ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 10
Research on the inhibitory effect of FAT-1 on endometrial cancer cell proliferation
Chao Han1, Wei-Min Kong1*, Yu-Hong Xin2*, Jing Jin1, Ting-Ting Liu1 and Ding Ding3
1Department of Gynecological Oncology, Beijing Obstetrics and Gynecology Hospital, Capital Medical University, Beijing, PR China
2Department of Obstetrics and Gynecology, the First People's Hospital of Xianyang, Xianyang, Shaanxi, PR China
3Department of Obstetrics and Gynecology, Care Women's and Children's Hospital, Beijing, PR China
- *Corresponding Author:
- Wei-Min Kong
Department of Gynecological Oncology
Beijing Obstetrics and Gynecology Hospital
Capital Medical University, PR China
Yu-Hong Xin
Department of Obstetrics and Gynecology
The First People's Hospital of Xianyang, PR China
Accepted date: February 25, 2017
Objective: This paper aims to discuss the n-3 fatty acid desaturase (FAT-1) expression in human endometrial cancer cell lines and its influences on endometrial cancer cell proliferation.
Methods: The endometrial cancer cell line Ishikawa was used as the study object. Ishikawa with stable FAT-1 expression was constructed using lentivirus transfection method. FAT-1 gene expression was tested using fluorescence-activated cell sorting and Western Blot (WB) methods. Proliferations of Ishikawa with stable FAT-1 expression at different time points were recorded, and the proliferation curve was drawn. At the same time, influences of FAT-1 on Ishikawa proliferation were tested via Cell Counting Kit-8 method. Related molecular regulation mechanism after FAT-1 overexpression was analysrd through WB analysis.
Results: Effective heterogeneous FAT-1 expression in Ishikawa was tested using fluorescence-activated cell sorting anzd WB methods. Results showed that proliferation of Ishikawa with stable FAT-1 expression dramatically slows down. Heterogenous FAT-1 overexpression can downregulate the β- catenin signal.
Conclusions: Heterogenous FAT-1 overexpression can inhibit endometrial cancer cell proliferation, and FAT-1 can regulate endometrial cancer cell proliferation through the β-catenin signaling pathways.
Keywords
Endometrial cancer cell, FAT-1, β-catenin
Introduction
Polyunsaturated Fatty Acids (PUFAs) are essential fatty acids that could not be synthesized by the human body [1,2]. PUFAs in foods mainly include n-6 series of arachidonic acids and n-3 series of Docosahexaenoic Acids (DHAs). n-3 Fatty Acid desaturase (FAT-1) can convert n-6 fatty acids in human bodies into n-3 fatty acids, thus enabling the regulation of fatty acid level in human bodies. Many n-3 PUFAs, such as eicosapentaenoic acid and DHA, can enhance body immunity, inhibit tumor cell proliferation and transfer, induce tumor cell apoptosis, and strengthen the therapeutic effect of medicines for tumors. Based on the latest studies, n-3 PUFAs and FAT-1 can significantly suppress many tumors, such as rectal, gastric, prostate, and breast cancers [3-5], among others.
This paper discusses FAT-1 expression in endometrial cancer cells and its relation to the proliferation of endometrial cancer cells. The molecular regulation mechanism of FAT-1 on endometrial cancer cell proliferation was further tackled. The research results can provide theoretical basis for clinical treatment and prevention of endometrial cancer.
Materials and Methods
Cell sources
Human embryonic kidney cells (293T) and endometrial cancer cell line (Ishikawa) were bought from the central laboratory of Xuzhou Medical University.
Reagents and instruments
Dulbecco’s modified Eagle medium (DMEM), McCoy’s 5a improved culture medium, RPMI-1640 culture medium, fetal calf serum (FBS), and 0.25% pancreatin were purchased from Gibco (USA). Cell Counting Kit (CCK-8) used for cell activity detection was procured from Biyuntian Bio-Technology Co., Ltd. The transfection reagent X-tremeGENE™ 9 was obtained from Roche. The mice monoclonal antibody FAT-1 was bought from Abcam. The rabbit monoclonal antibody β-catenin was procured from CST, Inc. The horseradish peroxidase (HRP)- labeled immunoglobulin G (IgG)/biotin second antibody and HRP-labeled IgG/alkaline phosphatase second antibody were purchased from Biyuntian Bio-Technology Co., Ltd. CO2 cell incubator was bought from Thermo Fisher Scientific. Electrophoresis apparatus, electrophoresis tank, and membrane transfer tank used for Western Blot (WB) were purchased from Bio-Rad (USA). The FACSCalibur flow cytometer was procured from BD Biosciences (USA).
Cell culture
Ishikawa was cultured using McCoy’s 5a improved culture medium containing 10% FBS, and 293T was cultured using DMEM culture medium containing 10% FBS. All cells were cultured in an incubator with 5% CO2 and 95% air at 37˚C.
Construction of cell line with stable FAT-1 expression
To construct a cell line with stable FAT-1 expression, pLVXinternal ribosome entry site (IRES)-Green Fluorescent Protein (GFP) (vector), pLVX-IRES-GFP-FAT-1 with complete coding DNA sequence of FAT-1 (FAT-1), and packaging plasmids (pSPAX2 and PMD2G) were transfected into 293T cells through the X-tremeGENE™ 9 transfection method on a 4:3:1 proportion. Virus supernatants at 48 and 72 h were collected and mixed with a complete medium on a 1:1 proportion. Next, 3 ml mixture was added into the six-hole plate coated with Ishikawa and cultured for 24 h. The second transfection was conducted in the same way. Infected cells were cultured in an incubator with 5% CO2 and 95% air at 37˚C for another 2 days. Green fluorescence expression was observed under fluorescence microscope.
Verification of FAT-1
GFP-positive cells in vector and FAT-1 were selected via fluorescence-activated cell sorting. The GFP-positive rate of these selected cells was further analysed using fluorescenceactivated cell sorting. Subsequently, sorted GFP-positive cells were centrifuged and collected. RIPA lysate was added. After 30 min splitting on ice, equal volume of 2X loading buffer was added. Following pyrolysis thermal deformation, 10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis and 100 min transverse flow transmembrane at a rate of 200 mA were conducted. The solution was closed in 5% skim milk at room temperature for 1 h. FAT-1 and β-catenin primary antibody diluent was added for overnight incubation at 4˚C and then washed with Tris-Buffered Saline (TBS) for three times at 5 min each. The corresponding second antibody was incubated at room temperature for 1 h and then washed with TBS for three times at 5 min each. HRP color reagent was utilized for scanning development.
Drawing of cell proliferation curve
Vector and FAT-1 cells were cultured in a six-hole plate. Each hole had a cell number of 1 × 106. In addition, each group has three accessory holes. Cells were collected, and their number was recorded every day. The cell proliferation curve was also drawn.
CCK-8 test of cell proliferation
Vector and FAT-1 cells were incubated on a 96 hole plate. A total of 2,000 cells were incubated on each hole. Moreover, each group of cells had 10 accessory holes, which were cultured under 37˚C for 48 h. Five accessory holes of each group were added with 10 μL CCK-8 solution. After 2 h incubation, absorbance at 450 nm was tested using a microplate reader. The relative value between FAT-1 and vector groups was calculated by subtracting the test values of CCK-8 groups from those of non-CCK-8 groups.
Statistical analysis
Statistical analysis of experimental data was conducted through GraphPad Prism 5, and the results were expressed as x ± SD. Intergroup data were tested using t-test of independent samples. *P<0.05 represents statistical difference.
Results
Preparation of FAT-1 virus
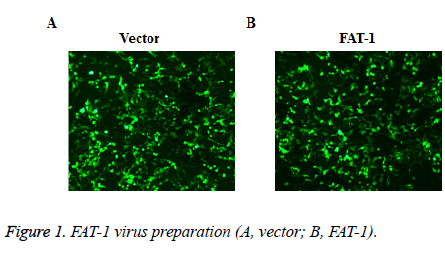
To disclose the effect of FAT-1 on Ishikawa, Ishikawa with stable FAT-1 expression was constructed through the lentivirus infection method. The lentivirus of vector and FAT-1 were prepared using the lentivirus package technique. Virus preparation effects were observed under an immunofluorescent microscope. The 293T cells with virus package were examined under a microscope. Most cells have GFP expressions, indicating a good virus preparation effect (Figure 1).
Detection of Ishikawa with stable FAT-1 expression
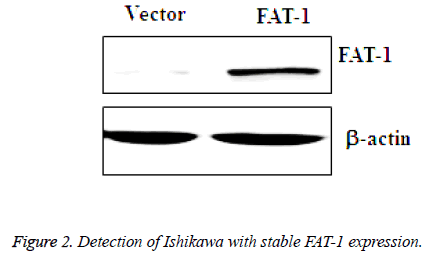
Prepared vector and FAT-1 viruses were collected and infected with Ishikawa, and then infected vector cells and FAT-1 cells were obtained. GFP-positive cells were screened using fluorescence-activated cell sorting and amplified. Next, amplified vector and FAT-1 cells were collected for further WB analysis of FAT-1 expression. Based on the WB results, FAT-1 expression in cells infected with FAT-1 lentivirus is significantly higher than that in vector (Figure 2), which suggests that FAT-1 has sustainable stable expression in Ishikawa.
Proliferation of Ishikawa with stable FAT-1 expression
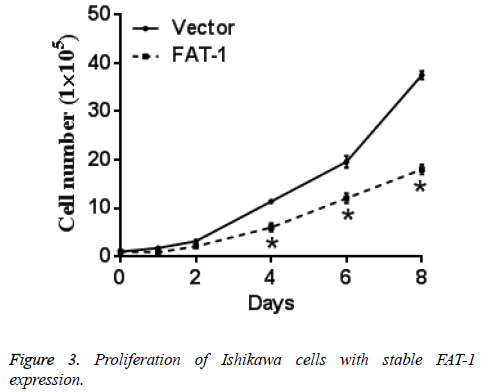
Same numbers of vector cells and Ishikawa cells with stable FAT-1 expression were cultured and counted every day. Ishikawa cells with stable FAT-1 expression significantly decreased with the increase of culture days compared with vector cells. In other words, proliferation of Ishikawa cells with stable FAT-1 expression is significantly lower than vector cells. Figure 3 shows the results.
CCK-8 test of Ishikawa cells with stable FAT-1 expression
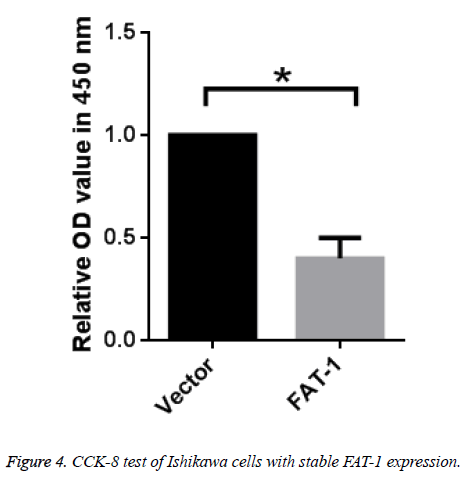
To further verify the inhibitory effect of FAT-1 on Ishikawa cell proliferation, vector cells and Ishikawa cells with stable FAT-1 expression were cultured. Proliferation of cells was tested using CCK-8 after 48 h. Ishikawa cells with stable FAT-1 expression have far lower proliferation than vector cells, which is in agreement with the above results (Figure 4).
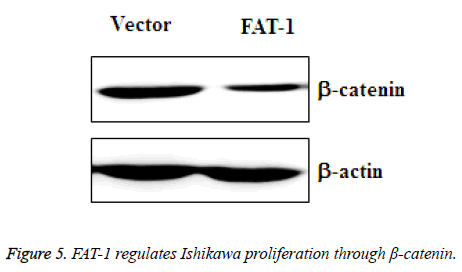
FAT-1 regulates Ishikawa proliferation through - catenin
To further determine the inhibitory mechanism of FAT-1 on Ishikawa proliferation, amplified vector cells and Ishikawa cells with stable expression of FAT-1 were collected. β-catenin changes in these two groups of cells were tested using WB. The result demonstrated a sharply downregulated β-catenin expression in Ishikawa cells with stable FAT-1 expression (Figure 5). This finding reflects the ability of FAT-1 to inhibit endometrial cancer cell proliferation through β-catenin downregulation.
Discussion
As a multiple malignant tumor in women, endometrial cancer has high morbidity and low survival rate characteristics and is a serious threat to women’s health [6]. At present, treatment to endometrial cancer mainly relies on surgical operation and medical chemotherapy. However, traditional surgical operation and medical chemotherapy could not significantly prolong survival period of patients. Therefore, the positive exploration of new treatment is still an important topic in the study of endometrial cancer. Gene therapy as tumor treatment has attracted considerable research attention. This treatment involves artificially inputting exogenous target genes at the DNA or RNA level of target cells, which could be expressed and have unique biological effect, thus realizing the goal of tumor treatment. At present, scientists are exploring gene therapy for endometrial cancer [7]. n-3 PUFAs has been reported to inhibit the development of many tumors [8]. Studies have discovered that FAT-1 can only be detected in most plants and few lower animals [9]. Heterogeneous FAT-1 expression can effectively inhibit the proliferation of a variety of tumor cells [10]. Therefore, n-3 PUFAs and FAT-1 gained the attention of scientists. In terms of the inhibitory effect of FAT-1 on Ishikawa proliferation, the WB results showed FAT-1 has successful exogenous overexpression in Ishikawa cells, indicating that FAT-1 can be transferred into endometrial cancer cells and achieve overexpression. This finding suggests that FAT-1 can have overexpression in human cells and further play its functions. The constructed cell line with stable FAT-1 expression is a beneficial tool for conducting follow-up research on endometrial cancer cell proliferation. Cell counts and CCK-8 test proved that FAT-1 can inhibit proliferation of endometrial cancer cells through β-catenin. The further mechanism of actions of FAT-1 requires deep discussions.
Conclusion
In this study, the inhibitory effect of FAT-1 on endometrial cancer cell proliferation is disclosed. The downstream-related signaling pathway of β-catenin is introduced preliminarily. The findings of this study showed that FAT-1 can be stably expressed in endometrial cancer cells and can play its functions normally. The research results provide theoretical basis for individualized gene therapy of endometrial cancer.
Conflicts of Interest
The following previous authors (Sheng Liang, Xiaoyuan Lu) state that we hold no conflict of interest at present or at future regarding any aspects (technical/academic) of this research.”
References
- Dai D, Wolf DM, Litman ES, White MJ, Leslie KK. Progesterone inhibits human endometrial cancer cell growth and invasiveness: down-regulation of cellular adhesion molecules through progesterone B receptors. Cancer Res 2002; 62: 881-886.
- Tehrani P, Shojaei S. RBC deformation analysing within capillary blood flow using FSI method. Biomed Res-India 2016; 27: 1000-1005.
- Khamesipour F, Tajbakhsh E. Analyzed the genotypic and phenotypic antibiotic resistance patterns of Klebsiella pneumoniae isolated from clinical samples in Iran. Biomed Res-India 2016; 27: 1017-1026.
- Oyebode KO, Tapamo JR. Selective cell segmentation using semi-automatic graph cut with adaptive distance penalties. Biomed Res-India 2016; 27: 1094-1098.
- Li K, Zhou N. Lung Cancer Drug-Drug Interaction (DDI) related with praeruptorin D. Lat Am J Pharm 2017; 36: 184-187.
- Chang J, Jia X, Wang S, Hou C. Kaempferol induces apoptosis in human breast cancer MDA-MB-231 cells by activating caspases and Bcl-2 family proteins and inhibiting NF-κB. Lat Am J Pharm 2017; 36: 109-114.
- Zhou Y, Liu Y, Liu SG. Bisphenol F Diglycidyl Ether (BFDGE) affects the activity of anti-colon cancer drugs-related drug-metabolizing enzymes (DMEs). Lat Am J Pharm 2016; 35: 1844-1849.
- Tehrani P, Shojaei S. RBC deformation analysing within capillary blood flow using FSI method. Biomed Res-India 2016; 27: 1000-1005.
- Dai D, Wolf DM, Litman ES, White MJ, Leslie KK. Progesterone inhibits human endometrial cancer cell growth and invasiveness: down-regulation of cellular adhesion molecules through progesterone B receptors. Cancer Res 2002; 62: 881-886.
- Hague S. Adrenomedullin inhibits hypoxic cell death by upregulation of Bcl-2 in endometrial cancer cells: a possible promotion mechanism for tumour growth. Oncogene 2001; 20: 2937-2945.