ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
- Biomedical Research (2008) Volume 19, Issue 3
Role of tocotrienol-rich palm vitamin E on pregnancy and preim-plantation embryos in nicotine-treated rats
Norfilza M Mokhtar*1, Mohd Hamim Rajikins2, Zaiton Zakaria1
1Department of Physiology, UKM Medical Centre, Universiti Kebangsaan Malaysia, Jalan Raja Muda Abdul Aziz, 50300 Kuala Lumpur, Malaysia
2Faculty of Medicine, Universiti Technologi MARA, 40450 Shah Alam, Selangor, Malaysia
- *Corresponding Author:
- Norfilza Mohd Mokhtar
Department of Physiology
Universiti Kebangsaan Malaysia
Jalan Raja Muda Abdul Aziz
50300 Kuala Lumpur, Malaysia
Phone: 006 -03 9289 7846, e-mail: norfilza@yahoo.co.uk
Accepted date: June 17 2008
The present study observed the effects of palm vitamin E (PVE) on pregnancy and embryo development in the nicotine-treated rat model. Sprague Dawley rats weighing 160 - 240 g (aged 3 – 6 months) were divided into four groups. Group A (control group), Group B had nicotine (5 mg/kg in 0.2 ml corn oil) sc/day. Animals of Group C received nicotine concur-rently with PVE at a dose of 60 mg/kg orally/day and Group D had PVE alone. To study the embryonic development, immature rats were superovulated following an identical treatment schedules as stated above. Nicotine treatment during pregnancy (from day 1 pc until term) reduced the pregnancy out-come to 33.3% whereas oral supplementation with PVE in nicotine-treated rats increased the percentage of pregnancy outcome to 83.3%. It was moreover found that 25.68 % em-bryos developed into 2- and 4-cell stage in the nicotine plus PVE-treated animals. In conclu-sion, PVE, an antioxidant, is found to be beneficial in neutralizing the nicotine-related ad-verse impact on female reproduction.
Keywords
Palm vitamin E, nicotine, embryo development, pregnancy, rat
Introduction
Female reproductive system is constantly exposed to mul-tiple deleterious factors including nicotine exposure. Among the 3,500 chemical substances, nicotine being an addictive agent retards fetal development, causes low birth weight and delays parturition [1-3]. In addition, ad-ministration of nicotine for 5 consecutive days in preg-nant rats could alter the rate of embryo proliferation, de-lays implantation and delivery process [4]. Nicotine when present in higher concentrations in oocytes cultured in vitro, causes perturbation in the first and second meiotic division [5].
The mechanism by which the nicotine could damage tis-sues is related to its effects on gamete cells’ viability or change in the oviductal epithelial function [6]. Earlier reports showed that nicotine could act as free radicals. These free radicals could damage the polyunsaturated fatty acids that are present in the cell membrane or as side-chains in certain chemical species [7]. Furthermore, administration of nicotine in rats leads to higher lipid per-oxidation with subsequent decrease in antioxidant en-zymes [8]. Lipid peroxidation could lead to a variety of toxicological effects such as impaired mitochondrial functions and inhibition of antioxidant enzymes. The end product of this process can be measured by using malondialdehyde (MDA) [9] To counter this condition, the effect of antioxidant such as vitamin E has been high-lighted. Vitamin E is known for its antioxidant properties since its discovery in 1922 [10]. Among all the compo-nents of vitamin E, α-tocopherol is widely reported to have the highest biological activities [11,12]. Recently, γ-tocotrienol has provoked many researchers to explore its beneficial effects in various human diseases [13].
A population study on the hypercholesterolemic patients treated with palmvitee (tocotrienol-rich fraction of palm oil capsule) demonstrates a significant reduction in total cholesterol (10%), low-density cholesterol (13%) and Apo B (7%) [14]. The significance of vitamin E in male reproduction has been emphasized when its deficiency is found to cause testicular degeneration in rats [15]. Evi-dences have therefore provided a new incentive to inves-tigate the role of palm vitamin E in combating the radical-driven oxidative events.
Aim of the present study was to investigate the possible protective profile of tocotrienol-rich palm vitamin E (PVE) on the embryo development and pregnancy out-come in nicotine-treated rats.
Materials and Methods
The experimental protocol was approved by the Universiti Kebangsaan Malaysia Animal Care and Use Committee (UKMACUC) and was conducted at the Animal Biotech-nology Laboratory, Department of Physiology, Universiti Kebangsaan Malaysia (UKM).
Twenty-four fertile female Sprague Dawley rats weighing 160 – 240 g (aged 3 – 6 months) were obtained from UKM animal unit. Animals were kept in the animal house at 25 – 30oC and had free access to rat chow and drinking water. Animals were divided into four groups of 6 rats in each. Fertile male rats were caged together for the pur-pose of mating.
Palm vitamin E (PVE) was a complimentary gift from Mr. Gapor Mat Top, Malaysian Palm Oil Board. PVE per 100 g contained: α-tocotrienol (17.3%), γ-tocotrienol (21.6%), δ-tocotrienol (15.3%), α-tocopherol (18.9%) and palm olein (26.9%). PVE was diluted with tocopherol-stripped corn oil (ICN USA) to obtain the desired concen-tration of 500 mg/kg rat weight. The dispensing volume was 0.2 ml.
Nicotine N-3876 (SIGMA chemical company, USA) was prepared weekly at a concentration of 20 mg/ml saline and stored away from light.
Effects of PVE on pregnancy outcome in rats treated with nicotine
Pregnant females were treated daily from day 1 pc until term. Animals of Group A (control) received 0.5 ml 0.9% sodium chloride (sc) and 0.2 ml tocopherol-stripped corn oil orally. Animals of Group B received nicotine [5 mg/kg body weight (sc)] concurrently with 0.2 ml tocopherol-stripped corn oil orally. Group C had nicotine [5 mg/kg body weight (sc)] and PVE at 60 mg/kg body weight oral-ly. Group D received 0.5 ml 0.9% sodium chloride (sc) and PVE at a dose of 60 mg/kg body weight orally. Vaginal smears were taken daily and subjected for Schorr’s staining. Sperm-positive vaginal smear was counted as day 0 of pregnancy. Upon delivery, anthropomet-ric measurements were performed on each pup.
Effects of PVE on embryo development in rats treated with nicotine
The distribution of animals groups was the same as de-scribed in the previous experiment. Immature female rats were 9-12 weeks old and weighing between 120-140g. Treatments were administered for 30 consecutive days. Animals were superovulated by an intraperitoneal injec-tion of 150 IU/kg body weight of PMSG and 48h later by another injection (sc) of hCG (75 IU/kg body weight). Animals were then mated with fertile males and sacrificed at 48 h post mating. The embryos were flushed from the fallopian tubes and examined under a dissecting micro-scope.
Venous blood samples were taken from the orbital sinus after the pregnant rats had delivered. Plasma levels of malondialdehyde (MDA) were measured as an indicator of lipid peroxidation [16]. In addition, vitamin E in plas-ma was also measured [17]. Cotinine level in urine was determined using a spectrophotometer according to the published protocol [18].
Values are given as median and the comparisons were analyzed by non-parametric Kruskal-Wallis analysis of variance on rank. Probability levels of less than 0.05 were taken as statistical significance.
Results
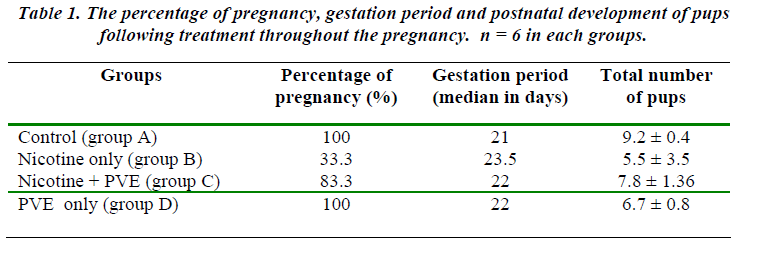
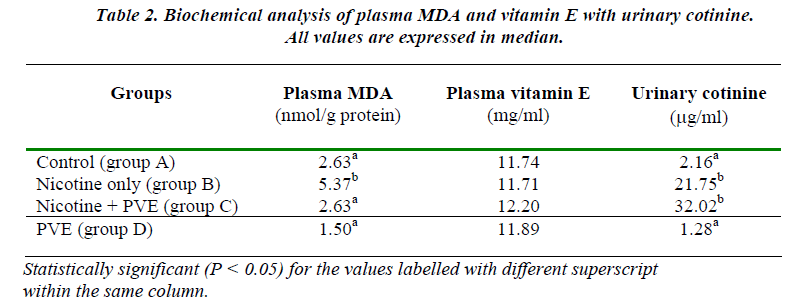
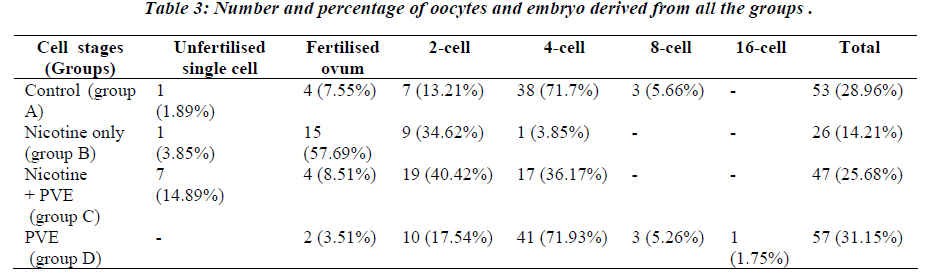
Nicotine treatment until term reduced the rate of preg-nancy outcome to 33.3% (Table 1). The pregnants (2/6) had longer duration of gestation (median 23.5 days) com-pared to controls (median 21.7 days). The harmful effect of nicotine as viewed by the lowest number of embryos survived in Group B which was 26 out of 183 (14.21%) [Table 3]. The extended length of gestation in the nico-tine-primed rats could possibly be due to delayed embryo cleavage in which 61.54% of retrieved embryos remained uncleaved. The excretion of cotinine as the metabolite of nicotine in the urine in Group B animals was found to be significantly higher compared to the controls (p<0.05) [Table 2]. The MDA levels were similarly recorded to be higher in Group B animals (P<0.05) [Table 2].
Supplementation of PVE in Group C rats improved the rate of pregnancy to 83.3% with the gestation length of median 22 days (Table 1). The number of retrieved em-bryos accounted for 47 out of 183 (25.68%). However, 76.56% of them were found to be in 2-cell and 4-cell stage (Table 3). The benefit of PVE supplementation was more obvious in Group D animals, 100% of them became pregnant with the highest number of retrieved embryos (57 out of 183). Furthermore, all the embryos obtained in this group were fertilized and cleaved. Ninety percent of them was found to be in 2-cell and 4-cell embryos and 7% had reached 8-cell and 16-cell stage.
Discussion
Present study particularly concentrated on the factors that could affect female reproduction. The idea was to investi-gate the effects of nicotine, a component of cigarette smoke, on pregnancy outcome. In addition, the effect of nicotine on pre-implantation embryo development had been programmed in order to observe whether the nico-tine could perturb the process of embryogenesis by free radical-mediated oxidation [7]. Many epidemiological studies have documented the negative outcomes between maternal smoking and pregnancy and the long-term ef-fects of nicotine dependence to the child’s behavior [2]. Despite all these known risks of smoking, there are still high percentage of women smoke during pregnancy [3]. Vitamin E acts as an antioxidant thereby protects the cell membranes by inactivating free radicals [12]. A study on thirteen healthy smokers who received α-tocopherol sup-plementation for two weeks, showed a significantly re-duction of lipid peroxidation [19].
It is evident from our preliminary study that nicotine treatment on first 7 days of pregnancy does not exert any remarkable impact on pregnancy in rats. However, nico-tine treatment from day 1 of pregnancy till term caused significant pregnancy wastage, only 33.3% of pregnancy continued until term. These findings correlate with the lesser number of embryos as retrieved (14.21%) from the superovulated rats that had nicotine treatment for 30 con-secutive days. It has moreover been documented that the nicotine exposure (5 mg/kg body weight) for 4 consecu-tive days produced less number of blastocyst as compared to control [20]. Subsequently, in another study the num-ber of collected oocytes for cytogenetic analysis is found to be lower among smokers [21]. Mice exposed to the cigarette smoke showed low fertilization and embryo de-velopment rate compared to the non-exposed animals (p<0.05) [12]. This effect is most likely to be nicotine related because nicotine could be detected in the uterine fluid and also around the blastocyst [22].
Another obvious parameter affected by nicotine admini-stration is the gestation length. Rats treated with nicotine throughout the duration of pregnancy showed a slightly longer gestation period (median 23.5 days) compared to controls (median 21.7 days). This extended length of gestation could possibly be the consequence of delayed em-bryogenesis, because forty-eight hours following su-perovulation, the embryos are supposed to attain a 4-cell stage.
In control animals (Group A) the number of retrieved em-bryos at 4-cell stage was 71.7%. However, nicotine treat-ment (Group B) remarkably attenuated the rate of embryo cleavage, 61.54% retrieved embryos remained as a single cell (not cleaved). A previous study also documented that nicotine injection for 5 consecutive days resulted in de-layed blastocyst hatching and loss of zona pellucida with delayed implantation [23]. Results of our study therefore conclude that PVE with a higher content of tocotrienol [24] is able to reverse the nicotine-induced retarded em-bryogenesis and consequently pregnancy loss in rats.
Acknowledgements
The authors wish to acknowledge with thanks for grant from Dr Ranjeet Bhagwan Singh (D/29/2000) foundation provided to use by the National Science Academy, Ma-laysia and also the Malaysian Palm Oil Board for the sup-ply of the vitamin E.
Also the valuable suggestion of Professor Amar Chatter-jee during preparation of this manuscript is highly appreciated
References
- Weisberg E, Smoking and reproductive health, Clin Reprod Fertil 1985; 3: 175-186.
- Hellstrom-Lindahl E, Nordberg A. Smoking during pregnancy: a way to transfer the addiction to the next generation?, Respiration 2002; 69: 289-293.
- Cooper AR, Moley KH. Maternal tobacco use and its preimplantation effects on fertility: more reasons to stop smoking, Semin Reprod Med 2008; 26: 204-212.
- Card JP, Mitchell JA. The effects of nicotine on im-plantation in the rat, Biol Reprod 1979; 20: 532-539.
- Racowsky C, Hendricks RC, Baldwin KV. Direct ef-fects of nicotine on the meiotic maturation of hamster oocytes, Reprod Toxicol 1989; 3: 13-21.
- Talbot P, Riveles K. Smoking and reproduction: the oviduct as a target of cigarette smoke, Reprod Biol En-docrinol 2005; 3: 52.
- Niki E, Yoshida Y, Saito Y, Noguchi N. Lipid peroxi-dation: mechanisms, inhibition, and biological effects, Biochem Biophys Res Commun 2005; 338: 668-676.
- Ashakumary L, Vijayammal PL. Effect of nicotine on antioxidant defence mechanisms in rats fed a high-fat diet, Pharmacology 1996; 52: 153-158.
- Gutteridge JM, Halliwell B. Free radicals and antioxi-dants in the year 2000. A historical look to the future, Ann N Y Acad Sci 2000; 899: 136-147.
- Sen CK, Khanna S, Roy S. Tocotrienol: the natural vitamin E to defend the nervous system?, Ann N Y Acad Sci 2004; 1031: 127-142.
- Brigelius-Flohe R, Traber MG. Vitamin E: function and metabolism, Faseb J 1999; 13: 1145-1155.
- Hassa H, Gurer F, Tanir HM, Kaya M, Gunduz NB, Sariboyaci AE, Bal C. Effect of cigarette smoke and alpha-tocopherol (vitamin E) on fertilization, cleavage, and embryo development rates in mice: an experimen-tal in vitro fertilization mice model study, Eur J Obstet Gynecol Reprod Biol 2007; 135: 177-182.
- Sen CK, Khanna S, Roy S. Tocotrienols in health and disease: the other half of the natural vitamin E family, Mol Aspects Med 2007; 28: 692-728.
- Qureshi AA, Bradlow BA, Brace L, Manganello J, Pe-terson DM, Pearce BC, Wright JJ, Gapor A, Elson CE. Response of hypercholesterolemic subjects to admini-stration of tocotrienols, Lipids 1995; 30: 1171-1177.
- Mason KE. The first two decades of vitamin E, Fed Proc 1977; 36: 1906-1910.
- Ledwozyw A, Michalak J, Stepien A, Kadziolka A. The relationship between plasma triglycerides, choles-terol, total lipids and lipid peroxidation products during human atherosclerosis, Clin Chim Acta 1986; 155: 275-283.
- Arafa HM, Hamada FM, Elmazar MM, Nau H. Fully automated determination of selective retinoic acid re-ceptor ligands in mouse plasma and tissue by reversed-phase liquid chromatography coupled on-line with solid-phase extraction, J Chromatogr A 1996; 729: 125-136.
- Peach H, Ellard GA, Jenner PJ, Morris RW. A simple, inexpensive urine test of smoking, Thorax 1985; 40: 351-357.
- Hoshino E, Shariff R, Van Gossum A, Allard JP, Pich-ard C, Kurian R, Jeejeebhoy KN. Vitamin E sup-presses increased lipid peroxidation in cigarette smok-ers, JPEN J Parenter Enteral Nutr 1990; 14: 300-305.
- Mitchell JA, Hammer RE. Effects of nicotine on ovidu-cal blood flow and embryo development in the rat, J Reprod Fertil 1985; 74: 71-76.
- Zenzes MT. Smoking and reproduction: gene damage to human gametes and embryos, Hum Reprod Update 2000; 6: 122-131.
- Zenzes MT, Reed TE, Wang P, Klein J. Cotinine, a major metabolite of nicotine, is detectable in follicular fluids of passive smokers in in vitro fertilization ther-apy, Fertil Steril 1996; 66: 614-619.
- Hammer RE, Mitchell JA. Nicotine reduces embryo growth, delays implantation, and retards parturition in rats, Proc Soc Exp Biol Med 1979; 162: 333-336.
- Edem DO. Palm oil: biochemical, physiological, nutri-tional, hematological, and toxicological aspects: a re-view, Plant Foods Hum Nutr 2002; 57: 319-341.