ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
- Biomedical Research (2012) Volume 23, Issue 2
Qualitative assessment of smooth muscle cells propagated on 2D-and 3Dpolycaprolactone polymers via scanning electron microscope
1Department of Physiology, University of Pretoria, Pretoria, Gauteng, South Africa.
2Council for Scientific and Industrial Research, Material Science and Manufacturing, PO Box 395, CSIR MSM, Polymers, Ceramics and Composites, Pretoria, Guateng, South Africa.
- *Corresponding Author:
- Anna Joubert
Department of Physiology
University of Pretoria
PO Box 2034, Pretoria, 0001
South Africa
Accepted Date: March 01 2012
Polycaprolactone (PCL) polymers which illustrate both biocompatibility and resorbability for replacement or bulking of damaged or diseased tissue are important in tissue engineering. Cytocompatibilty of these polymers was assessed on two-dimensional PCL disks and threedimensional PCL solid and PCL hollow microspheres using human uterine mixed leiomyosarcoma (SKUT-1) and hamster ductus deferens leiomyosarcoma (CRL-1701) cell lines. Possible PCL cytotoxicity and morphology were investigated in SKUT- and CRL-1701 cells. SKUT cells cultured in disk and microsphere extracts between 24 h and 5 day time periods displayed statistically increased metabolic activity, though activity decreased significantly on 1 month and 1 year extracts. However, the metabolic activity of CRL-1701 cells was similar to controls. Activity increased significantly on the 1 month extracts and decreased significantly on the 1 year extracts. Scanning electron microscopy illustrated increased cell density of cells attached to pre-conditioned disks. After 5 days, cells were spindle-shaped, following microspheres contours indicating high focal adhesion. Both cell lines migrated inside the hollow microspheres, indicating that they benefit from the sheltered environment. This in vitro study suggests that hollow microspheres allow for further cell expansion with a sheltered environment to protect cells from sheer stress experienced in vivo.
Keywords
tissue engineering, cytocompatibilty, biocompatibility, microspheres
Introduction
Tissue engineering is the science of replacing or bulking of damaged or diseased tissue. It requires the use of cells, growth factors and biomaterials individually or in combination to regenerate or replace lost tissue [1]. In its aim to regenerate and/or restore lost tissue function the use of polymer scaffolds can greatly assist in providing a supportive environment for cellular attachment and proliferation. These polymers or biomaterials can act as threedimensional (3D) scaffolds that enable cells to attach and configure into the correct anatomical shape of the tissue [2]. The molecular composition and morphology of the polymer play a key role in cellular invasion [3].
Bulking and regeneration of smooth muscle cells (SMC’s) is needed particularly in the gastrointestinal (GI) tract where defects are commonly found [4]. Smooth muscles have noticeable sarcomeres and stain positively for α- smooth muscle actin [5]. Smooth muscle tissue is responsible for involuntary contractions in the walls of the organs in which they are present [4].
The various smooth muscle sphincters are the lower oesophageal sphincter, pyloric sphincter, ileocecocolic sphincter, the internal anal sphincter and the urinary sphincter [4]. These sphincters act as one-way valves regulating the flow of gastrointestinal contents. At rest, they remain in a state of tonic contraction and closure [6]. This contractile function diminishes along with the ageing process. The incidence of physiological problems of dysphagia, constipation and incontinence also increase significantly with age. This ageing-related dysfunction mostly affects the upper GI tract (particularly the oropharynx and oesophagus) and the distal GI tract consisting of the colon and rectum [6]. Being able to restore the lost function would impact greatly on the quality of life of the many patients affected by these problems.
The ability of biodegradable and re-absorbable polymers to provide a regenerative scaffold for lost or wounded tissue whilst the polymer degrades with time makes the use of these polymers an attractive proposition. Scaffolds provide a supportive surface until the body’s own cells begin to replace the polymer as it degrades, thereby assisting and enhancing the body’s natural healing process. When the polymer degrades it breaks into fragments and loses its structural strength, resulting from a decrease in its molecular weight [7]. Biodegradable polymers e.g. poly(3-hydroxybutyrate), poly(glycolic acid) and poly(Llactide- co-caprolactone) are used in orthopaedic applications and in dentistry [8,9,10,11]. Once a polymer has degraded, it is optimal that the degradation products resorb into the body without resulting in a harmful or toxic effect to the body. Examples of non-toxic polymers that resorb are poly(DL-lactic acid) (PDLLA), poly(glycolic acid) (PGA) and poly(L-lactic acid) (PLLA) [12,13].
In this study smooth muscle cell interactions with poly(caprolactone) (PCL) as polymer were investigated. The latter is widely used as biodegradable polyester for medical applications because of its known biocompatibility and degradability [14]. The degradation product, lactic acid, can be metabolised by the body. Some reports suggest that this degradation product could cause an unsuitable microenvironment and may not be ideal for tissue growth [2]. However, as PCL’s degradation rate spans over more than a year, it becomes ideal for applications where a long-term scaffold or support is needed [15]. The slow degradation rate may cause relatively small local pH changes and may not exert a detrimental effect on the cells.
An important intention of in vitro cell culture is to reproduce the in vivo condition as closely as possible. Therefore, along with the usual route of two-dimensional (2D) in vitro investigations of the PCL polymer in the form of a flat PCL disk, it was attempted to compare these results with a three-dimensional (3D) model which included growing cells on 3D solid PCL microspheres (100-200 μm in diameter). Observing the manner in which the cells attach onto the PCL surfaces is indicative of whether the SMCs retain their characteristic spindle-shaped attachment formation. The density and concentration of attached cells also indicate as to how the cells differ in attachment on flat and curved surfaces. An intact cytoskeleton, as well as filopodia branching out from the cell body is indicative of cells that are migrating, spreading and proliferating well on polymer surfaces [16]. These microspheres aim to provide a larger surface area of growth and also allow for significant cell expansion, especially when they are engineered to be porous and/or hollow. Since these microspheres are small enough to be injected in a surgical environment, this creates potential for tissue regeneration in vivo where the filling of irregularly shaped defects is needed [17]. It also ensures a minimally invasive surgical procedure for the patient. Since the cells are only growing on the surface of the microsphere, lack of nutrient diffusion will not pose a problem. In effect, these microspheres will become cell microcarriers which could be implanted in the body and provide a bulking, functional effect [18,19]. This study aimed to understand the in vitro effects of PCL polymers on smooth muscle cells as possible future application such as scaffold/cell delivery vehicle in smooth muscle bulking where the muscle’s contraction/relaxation ability has become compromised.
Materials and methods
Cell lines. The SKUT-1 cell line is characterized as mixed leiomyosarcoma derived from the human uterus and the CRL-1701 cell line is characterized as a leiomyosarcoma derived from the Syrian hamster ductus deferens. Both cell lines were obtained from the American Tissue Cul- ture Collection (ATCC) (Maryland, United States of America).
Polycaprolactone polymers. Polycaprolactone (CAPA 6500, MW 50 000) was purchased from Solvay Chemi- cals (United States of America).
a) PCL was prepared as flat sheets by placing PCL beads (as obtained from the manufacturer) into a mould and then hydraulically pressing it at 80ºC for 15 min. The sheet was then cut into the correct size to fit into 24- well plates.
b) Solid PCL microspheres were prepared by dissolving the original PCL beads in dichloromethane (DCM – reagent grade, Sigma Aldrich, South Africa) at 25°C. This was added to 1% (w/v) poly vinyl alcohol (PVA – average MW 18 000 – 23 000, 87-89%, Sigma Aldrich, South Africa) in deionized water while magnetically stirring the mixture. The solvent was allowed to evaporate for 3 h and then the microspheres were subsequently filtered and rinsed several times. Microspheres were sieved in order to obtain the desired size range between 100 and 200μm.
Both PCL disks and solid microspheres were gamma- sterilized at 25 kGy at Isotron (Pty) Ltd. (Kempton Park, Johannesburg, South Africa), followed by 70% ethanol for 30 min and rinsed thrice in phosphate buffer saline (PBS) prior to testing.
Other reagents or kits
All chemicals were supplied by Sigma-Aldrich (St. Louis, United States of America) unless specified otherwise. Dulbecco’s Modified Eagles Medium (DMEM) with glucose, sodium pyruvate, L-glutamine and heat-inactivated fetal calf serum (FCS) were purchased from The Scientific Group (Johannesburg, South Africa). Penicillin, streptomycin and fungizone were supplied by Sigma- Aldrich (St. Louis, United States of America) and trypsin/ versene was obtained from Highveld Biological (Pty) Ltd (Sandringham, South Africa). Sterile cell culture flasks and plates were obtained through Laboratory Equipment Supplies (Germiston, South Africa) and 0.22 μm syringe filters from Aqualytic CC (Irene, South Africa).
General cell culture maintenance
Cells were grown in both 25 cm2 and tissue culture flasks at 37°C, 5% CO2 in a Forma Scientific water-jacketed incubator (Ohio, United States of America). The maintenance of both the SKUT-1 and CRL-1701 cell lines consisted of DMEM supplemented with 10% heat-inactivated FCS, penicillin (100 μg/l), streptomycin (100 μg/l) and fungizone (250 μg/l).
Since tissue culture plastic is known for optimal cell attachment cells were seeded in sterile 24-well plates as the 100% control with the PCL disks used as the experiment [20]. Cells were seeded onto these disks to serve as a 2D experimental setup. For the 3D experiments, 3.237 μg of solid PCL microspheres were weighed off in order to correspond with the surface area of one 24-well size and were placed in individually sterilized glass vials into which the cells were seeded.
All experiments were conducted on polymer samples that had been incubated in complete DMEM for 24 hr prior to seeding. Experiments consisted of the following time periods: 24 hr, 72 hr and 5 days. Cells were seeded at a density of 100 000 for 24 hr experiments, 50 000 cells for 72 hr and 25 000 cells for 5 day experiments.
Cytotoxicity studies
The effects of any possible residual solvent dimethyl sulphoxide (DMSO) present in the PCL solid microspheres were analyzed for cellular cytotoxicity. Since the PCL disks did not undergo the same solvent preparation procedure, it was considered a positive control.
Solid microspheres and PCL disks (0.01 g) were weighed off respectively and sterilized as described previously. The PCL was incubated in 10 ml cell culture medium for the following time periods: 24 hr, 72 hr, 5 days, and 1 month to allow for any residual solvent leaching. Medium was subsequently separated from the polymer and used as the experimental medium to conduct a 24 hr metabolic analysis. Medium that did not undergo polymer incubation served as the 100% control.
Metabolic activity
The 3-[4, 5 dimethyl thiazol-2-yl]-2, 5- diphenyl tetrazolium bromide (MTT) assay determines the amount of viable and metabolically active cells by monitoring formazan formation. MTT is cleaved in active mitochondria by the mitochondrial dehydrogenase enzyme to form dark purple formazan crystals when incubated with live cells [21]. The colour intensity is directly correlated to the amount of metabolically active cells and is quantified at the absorbance of 570nm (ref. 630nm) using an ELx800 Universal Microplate Reader from Bio-Tek Instruments Inc. (Vermont, United States of America).
SKUT-1 and CRL-1701 cells were trypsinized and seeded at 50 000 cells per well in 96-well plates and allowed to attach for 24 hr at 37°C. Subsequently, 20 l MTT (5 mg/ml in PBS) was added to 200 l medium in each well and left to incubate at 37°C for 3 ½ h. The supernatant was removed without disturbing the attached cells. Cells were gently washed with 200 l PBS, after which 200 l DMSO was added per well and shaken for 1 hr. Consequently, 100 l of the supernatant was then removed and transferred to a new 96-well plate in which the absorbance was measured. All experiments were conducted thrice with n=10.
24 h pre-conditioning of PCL polymers
It has been shown that PCL preconditioned with medium for 24 hr ensures extracellular matrix (ECM) deposition on the PCL prior to seeding in order to ensure and improve cell adhesion [21].
Cell attachment density
Observation of the cell by scanning electron microscopy (SEM) allowed for the qualification of cellular density as it illustrates surface observation of cells’ morphology and density and the substrate to which they are attached. Cells cultured on the 2D PCL polymer disk that has been treated in complete medium for 24 hr prior to cell seeding, were compared to those cells seeded on PCL disks that did not undergo the treatment. Cells cultured on disks that were treated with partial medium served as a control in order to rule out the effects of the proteins found in the FCS.
SKUT-1 and CRL-1701 cells were trypsinized and seeded at 100 000 cells per well in a 24-well plate. Cells were allowed to attach for 24 hr at 37°C. Experiments were terminated by rinsing the samples in PBS to remove nonadherent cells. Cells were fixed by placing samples in 2.5 gluteraldehyde in 1.15 M Na+/K+ buffer for 1 hr. Samples were rinsed thrice in 0.15 M Na+/K+ buffer and immersed in osmium tetroxide for 30 min. Subsequently samples were rinsed thrice in 0.15 M Na/K buffer, followed by a graded dehydration in ethanol from 30% to 100%. Samples were covered in hexamethyldisilazane, left to dry in a dessicator overnight, sputter-coated in gold and viewed under a JSM 840 Scanning Electron Microscope (JEOUL, Tokyo, Japan). All experiments were conducted thrice with n=3.
Time-based assessment of cell attachment characteristics and cell morphology
Cell attachment characteristics were analyzed when the cells were propagated directly on PCL disks and solid microspheres and SEM allowed for assessment of cellular morphology, enabling the comparison of cells cultured on the 2D PCL polymer disk to those cells cultured on the 3D PCL microspheres.
SKUT-1 and CRL-1701 cells were trypsinized and seeded in 24-well plate on PCL disks, and in glass vials on 3.237μg solid microspheres. The cells were allowed to attach for 1) 24 hr, 2) 72 hr and 3) 5days at 37°C. All experiments were conducted thrice with n=3.
Statistics
Analysis of data was conducted in consultation with Prof. Becker, a biostatistician at Medical Research Council (MRC) Pretoria, South Africa. Experiments were conducted as a factorial design with treatment at three levels (tissue culture plate, 2D PCL polymer disk, 3D PCL polymer microsphere), time at three levels (24 hr, 72 hr, 5 days) and cells at two levels (SKUT-1, CRL-1701). Data were analyzed using an appropriate analysis of variance for this 3x3x2 factorial design with five replicates. This design has 112 degrees of freedom for residual error which exceeds the conventional requirement of 30.
Results
Cytotoxicity- and metabolic studies
Viability of both the SKUT (Figure 1A) and CRL-1701 (Figure 1B) cell lines remained above 80% when grown in either the PCL disk or PCL microspheres extracts which had been incubated for 24 hr, 72 hr or 5 days.
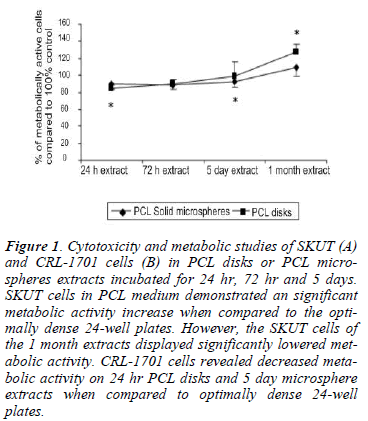
Figure 1: Cytotoxicity and metabolic studies of SKUT (A) and CRL-1701 cells (B) in PCL disks or PCL microspheres extracts incubated for 24 hr, 72 hr and 5 days. SKUT cells in PCL medium demonstrated an significant metabolic activity increase when compared to the optimally dense 24-well plates. However, the SKUT cells of the 1 month extracts displayed significantly lowered metabolic activity. CRL-1701 cells revealed decreased metabolic activity on 24 hr PCL disks and 5 day microsphere extracts when compared to optimally dense 24-well plates.
SKUT cells in the PCL medium extracts displayed a statistically significant increase in metabolic activity compared to the control, (between 120-130%). CRL-1701 cells cultured within the PCL extracts revealed decreased metabolic activities of between 80% and 98% when compared to the control. This was however, only statistically lower when cells were cultured on the 24 hr PCL disk extract and 5 day PCL microsphere extract.
The metabolic activity of the SKUT cell line decreased (80% on the PCL microsphere extract and 65% on the PCL disk extract) when cultured within the 1 month PCL disk and microsphere extracts when compared to the control. The CRL-1701 cell line displayed the opposite effect with an increase in metabolic activity over the control of 120% (P<0.05) when cultured in the PCL disk extract and 102% when cultured on the PCL microsphere extract respectively.
24 h pre-conditioning of PCL polymers
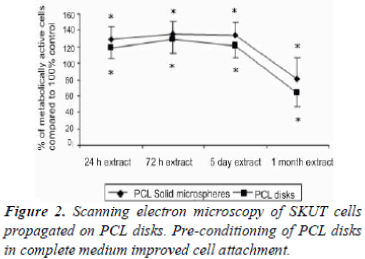
SKUT cells grown on PBS control PCL disks for 24 hr were viewed by means of SEM (100x (a) and 500x (b) magnification respectively of SKUT cells grown on PCL disks). PCL disks were pre-conditioned to partial medium and complete medium respectively to evaluate. Partial medium pre-conditioning was demonstrated to improve cellular attachment density when compared to the PBS control. When the PCL disks were pre-conditioned in complete medium, a notable difference was observed where the SKUT cells attached with a packed cell density (Figure 2). CRL-1701 cells grown on PCL disks viewed under SEM at 100x and 500x magnifications, where the PCL disks were pre-conditioned to partial medium and complete medium are illustrated in Figure 3C and 3D and Figure 3E and 3F respectively. Attachment density of the CRL-1701 cells was sparse on PCL disks pre-conditioned in PBS. However, an increased cell attachment density was observed on PCL disks treated in partial medium and complete medium. Once again, PCL disks preconditioned in complete medium supported the greater amount of initial cell attachment as qualitatively observed in the cell density.
Time-based assessment of cell attachment characteristics and cell morphology
SKUT and CRL-1701 cells were grown on PCL polymers for time frames of 24 h, 72 h and 5 days. Micrographs were taken at 100x magnification.
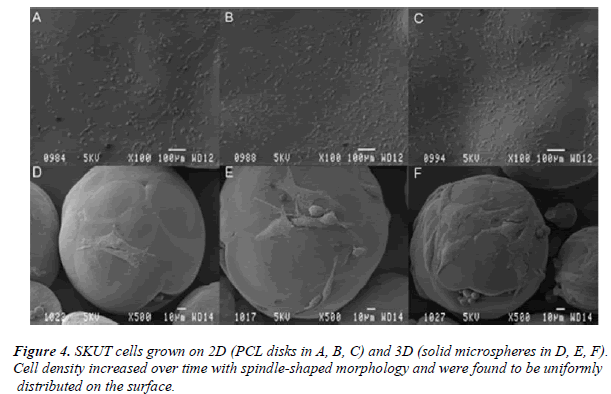
Figure 4 depicts SKUT cells grown on both the 2D (PCL disk: A, B and C) and 3D (PCL solid microspheres: D, E and F) PCL models. Cells that have attached for 24 h on
the PCL disk and PCL solid microspheres are illustrated in A and D respectively. Cells which have been allowed to proliferate for 72 hr on the PCL disk and PCL solid microspheres are observed in B and E respectively; followed by the micrographs where the cells proliferated for a 5 day time period on the PCL disk and PCL solid microspheres (C and F respectively).
SEM illustrated the density of the SKUT cells propagated on PCL disk increased over the 5 day time period. Cells PCL solid microspheres, density of the cells increased over time, though was never as populated as they were on displayed spindle-like morphology and were evenly distributed throughout the entire surface. When grown on the disk. Morphology remained spindle-shaped with several cells undergoing mitosis. Cells were observed to wrap around the entire solid microsphere surface.
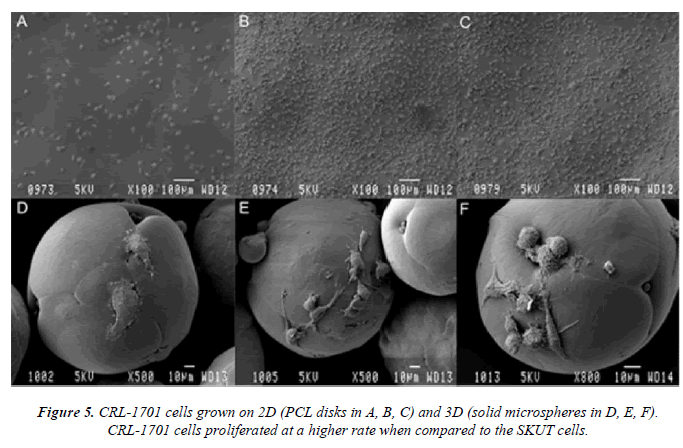
CRL-1701 cells depicted in figure 5 illustrate cells grown on both the 2D (disk: A, B and C) and 3D (solid microspheres: D, E and F) PCL models. Cells that have attached for 24 hr on the PCL disk and PCL solid microspheres are illustrated in A and D, respectively. Cells that have been allowed to proliferate for 72 hr on the PCL disk and PCL solid microspheres are seen in B and E respectively; followed by the micrographs. Cells proliferated for a 5 day time period on the PCL disk and PCL solid microspheres are shown in C and F respectively.
CRL-1701 cells proliferated at a faster rate than the SKUT cells and were observed to populate the entire surface of the PCL disk. These cells displayed a more fibroblast- like morphology which is characteristic of this particular cell line. Most cells were in mitosis. When grown on PCL solid microspheres, the density of the cell population on the surface increased over time. Cells maintained a mainly mitotic state. Many cytoplasmic extensions were illustrated; this allowed the cells to fuse microspheres to each other, forming larger cell/microsphere connections (data not shown).
Discussion
Previous studies have reported that the metabolic activity of SMC’s grown on PLLA polymers decreased after a 1 month period and was attributed to the possibility of lactic acid release from the polymer during degradation, along with the production of cellular lactic acid which occurs as cells proliferate [22].
Metabolic activity of both the SKUT and CRL-1701 cell lines remained above 70% (figure 1A and B) when cultured within 24 hr medium extracts to 1 month-old extracts. Initial degradation products and any chemical leaching from the PCL remained non-toxic towards the cells’ viability and therefore did not prove to be significantly cytotoxic. A significantly lowered metabolic activity was, however, observed when SKUT cells were cultured within the 1 month- old medium extracts (both the PCL disk and PCL solid microsphere).
Surface properties of implant material play a critical role in the progress of cell adhesion [23]. It was reported that cells attach to surfaces with a slight net negative charge and that cell adhesion is mediated by specific cell surface receptors for molecules in the extracellular matrix [24]. The PCL surface is hydrophobic and is therefore not ideally suited for cell attachment. Instead of radical chemical or surface modification treatments, preconditioning of the PCL surface with complete cell culture media has been previously illustrated to improve cell attachment density [22].
Cells attached with minimal density when the PCL disk was conditioned in the PBS control (Figure 2A and B). Density of both the SKUT and CRL-1701 cell lines increased when allowed to attach on the partial medium conditioned disks. Increased cell attachment density could be ascribed to the presence of sugars in DMEM leading to an increased surface hydrophilicity. Both the SKUT and CRL-1701 cells attached with a notable packed density when attached on the complete medium treated disks. This marked increased in cell attachment density is postulated to be due to proteins present in the FCS such as fibrinogen and albumin laying down an extracellular matrix that assist in cell attachment.
In vitro smooth muscle development yields two morphologically distinct smooth muscle phenotypes [25]. These include the synthetic smooth muscle cell which is noncontractile, highly proliferative, migratory, and is able to modify its local microenvironment by producing a wide range of molecules, including extracellular matrix and growth factors [4]. Also produced is the mature muscle cell which by comparison is highly contractile, less proliferative, and less synthetic and is represented by the classic mature smooth muscle spindle shape [22]. Mature gastrointestinal smooth muscle cells preserve the developmental prospective to be able to modulate their cellular phenotypes both in vivo and in vitro [4]. These cells are therefore seen to exist on a flexible differentiation range between smooth muscle myoblast and mature smooth muscle myocyte [4]. Cellular flexibility is important both for regular differentiation and maturation of gastrointestinal smooth muscle, and also plays a significant role in a variety of gastrointestinal diseases [4].
Morphologically flattened adherent characteristic cells (as opposed to rounded cells) are indicative of cells attaching to a particular surface [26]. The latter was observed in the SKUT cells adhered since these cells were mainly spread out and spindle-shaped (with a few cells in mitosis) when adhered to both the PCL disks and solid microspheres after 24 hr. By 5 days, the cells had proliferated and become more densely populated on the disk and sphere. The cells were well flattened and seen to closely follow the contours of the spheres, suggesting high focal adhesion. These adhesions are indicative that the cells proliferate and adhere strongly to the PCL microspheres [27]. The spherical shape observed suggests that the SMCs were in their mature, contractile phenotype. Cells encompassed most of the surface of the spheres and are anticipated to cover the entire surface if cultured for a longer time period.
In contrast, the CRL-1701 cells propagated on the PCL disk were observed to consist mainly of rounded mitotic cells, thus a proliferative, non-contractile phenotype. However, when the CRL-1701 cells were grown on PCL solid microspheres, it was observed that their morphology was mainly spread out and flattened. Many cytoplasmic extensions were noted, indicating that the cells maintained a contractile phenotype. This in vitro study suggests that hollow microspheres allow for further cell expansion with a sheltered environment to protect cells from sheer stress experienced in vivo.
The solid PCL microspheres provide a larger surface area for growth than a PCL disk would, allowing for significant cell expansion. When applied in biomedical applications, surgical procedures promise to be minimally invasive since microspheres are small enough to be injected in vivo. Microspheres can also be engineered to be hollow, porous and ported [28], yielding even higher surface area for cell growth while maintaining sufficient porosity for nutrient and oxygen supply and metabolite exchange and becoming cell microcarriers providing a bulking functional effect.
Acknowledgements
The Biomaterials and Polymer Division from the Council of Scientific and Industrial Research (Pretoria, South Africa), for the project concept, polymer design and bursary. The Department of Physiology, University of Pretoria (Pretoria, South Africa) for funding of the project and use of the Cell Culture Laboratory. The Microscopy Unit, University of Pretoria (Pretoria, South Africa) for use and assistance with the SEM preparation and image captivating.
References
- Jawad H, Lyon AR, Harding SE, Ali NN, Boccaccni AR. Myordial tissue engineering. Br Med Bul 2008; 97: 31-47.
- Sachlos E, Czernuszka JT. Making tissue engineering scaffolds work. Review on the application of solid free- form fabrication technology to the production of tissue engineering scaffolds. Eur Cells Mater J 2003; 5: 29-40.
- Wiesmann HP, Joos U, Meyer U. Biological and bio- physical principles in extracorporal bone tissue engi- neering. Part II. Int J Oral Maxillofac Surg 2004; 33: 523-530.
- McHugh KM. Molecular analysis of gastrointestinal smooth muscle development. J Pediatr Gastroenterol Nutr 1996; 23: 379-394.
- Glaser R, Lu MM, Narula N, Epstein JA. Smooth mus- cle cells, but not myocytes, of host origin in trans- planted human hearts. Circulation 2002; 106: 17-19.
- Bitar KN. Aging and GI smooth muscle fecal inconti- nence: Isbioengineering an option. Exp Gerontol 2005; 40: 643-649.
- Gogolewski S. Bioresorbable polymers in trauma and bone surgery. Injury 2000; 31: 28-32.
- Cao W, Wang A, Jing D, Gong Y, Zhao N, Zhang X.
- Banu N, Tsuchiya T, Sawada R. Effects of a biode- gradable polymer sythesized with inorganic tin on the chondrogenesis of human articular chondrocytes. J Bi- omed Mater Res 2006; 77: 84-89.
- Lin CY, Schek RM, Mistry AS, Shi X, Mikos AG, Krebsbach PH, Hollister SJ. Functional bone engineer- ing using ex vivo gene therapy and topology- optimized, biodegradable polymer composite scaffolds. Tissue Eng 2005; 11: 1589-1598.
- Zhu Y, Chian KS, Chan-Park MB, Mhaisalkar PS, Rat- ner BD. Protein bonding on biodegradable poly (L- lactide-co-caprolactone) membrane for esophageal tis- sue engineering. Biomaterials 2006; 27: 68-78.
- Kazarian SG, Chan KL, Maquet V, Boccaccini AR.
- Sittinger M, Reitzel D, Dauner M, Hierlemann H, Hammer C, Kastenbauer E, Planck H, Burmester GR, Bujia J. Resorbable polyesters in cartilage engineering: affinity and biocompatibility of polymer fiber struc- tures to chondrocytes. J Biomed Mater Res 1996; 33: 57-63.
- Lin WJ, Flanagan DR, Linhardt RJ. A novel fabrication of poly (e-caprolactone) microspheres from blend of poly (e-caprolactone) and poly (ethylene glycol)s. Pol- ymer 1999; 40: 1731-1735.
- Ria B, Ho KH, Lei Y, Si-Hoe KM, Ten CMJJ, bin Ya- cob K, Chen F, Ng FC, Yeoh SH. Polycaprolactone- 20% tricalcium phosphate scaffolds in combination with platelet-rich plasma for the treatment of critical- sized defects of the mandible: a pilot study. J Oral Maxilofac Surg 2007; 65: 2195-2205.
- Baxter LC, Frauchiger V, Textor M, ap Gwynn I, Rich- ards RG. Fibroblast and osteoblast adhesion and mor- phology on calcium phosphate surfaces. Eur Cells Ma- ter J 2002; 4: 1-17.
- Kang SW, Jeon O, Kim BS. Poly (lactic-co-glycolic acid) microspheres as an injectable scaffold for carti- lage tissue engineering. Tissue Eng 2005; 11: 438-447.
- Hong Y, Gao C, Xie Y, Gong Y, Shen J. Collagen- coated polylactide microspheres as chondrocyte car- riers. Biomaterials 2005; 26: 6305-6313.
- SenumaY, Franceschin S, Hiborn JG, Tissieres P, Bis- son I, Frey P. Bioresorbable microspheres by spinning disk atomization as injectable cell carrier: from prepa- ration to in vitro evaluation. Biomaterials 2000; 21: 1135-1144.
- Van de Loosdrecht AA, Beelen RH, Ossenkoppele GJ, Broekhoven MG, Langenhuijsen MM. A tetrazolium-
- Bacakova L, Filova E, Rypacek F, Svorcik V, Stary V. Cell adhesion on artificial materials for tissue engineer- ing. Physiol Res 2004; 53: S35-S45.
- McGlohorn JB, Holder WD, Grimes LW, Thomas CB, Burg KJL. Evaluation of smooth muscle response using two types of porous polylactide scaffolds with differing pore topography. Tissue Eng 2004; 10: 505-514.
- Zhang YM, Bataillon-Linez P, Huang P, Zhao YM, Han Y, Traisnel M, Xu KW, Hildebrand HF. Surface analyses of micro-arc oxidized and hydrothermally treated titanium and effect on osteoblast behaviour. J Biomed Mater Res 2004; 68: 383-391.
- Freshney RI. Culture of Animal Cells: a manual of
- Hedin U, Thyberg J. Plasma fibronectin promotes modulation of arterial smooth-muscle cells from con- tractile to synthetic phenotype. Differentiation 1987; 33: 239-246.
- Hunter A, Archer CW, Walker PS, Blunn GW. At-
- Williamson MR, Woollard KJ, Griffiths HR, Coombes AGA. Gravity spun polycaprolactone fibres for appli- cations in vascular tissue engineering: Proliferation and function of human vascular endothelial cells. Tissue Eng 2006; 12: 45-51.
- Naidoo K, Rolfes H, Easton K, Moolman S, Chetty A, Richter W, Nilen R. An emulsion preparation for novel micro-porous polymeric hemi-shells. Mater Lett 2008; 62: 252-254.