapy was obtained with fluvastatin and the combination of some statins with PEG-IFN and ribavirin seems to be beneficial in terms of SVR. Drugs that inhibit HCV entry into hepatocytes in association with standard double or triple drug combination are worth to be tested in large clinical trials since the results of the first study of this type have been promising
Combinations of a statin with a drug for which experimental studies have argued for anti-HCV action are worth to be tested in combination with standard therapy since chronic HCV infection is a global public health problem and improving the SVR is a main objective for all hepatologists.
Acknowledgements
Apologies to researchers who have worked in this field and whose articles were not cited in the text due to the selective thematic.
References
- Congdon D. Clinical inquiries. Can patients with steatohepatitis take statins? J Family Practice 2006; 55: 905-906.
- Musso G, Anty R, Petta S. Antioxidant Therapy and Drugs Interfering with Lipid Metabolism: Could They Be Effective in NAFLD Patients? Curr Pharm Des 2013; 19: 5297-5313.
- Arciello M, Petta S, Leoni V, Iannucci G, Labbadia G, Cammà C, Craxì A, Balsano C. Inverse correla-tion between plasma oxysterol and LDL-cholesterol levels in hepatitis C virus-infected patients. Dig Liv- er Dis 2012; 44: 245-250.
- Bassendine MF, Sheridan DA, Bridge SH, Felmlee DJ, Neely RD. Lipids and HCV. Semin Immunopa- thol 2013; 35: 87-100.
- Sun LJ, Li SC, Zhao YH, Yu JW, Kang P, Yan BZ. Silent information regulator 1 inhibition induces li- pid metabolism disorders of hepatocytes and en-hances hepatitis C virus replication. Hepatol Res 2013 Feb 7. [Epub ahead of print]
- Fabris C, Falleti E, Cussigh A, Bitetto D, Fontanini E, Colletta C, Vandelli C, Cmet S, Ceriani E, Smirne C, Toniutto P, Pirisi M. The interleukin 28B rs12979860 C/T polymorphism and serum choles- terol as predictors of fibrosis progression in patients with chronic hepatitis C and persistently normal transaminases. J Med Virol 2012; 84: 747-755.
- Ikeda M, Abe K, Yamada M, Dansako H, Naka K, Kato N. Different anti-HCV profiles of statins and their potential for combination therapy with inter- feron. Hepatology 2006; 44: 117-125.
- Ikeda M, Kato N. Life style-related diseases of the digestive system: cell culture system for the screen-ing of anti-hepatitis C virus (HCV) reagents: sup- pression of HCV replication by statins and synergis- tic action with interferon. J Pharmacol Sci 2007; 105: 145-150.
- Yano M, Ikeda M, Abe K, Dansako H, Ohkoshi S, Aoyagi Y, Kato N. Comprehensive analysis of the effects of ordinary nutrients on hepatitis C virus RNA replication in cell culture. Antimicrob Agents Chemother 2007; 51: 2016-2027.
- Pollock S, Nichita NB, Böhmer A, Radulescu C, Dwek RA, Zitzmann N. Polyunsaturated liposomes are antiviral against hepatitis B and C viruses and HIV by decreasing cholesterol levels in infected cells. Proc Natl Acad Sci U S A 2010; 107: 17176- 17181.
- Torres DM, Harrison SA. HCV replication and statin pleotropism: an adjuvant treatment panacea? Am J Gastroenterol 2008; 103: 1390-1392.
- Forde KA, Law C, O'Flynn R, Kaplan DE. Do stat- ins reduce hepatitis C RNA titers during routine clinical use? World J Gastroenterol 2009; 15: 5020-5027.
- O'Leary JG, Chan JL, McMahon CM, Chung RT. Atorvastatin does not exhibit antiviral activity against HCV at conventional doses: a pilot clinical trial. Hepatology 2007; 45: 895-898.
- Bader T, Fazili J, Madhoun M, Aston C, Hughes D, Rizvi S, Seres K, Hasan M. Fluvastatin inhibits hep- atitis C replication in humans. Am J Gastroenterol 2008; 103: 1383-1389.
- Mihăilă R, Nedelcu L, Frăţilă O, Rezi EC, Domnariu C, Ciucă R, Zaharie AV, Olteanu A, Bera L, Deac M, Mihăilă R. Lovastatin and fluvastatin reduce vi- remia and the pro-inflammatory cytokines in the pa- tients with chronic hepatitis C. Hepatogastroenterol- ogy 2009; 56: 1704-1709.
- Harrison SA, Rossaro L, Hu KQ, Patel K, Tillmann H, Dhaliwal S, Torres DM, Koury K, Goteti VS, Noviello S, Brass CA, Albrecht JK, McHutchison JG, Sulkowski MS. Serum cholesterol and statin use predict virological response to peginterferon and ri- bavirin therapy. Hepatology 2010; 52: 864-874.
- Mihaila RG, Nedelcu L, Fratila O, Retzler L, Dom- nariu C, Cipaian RC, Rezi EC, Beca C, Deac M. Ef- fects of simvastatin in patients with viral chronic hepatitis C. Hepatogastroenterology 2011; 58: 1296-1300.
- Patel K, Jhaveri R, George J, Qiang G, Kenedi C, Brown K, Cates C, Zekry A, Tillmann HL, Elliott L, Kilaru R, Albrecht J, Conrad A, McHutchison JG.Open-label, ascending dose, prospective cohort study evaluating the antiviral efficacy of Rosuvas- tatin therapy in serum and lipid fractions in patients with chronic hepatitis C. J Viral Hepat 2011; 18: 331-337.
- Malaguarnera M, Vacante M, Russo C, Gargante MP, Giordano M, Bertino G, Neri S, Malaguarnera M, Galvano F, Li Volti G. Rosuvastatin reduces nonalcoholic fatty liver disease in patients with chronic hepatitis C treated with α-interferon and ri- bavirin: Rosuvastatin reduces NAFLD in HCV pa- tients. Hepat Mon 2011; 11: 92-98.
- Rao GA, Pandya PK. Statin therapy improves sus- tained virologic response among diabetic patients with chronic hepatitis C. Gastroenterology 2011; 140: 144-152.
- Patel K, Lim SG, Cheng CW, Lawitz E, Tillmann HL, Chopra N, Altmeyer R, Randle JC, McHutchi- son JG. Open-label phase 1b pilot study to assess the antiviral efficacy of simvastatin combined with ser- traline in chronic hepatitis C patients. Antivir Ther 2011; 16: 1341-1346.
- Sheridan DA, Neely RD, Bassendine MF. Hepatitis C virus and lipids in the era of direct acting antivi- rals (DAAs). Clin Res Hepatol Gastroenterol 2013; 37: 10-16.
- Milazzo L, Meroni L, Galazzi M, Cesari M, Ca- ramma I, Marchetti G, Galli M, Antinori S. Does fluvastatin favour HCV replication in vivo? A pilot study on HIV-HCV coinfected patients. J Viral Hepat 2009; 16: 479-484.
- Milazzo L, Caramma I, Mazzali C, Cesari M, Oli-vetti M, Galli M, Antinori S. Fluvastatin as an adju- vant to pegylated interferon and ribavirin in HIV/hepatitis C virus genotype 1 co-infected pa- tients: an open-label randomized controlled study. J Antimicrob Chemother 2010; 65: 735-740.
- Sezaki H, Suzuki F, Akuta N, Yatsuji H, Hosaka T, Kobayashi M, Suzuki Y, Arase Y, Ikeda K, Miya- kawa Y, Kumada H. An open pilot study exploring the efficacy of fluvastatin, pegylated interferon and ribavirin in patients with hepatitis C virus genotype 1b in high viral loads. Intervirology 2009; 52: 43-48.
- Kondo C, Atsukawa M, Tsubota A, Itokawa N, Fu- kuda T, Matsushita Y, Kidokoro H, Kobayashi T, Narahara Y, Nakatsuka K, Kanazawa H, Sakamoto C. An open-label randomized controlled study of pegylated interferon/ribavirin combination therapy for chronic hepatitis C with versus without fluvas- tatin. J Viral Hepat 2012; 19: 615-622.
- Atsukawa M, Tsubota A, Kondo C, Itokawa N, Na-rahara Y, Nakatsuka K, Hashimoto S, Fukuda T, Matsushita Y, Kidokoro H, Kobayashi T, Kanazawa H, Sakamoto C. Combination of fluvastatin with pe- gylated interferon/ribavirin therapy reduces viral re- lapse in chronic hepatitis C infected with HCV geno- type 1b. J Gastroenterol Hepatol 2013; 28: 51-56.
- Yoshida EM. Rosuvastatin and chronic hepatitis C. Hepat Mon 2011; 11: 384–385.
- Wilby KJ, Greanya ED, Ford JA, Yoshida EM, Par- tovi N. A review of drug interactions with bocepre-vir and telaprevir: implications for HIV and trans- plant patients. Ann Hepatol 2012; 11: 179-185.
- Kunze A, Huwyler J, Camenisch G, Gutmann H. Interaction of the antiviral drug telaprevir with renal and hepatic drug transporters. Biochem Pharmacol 2012; 84: 1096-1102.
- Kohjima M, Enjoji M, Yoshimoto T, Yada R, Fujino T, Aoyagi Y, Fukushima N, Fukuizumi K, Harada N, Yada M, Kato M, Kotoh K, Nakashima M, Sa- kamoto N, Tanaka Y, Nakamuta M. Add-on therapy of pitavastatin and eicosapentaenoic acid improves outcome of peginterferon plus ribavirin treatment for chronic hepatitis C. J Med Virol 2013; 85: 250-260.
- Chen YW, Lai HW, Wang TD. Marked elevation of liver transaminases after high-dose fluvastatin un- masks chronic hepatitis C: safety and re-challenge. Acta Neurol Taiwan 2007; 16: 163-167.
- Andrus MR, East J. Use of statins in patients with chronic hepatitis C. South Med J 2010; 103: 1018- 1022.
- Khorashadi S, Hasson NK, Cheung RC. Incidence of statin hepatotoxicity in patients with hepatitis C. Clin Gastroenterol Hepatol 2006; 4: 902-907.
- Gibson K, Rindone JP. Experience with statin use in patients with chronic hepatitis C infection. Am J Cardiol 2005; 96: 1278-1279.
- Norris W, Paredes AH, Lewis JH. Drug-induced liver injury in 2007. Curr Opin Gastroenterol. 2008; 24: 287-297.
- Bima AI, Hooper AJ, van Bockxmeer FM, Burnett JR. Hypobetalipoproteinaemia secondary to chronic hepatitis C virus infection in a patient with familial hypercholesterolaemia. Ann Clin Biochem 2009; 46:420-422.
- Supala-Berger A, Fine E, Heffner R, Young-McLain E. Hyaline inclusion myopathy: unmasked by statin therapy. Muscle Nerve 2009; 40: 657-661.
- Lewis JH, Mortensen ME, Zweig S, Fusco MJ, Medoff JR, Belder R; Pravastatin in Chronic Liver Disease Study Investigators. Efficacy and safety of high-dose pravastatin in hypercholesterolemic pa- tients with well-compensated chronic liver disease: Results of a prospective, randomized, double-blind, placebo-controlled, multicenter trial. Hepatology 2007; 46: 1453-1463.
- Tolman KG. Defining patient risks from expanded preventive therapies. Am J Cardiol 2000; 85: 15E- 19E.