ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
- Biomedical Research (2010) Volume 21, Issue 4
Role of lipid peroxidation, glutathione and antioxidant enzymes in H1N1 Influenza.
1Department of Biochemistry Belgaum Institute of Medical Sciences, Belgaum. Karnataka, India
2Department of Microbiology Belgaum Institute of Medical Sciences, Belgaum. Karnataka, India
3Department of Medicine Belgaum Institute of Medical Sciences, Belgaum. Karnataka, India
H1N1 influenza tends to cause high morbidity but low mortality (1% to 4%) Based on its wide spread, the World Health Organization (WHO) has declared the 2009 outbreak of the novel H1N1 flu a global pandemic. On April 27, 2009, health services in India were put on high alert to guard against the entry of the disease into India. Pathogenesis of an infection and treatment is a challenge to infection management worldwide. The aim is to study the pathogenesis of an infection and find out supplementary treatment. The study period was 15th August 2009 to 25th October 2009. In present study 30 positive cases of H1N1 influ-enza was included. 30 normal healthy persons as per IFCC guidelines were included as control subjects. In present study oxidative stress was assessed by estimating lipid peroxi-dation product in the form of thiobarbituric acid reactive substances [TBARS], antioxi-dants in the form of reduced glutathione and enzymatic antioxidants in the form of super-oxide dismutase [SOD] and glutathione peroxidase [GPx] of plasma. All the data were ana-lyzed by using the statistical package SPSS-10.0. Results are reported as mean plus/minus standard deviation. The plasma TBARS were significantly high but glutathione levels and SOD and GPx activity were significantly lowered in H1N1 influenza when compared with control subjects. The study show that increased LPO levels and decreased levels of glu-tathione and activity of antioxidant enzymes raise oxidative stress in H1N1 Influenza. Ele-vated oxidative stress may be playing a role in natural killer (NK) cell loss and play a role in pathogenesis of H1N1 influenza virus. The present study suggests that use of Tamiflu (oseltamivir) or Relenza (zanamivir) with supplementation of antioxidants can recovers H1N1 influenza earlier than only antiviral drugs therapy by increasing the levels of NK cells and help to protect H1N1 viral infection Key words: H1N1 Influenza, pathogenesis, oxidative stress, antio
Keywords
H1N1 Influenza, pathogenesis, oxidative stress, antioxidants
Introduction
Novel H1N1 influenza is a highly contagious acute infec-tion caused by the swine influenza A virus.[1] It is an acute respiratory viral infection that continues to pose endemic, zoonotic, and pandemic threats to human health, with significant morbidity and mortality. H1N1 influenza tends to cause high morbidity but low mortality (1% to 4%). It’s unusual for humans to catch swine flu, but occa-sional cases occur, usually in people who have contact with infected pigs.[2,3,4] Like other flu viruses, the swine flu virus changes its RNA as it spreads, giving rise to a number of sub types.[5,6]
H1N1 can be sub divided according to the two proteins found on the surface of the virus: H (hemagglutinin) and N (neuraminidase). All the influenza strains contain these two protein structure, however- they vary from strain to strain in terms of the structure of the proteins. Each strain is assigned H and N numbers based on how many protein
structures they contain. [4] The outbreak of what is popu-larly called swine flu this involves in a new H1N1 type. A influenza strain that’s a genetic combination of swine, avian and human influenza viruses.[1,5,6] Based on its wide spread, the World Health Organization (WHO) has declared the 2009 outbreak of the novel H1N1 flu a global pandemic.[4] On April 27, 2009, health services in India were put on high alert to guard against the entry of the disease into India.[7] The H1N1 viral strain impli-cated in 2009 flu pandemic among humans often is called ‘Swine flu’ because initial testing showed many of the virus were similar to influenza viruses normally occurring in North American swine.[8] But further research has shown that the outbreak is due to a new strain of H1N1 not previously reported in pigs.
The new swine flu strain has also been called by various names: Swine origin influenza A, Swine influenza A (H1N1), influenza A / California / H1N1, Swine origin influenza virus, North American flu and influenza A (H1N1).[4,5,6,9]
The 1918 flu pandemic in humans was associated with H1N1 and influenza appearing in pigs, this may reflect a zoonosis either from swine to humans or from humans to swine. Although it is not certain in which direction the virus was transferred, same evidence suggests that, in this case, pigs caught the disease from humans.[10]
The main route of transmission is through direct contact between infected animals. Influenza spreads between hu-mans through coughing or sneezing and people touching something with the virus on it and then touching their own nose or mouth [11].
Natural killer (NK) cells develop in the bone marrow from the common lymphoid progenitor’s cell and circu-late in the blood. NK cells are key effector cells in innate immunity and play a critical role in the first line of host defence against acute viral infections by directly destroy-ing infected cells without the need for prior antigen stimu-lation. NK cells can recognize and kill influenza virus-infected cells. Two mechanisms are involved in the pro-tective effects of NK cells against viral infections; cyto-kine production and cytotoxic activity.[12] NK cells are to defend the body against infection with viruses and other pathogens. The role of NK cells in influenza A virus infection is poorly understood.[5,6,9,11] Pathogenesis of H1N1 infection and its treatment is a challenge to infec-tion management worldwide.
This study was undertaken in a 740 bedded teaching hos-pital located at Belgaum, Karnataka (India) to find out the role of oxidative stress and antioxidants in pathogenesis of H1N1 influenza.
Subjects and Methods
Study design
The study was conducted in the department of Biochemis-try at Belgaum Institute of Medical Sciences, Belgaum. The study period was 15th August 2009 to 25th October 2009. The ethical committee of the institute permitted to collect the blood samples of H1N1 influenza patients for this study. In present study 30 positive cases of H1N1 influenza was included who were admitted in H1N1 ward (swine flu ward) of District Hospital, Belgaum.
All these cases were in between the age group of 5- 55 years. Consent was obtained from all enrolled patients as well as controls. 30 normal healthy persons as per IFCC (International Federation of Clinical Chemistry) guide-lines were included by excluding history of alcohol, smoking and any infection as control subjects.
Sample collection
The diagnosis of H1N1 influenza cases were done by De-partment of Neurovirology, National Institute of Mental Health and Neurosciences (NIMHANS) Bangalore. After confirmation of diagnosis, blood samples were collected from H1N1 influenza patients under sterile condition. Just before starting any treatment 5 ml blood was taken in plain bulb. Plasma was separated and used for the estima-tion of TBARS (Thiobarbituric acid reactive substances) and reduced glutathione. Erythrocytes were washed 4 times with 0.9% NaCl solution and used for estimation of SOD and GPx.
Analytical Methods
As described by Burge JA, TBARS were estimated in plasma employing MDA as a reference standard.[13] Re-duced glutathione estimation was done by end point reac-tion method.[14] SOD was estimated in RBC according to RANSOD method by RANDOX laboratories Ltd.[15] GPx were assessed in RBC by UV method based on that of Paglia and Valentine.[16] All these investigations were carried out on semiautoanalyzer (Erba) and spectropho-tometer (Erba).
Limitations of the study
In swine flue ward 30 H1N1 positive patients within age 5-55 years were admitted. Hence this study was subjected to 30 cases. The laboratory is equipped with semiautoana-lyzer; hence all investigations were carried out on semi-autoanalyzer. The methods used in this study were stan-dardised in our laboratory, hence investigations were done by these methods.
Statistical Analysis
All the measurements were carried out by using Microsoft office ‘Excel’ with windows 2003 operating system and all the data were analyzed by using the statistical package for social sciences (SPSS) version10.0. Results are re-ported as mean plus/minus standard deviation.
Results
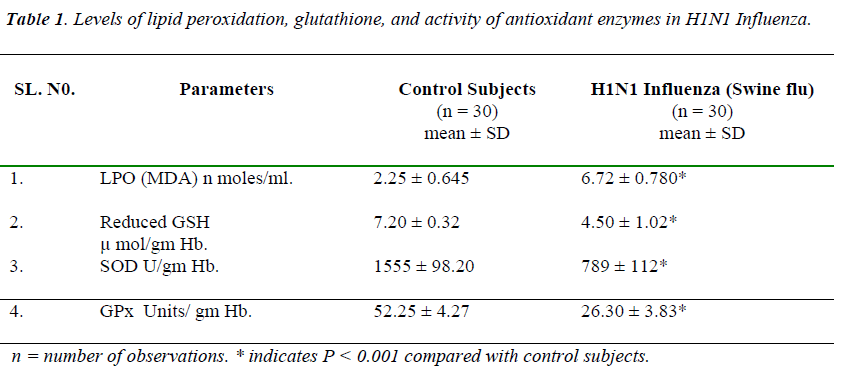
Table 1 shows in H1N1 influenza there was significantly increase in the plasma levels of TBARS on comparison with controls. Where as activity of erythrocyte SOD and GPx were significantly reduced, and levels of plasma re- duced glutathione were significantly lowered in H1N1 influenza.
Discussion
Influenza A virus is the major pathogen of humans caus-ing annual winter epidemics in the United States and has the potential to cause worldwide pandemics.[17]
MDA indicator of lipid peroxidation was significantly raised (6.72±0.780 MDA n moles/ml) in H1N1 influenza when compared with control subjects (2.25 ± 0.645 MDA n moles/ml).
The results of our study were similar to the study by Choi AM. Et al. They observed that increased lipid peroxida-tion in H1N1influenza and this is responsible for raised oxidative stress. Enhanced lipid peroxidation may occur as a result of the fact that naturally occurring scavenging mechanisms are suppressed and free radical generation processes are enhanced [18]
Natural killer (NK) cells are important effector cells in the innate immune response against infection and protect us from viruses during new infection. NK cells will encoun-ter influenza viruses and are important for host immunity during influenza infection. These cells can recognize and kill influenza virus-infected cells.[4,3,2,19] In H1N1 in-fection the increased free radicals may attack on the Natu-ral killer cells and decreases the mechanism of cytokine production and cytotoxic activity. This may induce marked apoptosis of NK cells. This is supported by the findings of Huawei Mao et. al., they states that influenza virus infection induced a marked apoptosis of NK cells which contributed to reduced NK cell cytotoxicity.[20] Thus oxidative stress may be responsible for contribution to H1N1 pathogenesis. This hypothesis is supported by Choi A.M. et al; he states that pathogenesis of influenza A virus of the lung is in part mediated by oxidative stress [21]
In H1N1 Influenza reduced glutathione levels were sig-nificantly lowered (4.50 ± 1.02 μ mol/gm Hb) when com-pared with control subjects (7.20 ± 0.32 μ mol/gm Hb). In H1N1 infection there may be inhibition of either alpha glutamylcystine synthase or glutathione synthase. Thus rate of synthesis of glutathione might be decreased; this decreased synthesis may be responsible for the lowered levels of glutathione and increased utilization of glu-tathione to detoxify the free radicals, can be responsible for the lowered levels of reduced glutathione.
Enzymatic antioxidant status was studied by estimating the erythrocyte SOD, and GPx activity. Erythrocyte SOD and Gpx activities were significantly lowered (789 ± 112 U/gm Hb and 26.30 ± 3.83 U/gm Hb) in the H1N1 Influ-enza on comparison with control subjects (1555 ± 98.20 U/gm Hb and 52.25 ± 4.27 U/gm Hb).
SOD is an important antioxidant enzyme having scaveng-ing effect against superoxide anion. Lowered activity of SOD may be due to increased utilization of SOD to de-toxify the superoxide radicals in H1N1 Influenza. The lowered levels of reduced glutathione in H1N1 Influ-enza may be responsible for decreased activity of glu-tathione peroxidase. Due to reduced levels of SOD, GPx, and reduced glutathione there was increased oxidative stress. Due to the raised oxidative stress and lowered an-tioxidants, free radicals may attack on NK cells. Thus decreased activity of SOD, GPx, and levels of reduced glutathione may be involved in NK cells death. The study indicates that, oxidative stress and antioxidants play an important role in H1N1 influenza. Elevated oxida- tive stress decreased activity of antioxidants may be indi-rectly responsible for NK cells loss and probably plays a role in pathogenesis of H1N1 influenza virus. The article published 2001, stated that support of prophylactic use of a carefully designed nutritional supplement formulation with glutathione may antagonize the major pathogenic processes of H5N1, influenza in humans.[22]
Conclusion
The study show that increased LPO levels and decreased levels of glutathione and activity of antioxidant enzymes raised oxidative stress in H1N1 Influenza. The study con-clude that elevated oxidative stress may be playing a role in natural killer (NK) cell loss and may play a role in pathogenesis of H1N1 influenza virus. The present study suggests that use of Tamiflu (oseltamivir) or Relenza (zanamivir) with supplementation of antioxidants can re-covers H1N1 influenza earlier than only antiviral drugs therapy by increasing the levels of NK cells and help to protect H1N1 viral infection
Acknowledgment
The author would like to thanks the staff of Biochemistry, Microbiology laboratory and staff of Medicine depart-ment for the technical help to make possible this study.
References
- Windler WG. Influenza in animals; its possible public health significance. J Wild Dis 1970;6 (4):239-42.
- Atonovics J, Hood ME, Baker CH. Molecular virology: was the 1918 flu avian in origin? Nature 2006; 44 0(7088): 9-10.
- Toubenberger JK, Morens DM. 1918 influenza: the mother of all pandemics. Emerg Infect Dis 2006; 12 (1):15-22.
- Mayo foundation for Medical Education and Research (MFMER). DSO1144. Sept. 17th 2009. www. mayo- clinic.com.
- Baurier NM, Palese P. The biology of influenza vi- ruses: Vaccine 2008; suppl 4: 49-53.
- Lynch JP, Walsh E. Influenza; evolving strategies in treatment and prevention. Semin Respir Care Med2007; 28 (2): 144-158.
- Ananthanarayan and Paniker’s. Text book of Microbi- ology. 8th edition, University Press India Pvt Ltd. 2009; 706-707.
- New York Times 2009-04-28. The naming of swine flu, a curious matter. http:/www. Hytimes.com /2009/ 04/29/world/asia/29 swine. Html. Retrieved 2009; 07-22.
- Gaydos JC, Top FH, Hodder RA, Russell PK. Swine influenza a outbreak, Fort Dix New Jensey, 1976. Emerging Infectious Diseases 2006; 12 (1): 23-28.
- Knobler S, Mack A, Lemon S, ed. 1. The story of In- fluenza. The Threat of Pandemic Influenza: Are we ready? Workshop summary. Washington, DC: The Na- tional Academies Press 2005; 75.
- Gray GC, Trample DW, Roth JA. Pandemic influenza planning: shouldn’t swine and poultry workers be in- cluded. Vaccine 2007; 25 (22): 4376-4381.
- Xiao Song He, Monia Draghi, Kutubddin Mohmod, Tyson H.Holmes, George W. Kamble. T-Cell depend- ent production of IFN – γ by NK cells in response to in- fluenza A virus. J Clin Investigation 2004; 114 (12):1812-1819.
- Burge JA. Aust AD. Microsomal lipid peroxidation. In methods Enzymes, Estbrooh RW, Pullman ME. New York: Acad press 1987: 302-310.
- Praful B. Godkar, Darshan P. Godkar. Text book of medical laboratory technology. 2nd edition Reprint Sept. Published by Bhalani Publishing house Mumbai. 2009; 824.
- Kono Y. Generation of superoxide radical during auto- oxidation of hydroxylamine and an assay for superox- ide dismutase. Arch Biochem Biophysics 1978; 186: 189-195.
- Paglio DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glu- tathione peroxidase. J Lab Clin Med 1967; 70: 158-159.
- Lamb R. A. and Krug R. M. Orthomyxoviride: the vi- ruses and their replication. In fields virology. DM Knipe, PM Howlyey; editor. Lippincott Williams , Wilkins. Philadelphia, Pennsylvania. USA. 2001, 1533-1579.
- Jayakumari N, Ambikakumari, Iyer BK. Antioxidant status in relation to free radical production during sta- ble and unsuitable enigma syndromes. Atherosclerosis 1992; 94: 183-190.
- Mao H, Tu W, Oin G, Law HK, Sia SF, Chan PL, Liu Y, Lam KT et al. Influenza virus directly infects human natural killer cells and induces cell apoptosis. J Virol 2009 July 18th (Epub ahed of print).
- Huawei Mao, Wenwei Tu, Gang Qin, Helen Ka Wai Law, Sin Fun Sia, Ping-Lung Chan, et al. Influenza vi-rus directly infects human natural killer cells and in- duces cell apoptosis Journal of Virology 2009;83(18): 9215-9222.
- Choi AM, Knobil K, Otterbein SL, Eastman DA, Jacoby DB. Oxidant stress responses in influenza virus pneumonia: gene expression and transcription factoractivation. AM J Physiol 1996; 271 (3 pt 1): L383-391.
- Neumann G, Takeshi N, Yoshihiro K. Emergence and pandemic potential of swine-origin H1N1 influenza vi- rus. Nature 2009; 459: 931-939.