ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
- Biomedical Research (2013) Volume 24, Issue 2
Quantitative augmentation of immune cells in elderly normal mice by short-term, daily consumption of an extract of North American ginseng (Panax quinquefolius).
Department of Anatomy and Cell Biology, McGill University, Montreal, QC, Canada
- *Corresponding Author:
- Sandra C. Miller
Department of Anatomy & Cell Biology
Room No. 1/61, McGill University
3640 University Ave.
Montreal, Quebec, CANADA
Tel: 514-398-6358
Fax: 514-398-5047
Accepted date: January 05 2013
As mammals age, the cells of the immune system (IS) decline quantitatively and functionally, concomitant with which diseases such as cancer progressively increase in frequency. We have previously demonstrated in normal, juvenile mice that a specific, proprietary extract of North American ginseng, i.e., CVT-E002, is capable of significantly, quantitatively stimulating IS cells. We have previously revealed that the mechanisms responsible for the low numbers of immune cells seen in both juvenile and aging mice are very different. In the present study, we assessed the ability of CVT-E002 to restore/stimulate the declining immune cell numbers of aged mice. CVT-E002 (80mg in 6 gm chow/mouse) was added to the chow daily of 22 month old (= approximately 70 human years) normal, female C3H/HeN mice for 5 weeks. At 5 wk, the spleen and bone marrow were analyzed quantitatively for their content of lymphocytes, NK cells (the first line of defense against developing neoplasms), and “other” cells, i.e., those of the various hemopoietic lineages. Since the bone marrow is the central generating site of all NK cells, the observed significant augmentation in the numbers of these cells in that organ after consuming CVT-E002 for only 5 wk, necessarily reflects a CVT-E002-mediated stimulation of the production of new cells in this important tumor defense mechanism.
Keywords
Elderly, ginseng, immunity, animal model, in vivo
Introduction
The immune system declines with advancing age in mammals, and it is believed to be more than coincidental that various pathologies, not the least of which is cancer, concomitantly increase with advancing age. We have already demonstrated in a mouse model species, that the cells of the immune systems of pre-weaned, juvenile, and young adult mice can be quantitatively enhanced, in vivo, when the animals are administered via the diet (juvenile, young adult), or via intraperitoneal injection (preweaned), a proprietary extract of North American ginseng (Panax quinquefolius), i.e., CVT-E002 [1–4]. Specifically increased in CVT-E002-consuming mice were natural killer (NK) cells. These important cells act as the first line of defense against developing neoplasms and consequently, any elevation in the numbers of these cells in aged animals would be of potentially great significance in abating and/or preventing the development of cancers. The C3H/HeN mouse which, in old-age, develops assorted cancers with high frequency relative to the juvenile and young adult members of this strain was used in ourstudy as a model paralleling this phenomenon in humans.
We hypothesized that a derivative of the North American ginseng plant, Panax quinquefolius, i.e., CVT-E002, would enhance the cells of the degenerate immune system of aged, normal mice. The product was developed and marketed as Cold fX ® by Afexa Life Sciences, Inc. for human use in the amelioration of virus-induced respiratory ailments. CVT-E002 is the active agent in Cold fX®. This product has been shown, in vitro, to exert its effect on pathogenic agents, indirectly, via immune cell stimulation [5,6].
However, in spite of the proven effectiveness of CVTE002 as an immuno-stimulant in normal infant, juvenile and young adult mice [1,4], and in leukemic juvenile and adult mice [2,3], CVT-E002 has yet to be shown effective as a similar stimulant in elderly animals. The reason for this study, therefore, is to test our hypothesis that the declining immune cell numbers in aged, normal animals could be augmented by short-term, daily feeding of CVTE002.
Materials and methods
Animals
Female retired breeders of the C3H/HeN strain were purchased from Charles River Laboratories, St. Constant, QC, Canada. This strain was selected because as females age, they are prone to the development of assorted cancers, especially breast cancers. All mice were maintained under controlled environmental conditions of temperature and humidity, in microisolator cages on a 12 hr light/dark cycle. In-house veterinary and technical services in the McGill University Animal Care Facility ensured, moreover, that all edicts issued by the CCAC (Canadian Council on Animal Care) were strictly adhered to. All personnel involved in the handling of mice in this study have passed the required training courses in rodent husbandry. Sentinel mice in the same Facility, regularly demonstrated the absence of all common mouse pathogens. Euthanasia was carried out in a CO2 chamber by methods fully approved of by the CCAC.
The dietary additive
CVT-E002 is a proprietary extract of North American ginseng (Panax Quinquefolius), produced by Afexa Life Sciences, Inc., Edmonton, AB, Canada. CVT-E002 consists of specific polysaccharides (poly-furanosylpyranosyl- saccharides). Standard chemical and biological assays were applied in combination with consistent manufacturing processes to ensure that both the composition and the pharmacological activity of each lot of CVT-E002 are identical [5,7], and free of microbial contaminants. The extract is a strong immune stimulant in vivo and in vitro, resulting in the augmented production of several lymphocyte-stimulating cytokines [5,6]. CVT-E002 is a very effective stimulant especially of NK cells, elevating their numbers in vivo to significantly super-normal levels [1,2,4].
Treatment
A homogenized mixture of powdered standard mouse chow (Lab Chow, Agribrands, Inc., Woodstock, ON, Canada) was provided daily as follows. Mice were fed daily 6 gm of powdered chow each to which was added CVT-E002 (80mg/6gm chow/day/mouse). Since mice were housed 2/cage, each daily provision/cage consisted of 12 gm chow + 160mg CVT-E002. Control cage mates were identical in every way (strain, age, gender, husbandry) except that their daily chow did not contain CVTE002.
Healthy, elderly female mice (22 mo. of age) were given chow containing CVT-E002 for a period of 5 weeks. All mice (CVT-E002-consuming and control-diet-consuming) were euthanized when they were just over 23 mo. of age. At that time, the spleen, bone marrow and blood were taken for analysis of their content of lymphocytes, NK cells and a third category consisting of all hemopoietic (non-immune), cells.
Preparation of the organs for analysis of immune and hemopoietic cell content
For each mouse, at the time of euthanasia, single cell suspensions of the spleen and bone marrow were prepared by our standard laboratory methods [8-10]. Each spleen, upon removal was pressed through a stainless steel mesh into medium (GIBCO Invitrogen Corp,, Burlington, ON, Canada). Both femurs (bone marrow source) from each mouse were aseptically removed and transferred to ice cold medium containing 10% heat inactivated newborn bovine serum (NBS – GIBCO). The bone marrow was expressed from the femurs by repeated flushing with medium. Free cell suspensions from both organs were obtained by gentle, repeated pipeting. Each suspensions was then layered for 7 min onto 1.5 ml pure NBS to allow the sedimentation of any remaining non-cellular debris into the NBS. The aggregate-free supernatants were then removed and centrifuged for 7 min (1100rpm, 4 C) and the resulting pellet was re-suspended in a fixed volume of fresh medium + NBS. The total number of nucleated cells was obtained by means of a hemocytometer (American Optical Co., Buffalo, NY, USA). The total number of nucleated cells in each spleen and in both femurs, for every mouse (CVT-E002-consuming and control-diet) can then readily be calculated. Immediately prior to euthanasia in each mouse for extraction of the spleen and bone marrow, blood was extracted from the lateral tail vein.
Subsequently, from each organ (spleen, bone marrow) of every mouse, smears were made from the clean cell suspensions, onto Superfrost Plus® microscope slides (Fisher Scientific, Whitby, ON, Canada). Blood was directly smeared onto slides at the moment of extraction in all cases. All smears (spleen, bone marrow, blood) were then stained for morphological identification of lymphocytes, NK cells and “other” hemopoietic cells grouped into a single category, using MacNeal’s tetrachrome hematologic stain (Sigma-Aldrich, Oakville, ON, Canada). This stain provides a 4-color discrimination of the key cellular components (DNA, RNA, protein), as well as providing a clear distinction of cells’ size, shape and anatomical components, i.e., cytoplasmic granules, Golgi zone, stage of mitosis, etc. Although NK cells are identical to other lymphocytes, i.e., T and B, only NK cells contain readily identifiable cytoplasmic granules. We have recently used this feature to monitor NK cells, distinct from other lymphocytes [4]. Each smear from all 3 organs was then “read” for its proportions of lymphocytes, NK cells, and a third category composed of all hemopoietic cells. The latter category was composed of granulocytes, monocytes, and erythroid cells, including their mature, maturing and precursor forms. Non-nucleated red blood cells and platelets were not included or recorded.
From 500 total blood cells/smear, 1000 total spleen cells/smear and 2000 total bone marrow cells/smear, the proportions (%) of cells in each of these cell categories were obtained from every smear from all 3 organs (spleen, bone marrow, blood) of every mouse. NK cells were identified by virtue of their being small to medium sized cells (7-9 microns in nuclear diameter and of lymphocytic morphology, and bearing 2-5 stained cytoplasmic granules [4,11] clearly visible at x100 in the light microscope. Other lymphocytes (T, B) are morphologically identical to NK cells, but never contain granules, irrespective of the developmental stage in their cell cycle. All hemopoietic cells after staining are morphologically clearly distinct and discernable from lymphocytes and NK cells [8-10].
Finally, the absolute numbers of cells in each of these 3 categories (lymphocytes, NK cells and “other” hemopoietic cells), were obtained for each mouse, for the spleen and bone marrow (both femurs/mouse), by converting the percentage values obtained from the microscopic “reading” of the smears from each organ, via the known total cell content of each organ, the latter values having been recorded from the hemocytometer at the time of organ extraction. In the case of the blood analysis for every mouse, the percentage values of these 3 categories of cells were recorded as relative (%) values, it not having been possible to obtain the total blood volume of blood per mouse.
Where cell population sizes are large, methods which are less labor intensive than those described above can be used for quantifying cell populations. Thus, the florescence activated cell sorter (FACS) can be used. However, FACS is inaccurate for enumerating very small populations such as NK cells. Their numbers are usually lost in the error range of the sorter. Moreover, FACS relies on a specified cell surface marker and assumes that the marker chosen is not present on more than one cell type. This is certainly not the case with NK cells which share markers with other immune and hemopoietic cells. Finally, immature cells of several populations (hemopoietic and immune) share the same markers. These phenomena, when using FACS analysis to sort for very small populations of cells, confound the results such that the quantitative data are not accurate.
Statistical analysis
The mean ± s.e. of the absolute numbers of lymphocytes, NK cells and “other” hemopoietic cells in the spleen and bone marrow were then calculated. In the case of the blood values, the mean ± s.e. represent the percentage values of cells in each category. The effect of dietary CVT-E002 in each group of mice was compared to the appropriate control and the two-tailed Student t-test was applied. Comparisons were considered statistically significant at probability (p) values of <0.05.
Results
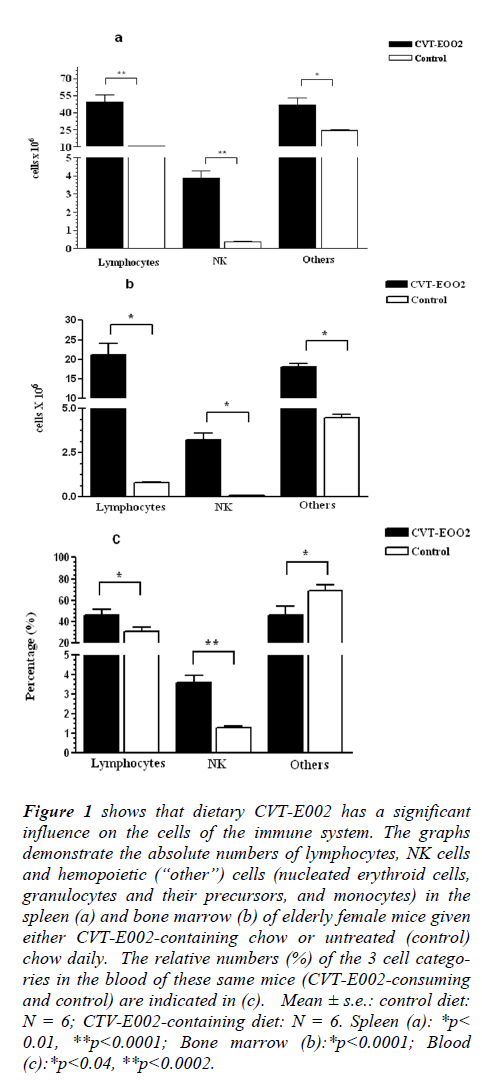
Fig. 1 (a-c) indicates the effect of short term (5 wk) daily consumption of CVT-E002 by elderly (22 mo old), normal mice when compared with identically aged, normal cage-mates on the control diet (no CVT-E002). Mice of this age correspond to the human age of 70 years (Jackson Laboratories, Bar Harbor, ME). In spite of their advanced age, all mice in this study were tumor-free as confirmed at whole-body autopsy at the time of euthanasia, i.e., at 5 wk on the CVT-E002-containing or control diet.
Figure 1: shows that dietary CVT-E002 has a significant influence on the cells of the immune system. The graphs demonstrate the absolute numbers of lymphocytes, NK cells and hemopoietic (“other”) cells (nucleated erythroid cells, granulocytes and their precursors, and monocytes) in the spleen (a) and bone marrow (b) of elderly female mice given either CVT-E002-containing chow or untreated (control) chow daily. The relative numbers (%) of the 3 cell categories in the blood of these same mice (CVT-E002-consuming and control) are indicated in (c). Mean ± s.e.: control diet: N = 6; CTV-E002-containing diet: N = 6. Spleen (a): *p< 0.01, **p<0.0001; Bone marrow (b):*p<0.0001; Blood (c):*p<0.04, **p<0.0002.
Lymphocytes, i.e., a group of immune cells consisting of virgin and recirculating B cells and re-circulating T cells, were significantly elevated in CVT-E002-consuming mice, vs control, in both the spleen (Fig. 1a) and the bone marrow (Fig. 1b). The bone marrow is the birth site of (i) virgin (naïve) B lymphocytes, (ii) the earliest precursors to T lymphocytes (pro-T cells), as well as re-circulating, mature B and T lymphocytes. The proportion (%) of lymphocytes in the blood (Fig. 1c) reflects the quantitative increase in these cells in both key organs where they are generated (Fig. 1a) and/or housed (Fig. 1b).
In the spleen (Fig.1a), the absolute numbers of NK cells in CVT-E002-consuming, healthy, aged mice were significantly higher than those in control-diet cage-mates. Fig. 1b shows that the absolute numbers of these cells in the bone marrow (production organ of new NK cells) of CVT-E002-consuming mice were also significantly elevated over those of mice consuming the control-diet. In the blood of these mice (Fig. 1c), the elevated proportion (%) of NK cells reflects the transport of their increased production in the bone marrow, en route toward their primary depot, the spleen. Unlike other lymphocytes, NK cells do not re-circulate. Instead, they transit via the blood, unidirectionally, from their bone marrow birth site, to the spleen, [8,12,13], where the vast majority of them reside in wait for offensive cells such as tumor cells which have metastasized from a primary tumor site located elsewhere in the body. NK-mediated, tumor cytotoxicity occurs in the spleen upon encounter with metastasized tumor cells.
Fig. 1a reveals the absolute numbers in the spleen of “other” cells, i.e., hemopoiteic cells which are neither NK, nor lymphocytic. Consuming CVT-E002 has significantly increased this population (nucleated red blood cell precursors, monocytes, and granulocytes, including both their mature and precursor forms).
Similarly, in the bone marrow (Fig. 1b), the absolute numbers of cells in this group is also significantly elevated over mice consuming the control diet. However, the blood (Fig. 1c) of CVT-E002-consuming mice reveals that the proportion (%) of these cells, collectively, is lower than the proportion found in the blood of mice on the control diet. The reason for this is readily explicable given the fact that the immature/precursor forms to these hemopoietic lineages are not found in the blood of healthy animals, i.e., immature/precursor cells do not leave their organ of generation – only the mature forms (progeny) of these lineages are found in normal blood. CVT-E002 has elevated the production of the cells in these hemopoietic lineages, and it is the immature/precursor cells which accounts for the significant elevation in the absolute numbers of hemopoietic cells in the bone marrow and spleen.
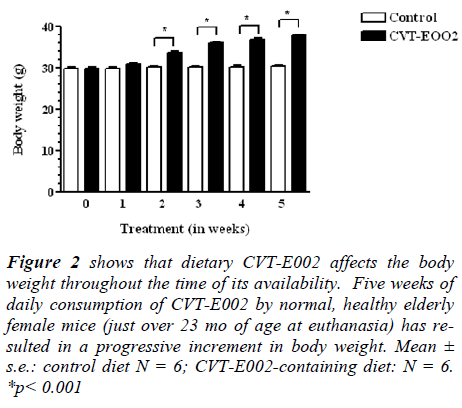
Fig. 2 reveals that consuming CVT-E002 has resulted in an early and sustained onset in body weight gain. This gain was significant, relative to mice on the control diet, and was observed as early as 2 wk after beginning feeding and remained elevated until the end of the study (5 wk), at which time these mice were euthanized for tissue preparation, to produce the data of Fig. 1 (a-c). In spite of weight gain during the 5 wk period, CVT-E002-consuming mice were clinically as healthy as their cage-mates consuming the control diet, in all parameters assessed, i.e., food/water intake, level of activity, fur quality and social behavior.
Figure 2: shows that dietary CVT-E002 affects the body weight throughout the time of its availability. Five weeks of daily consumption of CVT-E002 by normal, healthy elderly female mice (just over 23 mo of age at euthanasia) has resulted in a progressive increment in body weight. Mean ± s.e.: control diet N = 6; CVT-E002-containing diet: N = 6. *p < 0.001
Discussion
The plasticity of the immune system has been revealed in this study wherein we have shown that the long-observed and well known decline in the aged immune system can be significantly reversed. This is especially relevant in terms of quantitatively augmenting certain key cells involved at the first line of defense against cancer, i.e., NK cells. To date, there is little or no information available on possible methods of fortifying the aged immune system yet such studies would be of considerable importance given the concomitant increase in assorted pathologies, including cancers that accompany the age-related decline in immunity in mammals including humans.
Although this study is based on elderly female mice, we believe that similar observations would be found in elderly male mice. The reason is that far beyond the breeding stage, such as is the case in the females used in this study, the likelihood of female hormones confounding the results is minimal. Indeed, elderly C3H females undergo significant hair (fur) loss as they progressively age – hair thinning being a condition common to both genders in aging humans. It has been shown both in vitro and in vivo [1,2,4-6] that immune cells from both genders are stimulated by CVT-E002. Moreover, several years ago, it was demonstrated] that elevated NK cell numbers do indeed reflect elevated NK cell function, i.e., tumor cytotoxicity [referenced in 1, 2]. Furthermore, in our recent studies [14,15], we have shown in vivo, an inverse relationship between NK cell numbers and the presence of tumors. Thus, when NK cells are quantitatively elevated, tumors are simultaneously reduced or eliminated.
Nevertheless, a search of the literature, together with the findings of this study, may shed light on - and suggest possible mechanisms - by which the aged immune system can be manipulated. It has been well established for decades that the central generating site for both NK cells and B lymphocytes is the bone marrow. Thus, quantitative increases in these 2 populations seen in the present study necessarily means the production of new, naïve (virgin) B lymphocytes and NK cells. NK cells travel unidirectionally from the bone marrow and reside primarily in the spleen – the organ in which they intercept blood-borne, metastatic tumor cells. NK cells have no “memory” component and do not re-circulate They are rapidly renewed and replaced, and have a short life span of approximately 2 days [8,12].
That this significant increase in the bone marrow NK cells has occurred is of fundamental importance since all newly generated NK cells, exit that organ, never to re-enter it. Consequently, the quantitative elevation in NK cells necessarily means increased NK cell production has occurred in the presence of CVT-E002. This may be explained by the following, previously defined phenomena. The mechanism by which CVT-E002 exerts its significant stimulatory influence on NK cells for instance, is believed to involve macrophages (the circulating, mature forms of monocytes). However, potentially even more crucial are the stromal cells in the bone marrow microenvironment and to a lesser extent, those of the spleen. Both these organs have been known for decades to govern the production of cells classified in the present study as “other”, i.e., the hemopoietic cells [16,17]. Subsequently, it was established that the stromal microenvironment of the bone marrow also governed the production of new NK cells [8,12,13], and virgin (naïve) B lymphocytes [18,19].
It has been shown that in the presence of CVT-E002 in vitro, mixtures of spleen cells containing macrophages, stromal cells, and assorted hemopoietic and immune cells, release immune cell-stimulating cytokines, i.e., IL-2, IL- 1, IL-6, IFNγ, TNF-α [5,6], some of which directly link to NK cell receptors. Such stimulation with CVT-E002 releases a cascade of these and other cytokines which directly or indirectly influence NK cells and other cells of the immune system. Moreover, the cytokines IL-1, IL-6 and TNF-α (augmented in the presence of CVT-E002), are known to directly stimulate stromal cells themselves [20,21]. Such stimulated stromal cells, in turn, secrete factors which then drive the production of new cells of the immune and hemopoietic lineages.
It is reasonable, therefore, to conclude that the elevated levels of NK cells, non-NK lymphocytes and “other” hemopoietic cells in the spleen and bone marrow in CVTE002- consuming mice are the indirect result of stimulation by this agent of cells of the microenvironment, i.e., the stromal cells.
The progressive increase in body weight in CVT-E002- consuming, elderly mice may indicate interference by this herb in lipid physiology. Indeed at autopsy, CVT-E002- consuming mice showed an increase in fat deposition around the viscera, a phenomenon not seen in cage-mates consuming the control diet. It is possible the CVT-E002 may activate the fatty acid and lipid synthesis pathway via acetylCoA carboxylase and malonylCoA. Moreover, CVT-E002 may influence insulin secretion – also a measureable phenomenon.
It could be logically speculated that this weight gain could be estrogen-driven. It is known that estrogen is related to lipid deposition in vivo and like many herbs, Panax quinquefolius (from which CVT-E002 is derived), has phytoestrogenic properties [22,23]. However, CVT-E002 is a specific extract containing only polysaccharides (polyfuranosyl- pyranosyl-saccharides), having no known estrogenic properties.
Another theoretical possibility could be that CVT-E002 is stimulating the appetite of the mice which consume it, and this could explain the increase in weight gain. However, this too is not a defendable hypothesis for the following reason. Every mouse (control diet and CVTE002- consuming) is provided daily with the identical quantity (6 gm, i.e., 6,000 mg) of chow (± 80 mg CVTE002). Consequently, no mouse “can overeat”. Indeed, monitoring of the chow containers each morning (eating occurs during the dark hours since mice are nocturnal), revealed that no food remained in any container in the cages of either the mice consuming the control diet, or those consuming the CVT-E002-containing diet.
Thus, the answer to this peculiar observation of progressive body weight increase, with no clinical signs of illhealth, in mice fed CVT-E002, may only be revealed in the biochemistry lab. This latter feature (weight gain) may also be of clinical significance for aged human individuals suffering from wasting conditions, i.e., cachexia during chemotherapy.
It would appear instructive to formally study this potential, directly employing human subjects.
Once the mechanism responsible for CVT-E002-mediated weight gain is defined, it would then be reasonable to assess its effect in humans, especially since CVT-E002 is the active ingredient in a product (Cold fX ®) which is already in the marketplace.
Acknowledgements
This work was financially supported (materials, supplies and technician) by Afexa Life Sciences, Inc.
Conflict of Interest
The authors report no conflict of interest in any part of this study with any commercial or academic establishment.
References
- Miller SC, Delorme D. An extract from North American ginseng stimulates spontaneous immunity in infant mice: sustained, augmented immunity in adulthood long after withdrawal of the extract. J Comp Integ Med 2008; http://www.bepress/com/jcim/vol5/iss1/6
- Miller SC, Delorme D, Shan JJ. CVT-E002 stimulates the immune system and extends the life span of mice bearing a tumor of viral origin. J Soc Integ Oncol 2009;7(4): 127-136.
- Miller SC, Delorme D, Shan JJ. A proprietary extract of North American ginseng (Panax quinquefolium) administered to leukemic juvenile mice extends their life in a dose-dependent manner. J Comp Integ Med. 2010; http://www.bepress.com/jcim/vol8/1/10
- Miller SC, Ti L, Shan JJ. Dietary supplementation with an extract of North American ginseng in adult and juvenile mice increases natural Killer cells. Immunol Invest 2012; 41(2): 157-170.
- Wang M, Guilbert LJ, Ling L, Li J, Wu Y, Xu S, Pang P, Shan JJ. Immunomodulating activity of CVT-E002, a proprietary extract from North American ginseng (Panax quinquefolius). J Pharm Pharmacol 2001; 22: 1515-1523.
- Wang M, Guilbert LJ, Li J, Wu Y, Pang P, Basu TK, Shan JJ. A proprietary extract from North American ginseng (Panax quinquefolius) enhances IL-2 and IFN-γ production in murine spleen cells induced by Con-A. Int Imunopharm 2004; 4: 311-315.
- Shan JJ, Rodgers K, Lai C-T, Sutherland SK. Challenges in natural health product research: The importance of standardization. Proc West Pharmacol Soc 2007; 50: 24-30.
- Miller SC. Production and renewal of murine natural killer cells in the spleen and bone marrow. J Immunol 1982: 129(5): 2282-2286.
- Miller SC, Kearney SL. Effect of in vivo administration of trans retinoic on the hemopoietic cell populations of the spleen and bone marrow: Profound strain differences between A/J and C57Bl/6J mice. Lab Animal Sci 1997; 48(1): 74-80.
- Sun LZ-Y, Currier NL, Miller SC. The American cone flower: A prophylactic role involving non-specific immunity. J Alt Comp Med 1999; 5(5): 437-448.
- Babcock GF, Phillips JH. Human NK cells: Light and electron microscopic characteristics. Immunol Res 1983: 2(1): 88-101.
- Kalland T. Generation of natural killer cells from bone marrow precursors in vitro. Immunology 1986; 57: 493-498.
- Rosmaraki EE, Douiagi I, Roth C, Colucci F, Clumano A, DiSanto JP. Identification of committed NK cell progenitors in adult murine bone marrow. Eur J Immunol 2001; 31(6): 1900-1909.
- Durairaj P, Miller SC. Inhibition/prevention of primary liver tumors in mice given a daily dietary extract of North American ginseng (Panax quinquefolius) following a hepatoma-inducing agent. Biomedical Research 2012; 23(3): 430-437.
- Durairaj P, Miller SC. Neoplasm prevention and immuno-enhancement mediated by daily consumption of a proprietary extract from North American ginseng by elderly mice of a cancer-prone strain. Phytother Res 2012; doi:10.1002/ptr.4880.
- Trentin JJ. Influences of hematopoietic organ stromas (hematopoietic inductive microenvironments) on stem cell differentiation: In: Regulation of Hematopoiesis. Gordon AS ed Appleton-Century-Crofts, New York 1970; pp 161-215.
- Wolf NS, Trentin JJ. Hemolpoietic colony studies. V. Effects of hemopoietic organ stroma on differentiation of pluripotent stem cells. JExp Med 1968; 127(1): 205-214.
- Fauteux LJ, Osmond DG. Interleukin-1 as a systemic stimulant of B lymphopoiesis: IL-1α binds to stromal cells and sinusoid endothelium of the bone marrow and perturbs precursor B cell dynamics. J Immunol 1996; 156: 2375-2383.
- Hayashi S, Kunisada T, Ogawa M, Sudo T, Kodama H, Suda T, Nishikawa S, Nishikawa S. Progression of B lineage differentiation supported by interleukin-7 and other stromal cell molecules. J Exp Med 1990; 171: 1683-1695.
- Gronthos LS, Simmons J. The growth factor requirements of STROM-I-positive human bone marrow stromal precursors under serum-deprived conditions in vitro. Blood 1995; 85: 929-940.
- Hubin F, Humblet C, Belaid Z, Lambert C, Boniver J, Thiry A, Defresne MP. Murine bone marrow stromal cells sustain in vivo the survival of hematopoietic stem cells and the granulopoietic differentiation of more mature progenitors. Stem Cells 2005; 23(10): 1626-1633.
- Duda RB, Zhong Y, Navas V, Li MZC, Toy BR, Alavarez JG. American ginseng and breast cancer therapeutic agents synergistically inhibit MCF-7 breast cancer cell growth. J Surg Oncol 1999; 72: 230-239.
- King ML, Adler SR, Murphy LL. Extraction-dependent effects of American ginseng (Panax quniquefolium) on human breast cancer cell proliferation and estrogen receptor activation. Int Cancer Ther 2006; 5(3): 236-243.