ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
- Biomedical Research (2013) Volume 24, Issue 4
Quantitative assessment of erythrocytes and leucocytes in CPD- A stored blood.
Oluyombo R1*, Oluyombo O2, Uchegbu OO3, Adegbamigbe O4, Ayodele OE5
1Renal Unit, Department of Internal Medicine, Federal Medical Center, Ido- Ekiti, Ekiti state, Nigeria.
2Department of Medical Microbiology, Ladoke Akintola University of Technology Teaching Hospital, Osogbo, Osun State, Nigeria.
3Department of Haematology and Serology, Obafemi Awolowo University Teaching Hospital complex, Ile-Ife, Osun State, Nigeria.
4Department of Haematology, Federal Medical Centre, Ido- Ekiti, Ekiti State, Nigeria.
5Department of Internal Medicine, College of Health Sciences, Ladoke Akintola University of Technology, Osogbo, Osun State, Nigeria
- Corresponding Author:
- Oluyombo Rotimi
Renal Unit, Internal Medicine Department
Federal Medical Centre, Ido-Ekiti
Ekiti State, Nigeria
Accepted Date: June 01 2013
Citation: Oluyombo R, Oluyombo O, Uchegbwu OO, Adegbamigbe O, Ayodele OE. Quantitative assessment of erythrocytes and leucocytes in CPD- A stored blood. Biomedical Research 2013; 24 (4): 503-508.
Blood is stored to achieve a good post-transfusion survival. The ability of stored whole blood or blood components to fulfill their functions after transfusion can be studied during the period of storage. Transfusion of whole blood is currently being practiced in many centers in Nigeria despite the sustained short supply from voluntary donation. Studies have shown that leucocytes in stored blood can produce adverse effects and influence patient outcome. This study explores quantitative assessment of haematocrit values and leucocytes properties in a large number of blood units and aims at refreshing several years’ knowledge about quantitative changes in erythrocytes and leucocytes during storage. This will hopefully rekindle the need for widespread component transfusion in Nigeria. One hundred whole blood samples collected in CPDA-1 bags from individual, non-repeat, qualified donors were studied. Haematocit values and leucocytes count were done on days 0, 7, 14, 21, 28 and 35. Mean haematocrit and leucocytes count of donors on day 0 were 38.81 ± 3.31% and 5.1 ±1.9x 109 cells/l respectively. Mean difference of haematocrit and leucocytes between day 0 and 35 of storage were 2.25 ±1.07 % and 5.1 ± 1.9x109 cells/l respectively. Mean difference of haematocrit of 0.1± 0.4% on day 14 was statistically significant. While 49% of leucocytes were remaining by day 14, seventy five percent of neutrophil have disappeared by day 7. The number of leucocytes remaining in the blood unit on day 35 has inverse proportion to the haematocrit value on day 35 of storage. Significantly, neutrophil count on day 35 was negatively associated with the haematocrit value of day 35. We conclude that the seemingly subtle changes in the haematocrit values and remarkable loss of leucocytes in the stored whole blood have association and are significant. Also, to improve the benefit from the limited blood transfusion services, component transfusion might be a good option.
Keywords
Erythrocytes, leucocytes, CPD-A
Introduction
Blood is stored to achieve a good post-transfusion survival. To attain this, the cellular integrity and chemical components of the blood must be maintained. This keeps blood in a life-like manner in-vitro and maintains its physiological function. The ability of stored blood components or whole blood to fulfill their functions after transfusion can be studied during the period of blood stor Citrate phosphate dextrose-Adenine is the most commonly used anticoagulant preservative in Nigeria. Evidence given by Joint Task Force of USA and other authors shows that CPDA-1 provides an anticoagulant preservative system with properties consistent with safety and efficacy when used for 35-day storage of whole blood at 1- 60 C [2-4].
Blood supply is generally limited in Nigeria and there is need to make the best use of the available supply. Furthermore, transfusion services involving blood compo nents are not readily available in many centers, thereby limiting service providers to storage and transfusion of whole blood. However, many patients seldom need whole blood transfusion. Literature has shown that the presence of leucocytes in stored blood compromise the quality of the blood and this is associated with adverse effects to the recipients. Cellular components of stored blood undergo pathogenic changes with enzymes and other substances released which affect other components, their functions and invariably the clinical outcomes of recipients [5-7]. The overall benefit(s) from the limited supply of blood is affected. To the best of our knowledge, there is a dearth of information on the benefits of stored blood in Nigeria. In this study we look at the quantitative assessment of leucocytes and haematocrit of whole blood stored in CPD-A in a health facility in South West Nigeria. Proposed study can improve our knowledge about quantitative changes in erythrocytes and effect of leucocytes during their storage thus emphasizing the need for improved blood transfusion services and importantly, widespread component transfusion in Nigeria.
Methodology
It is a single centre study conducted in Obafemi Awolowo University Teaching Hospital Complex, Ile-Ife, Osun State, Nigeria. Blood samples from 100 qualified donors were collected in CPDA-1 bags (HELM)) after consent had been taken. Standard procedure of blood collection was followed. Samples were collected under aseptic condition. The CPDA-1 blood was stored as whole blood for 35 days. Before mixing the blood in the bags, sampling supernatant was examined visually for presence of haemolysis. After gentle mixing, aliquot of 5ml was taken from freshly collected whole blood after 30 minutes of holding in CPDA -1and at a 7-day interval for measurements of haematocrit, white blood cells and differential count from each CPDA-1 blood bag via the connecting tube. Blood bags were stored in blood bank refrigerator (Sanyo Inc.)
Measurement of Haematocrit.
Haematocrit was measured using microhaematocrit method. Plain capillary tubes were filled with whole blood and sealed with plasticine. Samples were centrifuged for 5 minutes at 10,000 rpm. The level of the packed cells in the tubes was read with microhaematocrit reader to the nearest 1 %.
Leucocyte count
White blood cell count was done with whole blood samples (50μl) diluted with Turk’s solution (950μl). Cells were counted using improved Neubaeur counting chamber. The differential leucocyte count was done using dry Leishman’s stained thin film. Battlement method of counting was used to count cells on the film.
Data Analysis
Data analysis was done and presented as mean values ± SD. The difference in mean values at a particular storage time was determined using t-test with a significance p ≤ 0.05.
Results
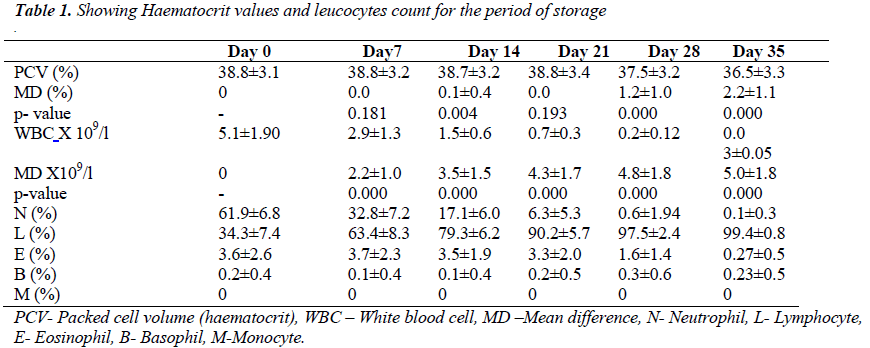
Ten of the blood bags showed visual evidence of haemolysis. There were 6 on day 28 and another 4 on day 35 of storage. The mean ± SD of haematocrit on day 0 and 35 were 38.77 ± 3.13% and 36.52 ± 3.32% respectively. The means and mean difference of haematocrit, total white blood cell count and differential counts are as shown in the table below. There was no significant statistical difference in mean of haematocrit until 14th day of storage of whole blood (0.12 %, t = 2.93, p < 0.004).
The mean difference between haematocrit value of day 0 and 35 was 2.25 ± 1.07 % (t = 20.89, p < 0.05). In fig 1, haematocrit values of day 0 have strong positive correlation with subsequent measurements throughout the period of storage (r = 0.946, p<0.05).
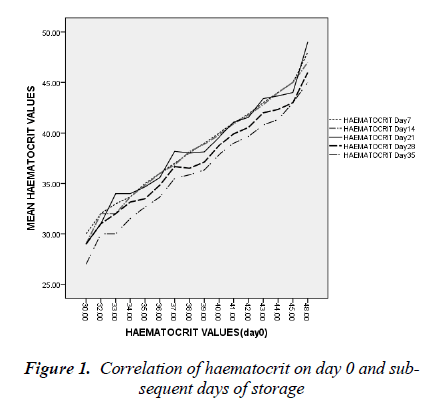
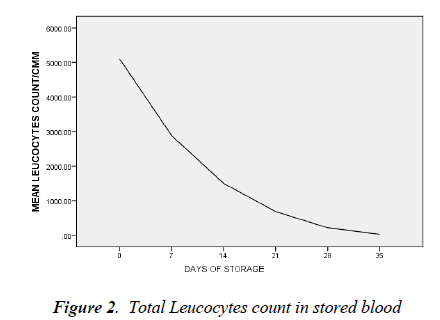
day 35 was 5.0 ± 1.8 cells/l (t=26.692, p < 0.05). By day 7 of blood storage, 50 % of leucocytes have disappeared and only 29% was remaining by 14th day of storage. Of all the leucocytes, neutrophil showed the most rapid disappearance from stored blood (Figure 3). By the 7th day, 75% of neutrophils have disappeared from the stored blood with numerous basket cells seen. And only about 8% was remaining on day 14. Contrariwise, though, lymphocyte constituted 33% of total leucocytes on day 0, by day 21, 28 and 35, it constituted 90, 97 and 99.4% respectively of total leucocytes count (Figures 3).
Although, other components of white cells were negatively correlated with haematocrit on day 35, it was not statistically significant.
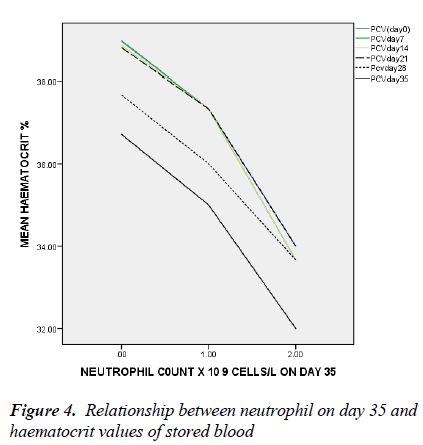
In figure 4, only the neutrophil count of day 35 has significant negative association with haematocrit on day 35 with linear regression (t = -2.048, ANOVA = 0.027, p = 0.043).
Discussion
This study shows that significant reduction in haematocrit is not noticed until 14th day of blood storage. This could be explained partly by reduced deformability of stored erythrocytes which are almost completely packed. Also about 1/120 of the donated erythrocytes is already at the end of its useful life because of life span of the cells [7, 8]. Haemolysis might also have contributed to the decrease in haematocrit. Moroff et al reported mean percent haemolysis after 35 days of storage as 0.58 ±0.23 % [9].
Leakage of haemoglobin occurs during storage. Although, this is with variation, variable mechanical injury may be responsible [10]. Handling of blood bags and repeated exposure to higher than expected ambient temperature could have added to haemolysis [11, 12]. Other changes such as sphering and crenation are due to anticoagulants and other biochemical changes such as reduction of 2,3- DPG and ATP [9, 13]. Most of these biochemical changes occur between the second and third week of storage [10]. This is in spite of mechanisms put in place to reduce haemolysis on storage.
In addition, leucocytes break down to release their constituents such as hydrogen peroxide and proteases which damage the erythrocytes [5, 15-17]. The degree of haemolysis is shown to be related to the levels of leucocytes in additive suspended units [5, 16, and 17]. There are improved biochemical changes with associated reduction in leucocyte enzymes such as elastase and chymotrypsin-like enzymes when units are leucodepleted. The presence of leucocytes is associated with membrane lesion with attendant potassium leakage, increased glycolysis and compromised ATP preservation. A significant benefit and better blood preservation when blood components are separated at the time of collection to liquid or freeze preserved [15-17] has therefore been reported. The viability and stability of stored erythrocytes is improved.
There is progressive drastic reduction in the differential count of neutrophils and by day 14 it constituted only 8% while the relative proportion of lymphocyte differential was progressively increasing. Studies have shown that polymorphonuclear cells are reduced to 50% of their number in 48 hours and only 25% would remain after the seventh day of storage [18, 19]. And as shown in this study, 75% of granulocytes have disappeared by day 7 of storage; little benefit is therefore derived in leucopenia or agranulocytosis when blood has been stored for more than two days.
The proportion of lymphocyte differential by 35th day of storage was 99.4%. This is similar to the finding of Sagir et al [20]. Lymphocyte has the longest half life both invivo and in-vitro. By implication, the risk of graft- versushost reaction will increase with transfusion of whole blood and consequently increased graft rejection rate of marrow or kidney transplantation [21].
There is a negative correlation between the leucocytes and more importantly neutrophil count on day 35 and the haematocrit values from day 0 to day 35 of storage. This implies that the leucocytes and specifically neutrophils negatively affect the erythrocytes and/or the milieu in which the cells are stored. The degree of haemolysis has been previously shown to be related to the levels of leucocytes [21-23]. Reduced exocytic vesicle formation, K+ release, haemolysis and improved morphology scores was reported by Greenwalt et al in leucocyte-depleted red blood cells unit [24]. And in order to reduce the haemolytic effects of leucocyte on erythrocytes in stored blood, Hogman et al reported effectiveness of adding synthetic enzyme inhibitors of chymotrypsin-like enzyme [21]. Transfusion of aged stored blood is associated with many pro-inflammatory effects likely because of the substances released during storage. Neutrophil is a major destroyer of erythrocyte membranes when activated as a number of toxic radicals are released [21-23].
This is also evidenced in this study where neutrophil count on day 35 of storage was independently negatively associated with haematocrit value. Leuco-depletion delays the appearance of storage lesions. Recipients of leucocyte- depleted blood have reduced metabolic burden of transfusion as in-vitro parameters such as PH, K+ and haemolysis levels were also improved [13].
Reduction in leucocytes also reduces the risk of HLA immunization [25-29]. Risk of transmitting parasitic, viral and bacterial infections such as Trypanosoma cruzi, CMV, HTLV-1 and HTLV-11 is also reduced [25, 26]. This may well be that patients envisaged for organ transplantation such as renal allograft recipients will be better transfused [27], with leuco-depleted red cells. Sircha et al 1990 reported reduced febrile, non-haemolytic reactions in leuco-depleted blood products. In addition post-storage ATP is less in unfiltered blood units. Separation of leucocyte from blood at collection has been reported by many authors as a better way to preserve erythrocytes and consequently increase post transfusion survival. It also reduces burden of transfusion.
In conclusion, this study has shown that there are significant cellular changes in stored whole blood. It has also suggested that these changes affect the quality of the blood. There is a significant interaction in the components of the blood in vitro. In order to optimize the limited blood supply for numerous recipients, component therapy would be a better option in our transfusion services. This would increase the yield from blood transfusion services when components are available to treat other medical conditions. Blood safety would also improve when leucocyte depleted blood are transfused. The more common practice in developed nations is blood components for transfusion [30].
There is a challenge of inadequate supply of blood for transfusion in our health centers partly because of cultural reasons [31] but the available few pints should be judiciously and adequately utilized to meet the ever growing need of transfusion of blood and its products.
References
- Hogman CF, de Verdier CH, Borgstrom L. Studies on the mechanism of human red cells loss of viability during storage II. Relation between cellular morphology and viability. Vox Sang 1987: 52; 20-23
- Federal Register, 43 (151) 34457, Part 640, August 4, 1978.
- Zuck TF, Bensinger TA, Peck CC, Chillar RK, et al. The in vivo survival of red blood cells stored in modified CPD with adenine: Report of a multi institutional cooperative effort. Transfusion 1977; 17: 374.
- Moore GL, Peck CC, Sohmer PR, Zuck TF. Some properties of blood stored in anticoagulant CPDA-1 solution. A brief summary. Transfusion. 1981; 21 (2): 135-137.
- Heaton WAL, Holme S, Smith K, Brecher ME, Pineda A, Aubuchon JP, Nelson E. Effects of 3-5 log 10prestorage leucocyte depletion on red cell storage and metabolism. British Journal of Haematology, 1994; 87: 363-368.
- Pavenski K, Saidenberg E, Lavoie M, Tokessy M, Branch DR. Red blood cell storage lesions and related transfusion issues: a Canadian Blood Services research and development symposium. Transfus Med Rev. 2012; 26(1): 68-84.
- The Age of Blood Evaluation (ABLE) randomized controlled trial: Study design. In: Lacroix J, Hébert P, Fergusson D, Tinmouth A, Blajchman MA, Callum J, Cook D, Marshall JC, McIntyre L, Turgeon AF; ABLE study group. Transfus Med Rev. 2011; 25(3): 197-205.
- Mollison PL. Further observations in the normal survival curve of 51Cr-labelled red cells. Clin Sci 1961; 21: 21.
- Moroff G, Morse EE, Katz AJ, Kahn RA et al. Survival and biochemical characteristics of stored red cells preserved with citrate phosphate dextrose adenine one and two and prepared from whole blood maintained at 20- 24o C for eight hours following phlebotomy. Transfusion 1984; 24: 115-9
- Sawant RB, Jathar SK, Rajadhyaksha SB, Kadam PT. Red cell hemolysis during processing and storage. Asian J TransfusSci 2007; 1(2): 47-51.
- Beaujean F, Segler JM, le Forestier C, Nuedari N. Leucocyte depletion of red cell concentrates by filtration: influence of blood product temperature. Letter Vox Sanguis 62, 242-243.
- Peck CC, Moore GL, Bolin RB. Adenine in blood preservation. CRC Crit Rev Clin Lab Sci 1981; 13: 173- 212.
- Moroff G and Dende D. Characterization of biochemical changes occurring during storage of red cells: Comparative studies with CPD and CPDA-1 anticoagulantpreservative solutions. Transfusion. 1983; 23(6): 484- 489.
- AuBuchon JP, Estep TN, Davey RJ: The effect of the plasticizer di-2- ethylhexyl phthalate on the survival of stored RBCs. Blood 1988; 71: 448-452
- Beutler E, Meul A, Wood LA. Depletion and regeneration of 2,3 diphosphoglyceric acid in stored red blood cells. Transfusion. 1969; 9: 109-115.
- Brecher ME, Pineda AA, Torloni AS, Harbaugh CA, Emery PL, Moore SB, Carmen R, Ne
- lson E. Prestorage leucocyte depletion: effect on leucocyte and platelet metabolites, erythrocyte lysis, metabolism and in vitro survival. Seminars in Haematology 1991; 28(suppl 5): 3-9
- Rogers SE, Edmondson D, Goodrick MJ, Standen GR, Franck V, Reppucci A, Pamphilon DH. Prestorage white cell reduction in saline-adenine-glucose-mannitol red cells by use of an integral filter: evaluation of storage values and in vivo recovery. Transfusion 1995; 35: 727-733.
- Crosbie A and Scarborough H. Studies on stored blood: Leucocytes in stored blood. Edinburgh Medical J. 1940; 47: 553.
- Young JA, Rudmann SV: Blood Component preservation and storage. In Rudmann SV: Textbook of Blood Banking and Transfusion Medicine. Philadelphia WB Sanders, 1995.
- Ahmed SG, Orakah JA.Cellular Changes In Stored Whole Blood And The Implication On Efficacy Of Transfusion Therapy In Nigeria. The Internet Journal of Third World Medicine. 2009; 8(2) doi:10.5580/b40.
- Hogman CF, Hedlund K, Akerblom O, Venge P. Red blood cell preservation in protein-poor media: I. Leukocyte enzymes as a cause of haemolysis. Transfusion 1978; 18: 233-241.
- Chu RW. Leukocytes in blood transfusion: adverse effects and their prevention. HKMJ 1999; 5(3). 24.
- Frake PC, Smith HE, Chen Lii-Fang, Biffl WL. Prestorage leuko-reduction prevents accumulation of Matrix Metalloproteinase 9 in stored blood. Arch Surg 2006; 141: 396-400
- Greenwalt TJ, Zehner Sostock C, Dumaswala UJ. Studies in red blood cells preservation. Effect on the other formed elements. Vox Sang 1990; 58: 85-89.
- Schiffler CA, Dutcher JP, Aisner J, Hogge D, Wiernik PH, Reilly JP. A randomized trial of leucocyte depleted platelet transfusion to modify alloimmunization in patients with leukaemia. Blood 1983; 62: 815-820.
- Kooy MM, vaProoijen HC, Moes M, Bosma-Stants I, Akkerman JWN. Use of leucocyte-depleted platelet concentrates for the prevention of refractoriness and primary HLA alloimmunization: a prospective randomized trial. Blood. 1991; 77: 201-205.
- Bowden RA, Slichter SJ, Sayers M, Weisdorf D, Cays M, Schoch G, Banaji M, Haake R, Welk K, Fisher L, McCullough J, Miller W. et al. A comparison of filtered leukocyte-reduced and cytomegalovirus (CMV) seronegative blood products for the prevention of transfusion- associated CMV infection after marrow transplant. Blood. 1995; 86: 3598-3603.
- Moraes-Souza H, Bordin JO, Bardossy L, MacPherson DW, Blajchman MA. Prevention of transfusionassociated Chagas' disease: efficacy of white cellreduction filters in removing Trypanosomacruzi from infected blood. Transfusion. 1995; 35: 723-726.
- Sirchia G, Wenz B, Rebulla P, Parravicini A Carnelli V, Bertolini F. Removal of white cells from red cells by transfusion through a new filter. Transfusion 1990; 30: 30-33
- Erhabor O, Adias TC. From whole blood to component therapy: the economic, supply/demand need for implementation of component therapy in sub-Saharan Africa. Transfus Clin Biol. 2011; 18(5-6): 516-526.
- Egbewale EE, Ogunro PS, Muhibi MA. Knowledge, Attitude and Practice of Blood Donation In South West Nigeria. Nigerian Hospital Practice, 2008; 2: 132- 136.