ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
- Biomedical Research (2015) Volume 26, Issue 2
Protective effect of polysaccharides from Cortex Eucommiae on exhaustive exercise-induced oxidative stress in mice.
1Physical Education Institute, Henan University of Technology, Zhengzhou 450001, P. R. China
2Department of Physical Education, Central South University, Changsha 410083, P. R. China
- *Corresponding Author:
- Bo Qi
Department of Physical Education
Central South University
Changsha 410083, P. R. China
Accepted date: March 10 2015
Cortex Eucommiae is well known in traditional Chinese herbal medicine. The present study investigated the effects of polysaccharides from Cortex Eucommiae (PCE) on exhaustive exercise- induced oxidative stress in mice by measuring the changes in the activities of superoxide dismutase (SOD), glutathione peroxidase (GPX) and catalase (CAT) and levels of malondialdehyde (MDA) and 8-hydroxydeoxyguanosine (8-OHdG). The mice were randomly divided into four groups: a negative control group, a low-dose PCE intervention group, a medium-dose PCE intervention group and a high-dose PCE intervention group. The mice in the control group were given distilled water whereas those in the three intervention groups were given different doses of PCE (10, 50 and 100 mg/kg). After 28 days, the mice were made to perform an exhaustive swimming exercise. Changes in the activities of the main antioxidant enzymes and the levels of MDA and 8-OHdG in the blood, liver and muscle of the mice were measured. The results of the study showed that PCE increased the activities of SOD, GPX and CAT and decreased the levels of MDA and 8-OHdG in mice, suggesting that PCE has a protective effect on exhaustive exercise- induced oxidative stress.
Keywords
polysaccharides, Cortex Eucommiae, oxidative stress, exhaustive swimming exercise, superoxide dismutase, glutathione peroxidase, catalase, malondialdehyde, 8-hydroxydeoxyguanosine
Introduction
Cortex Eucommiae (also known as Du-Zhong or Tu-chung), the dried bark of Eucommia ulmoides Oliv. (family: Eucommiaceae), is one of the oldest tonic herbs in traditional Chinese herbal medicine [1]. It is widely used to strengthen muscles and lungs, decrease blood pressure, prevent miscarriages, improve the function of the liver and kidneys, and increase longevity [2]. Recently, several studies have shown that the extracts of Cortex Eucommiae have multiple pharmacological properties such as anti-oxidant, anti-hypercholesterolaemic, anti-obesity, anti-complementary, anti-microbial, anti-inflammatory, hypoglycaemic and hypolipidaemic properties and that they protect against ultraviolet irradiation [3-6]. Cortex Eucommiae contains many phytochemicals such as lignans, iridoids, flavonoids, polysaccharides and terpenes. To date, the pharmacological properties of Cortex Eucommiae have mainly been attributed to lignans and flavonoids. Recent studies have suggested that polysaccharides from Cortex Eucommiae (PCE) also exhibit significant pharmacological properties such as antioxidant, anti-tumour and anti-complementary properties [7,8]. However, the effects of PCE on exercise- induced oxidative stress have not yet been reported. Hence, the present study aimed to evaluate the effect of PCE on oxidative stress induced by exhaustive swimming exercise in mice.
Materials and Methods
Plant material
Dried Cortex Eucommiae was purchased from a local herbal drug market (Changsha, China) and was identified by Dr Meng YJ, a botanist at the Central South University (Changsha, China). Voucher specimens (KIM-NA-9132) were preserved in the herbarium of the Central South University. The dried Cortex Eucommiae was ground to fine powder and was stored at 5 °C until further use.
Chemicals and reagents
Assay kits for superoxide dismutase (SOD), glutathione peroxidase (GPX), catalase (CAT) and malondialdehyde (MDA) were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). The assay kit for 8-hydroxydeoxyguanosine (8-OHdG) was purchased from Japan Institute for the Control of Aging (Fukuroi, Japan). All other chemicals used in this research were of analytical grade and were obtained from Hunan Chemical Reagent Co. (Changsha, China).
Experimental animals
Healthy male Kunming mice (weight, 18-22 g) were obtained from the experimental animal centre of the Henan University of Technology. The mice were housed with ad libitum access to food and water and were maintained under constant environmental conditions (temperature, 22 °C ± 2 °C; humidity, 50% ± 5%; and 12-h light:12-h dark cycle starting at 07:00). Experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals of the Central South University and were approved by the Ethics Committee.
Extraction of PCE
PCE was extracted as described previously [9], with slight modifications. Powdered Cortex Eucommiae was soaked in 95% ethanol to remove pigments and small lipophilic molecules. The residue was then washed thrice with distilled water (1:15, v/v ) at 100 °C for 30 min to extract PCE. All the water extracts were combined, filtered, concentrated and precipitated using absolute ethanol (1:4, v/v) and stored at 4°C overnight. The precipitate was collected by centrifugation (8000 × g for 30 min) and was deproteinated using Savage method [10]. Finally, the supernatant was lyophilised to obtain crude PCE.
Experimental design
After one week of accommodation, the mice were randomly divided into the following four groups (10 mice in each group) based on their body weight: negative control (C) group, low-dose PCE intervention (LP) group, medium- dose PCE intervention (MP) group and high-dose PCE intervention (HP) group. The mice in the C group were given distilled water (2.0 mL) whereas those in the three intervention groups were given different doses of PCE (10, 50 and 100 mg/kg). Samples were administered once a day for 28 consecutive days by gavage using a feeding needle. PCE solutions used in intervention groups were prepared by dissolving PCE in 2.0 mL distilled water.
After 28 days, the mice were made to perform an exhaustive swimming exercise, as described previously [11]. Briefly, 30 min after the last treatment, the mice were placed individually in acrylic plastic tanks (50 cm × 50 cm × 40 cm) containing 30-cm deep water that was maintained at 25°C ± 1°C. The mice were loaded 7% of the body weight of lead threads at the bases of the tails. The mice were identified as being exhausted when they sunk into the water and could not rise to the surface within a 10-s period.
Assay of biochemical parameters
After completing the exhaustive swimming exercise, all the mice were immediately anesthetised using ethyl ether and sacrificed by exsanguination via the abdominal aorta. Blood samples were collected and serum was immediately separated by centrifugation at room temperature (2000 × g for 10 min). Next, the liver and hindlimb skeletal muscles were carefully removed and rinsed in ice cold physiological saline solution (0.9% NaCl), blotted dry and stored at -80°C until biochemical parameters were analysed. Next, the activities of SOD, GPX, and CAT and the levels of MDA and 8-OHdG in the blood, liver and muscle were measured using procedures recommended in the commercial assay kits.
Statistical analysis
All data are expressed as mean ± standard deviation (SD). Statistical comparisons were made using one-way analysis of variance. P values of <0.05 were considered statistically significant. SPSS software version 17.0 (SPSS Inc., Chicago, Illinois) was used for all analyses.
Results
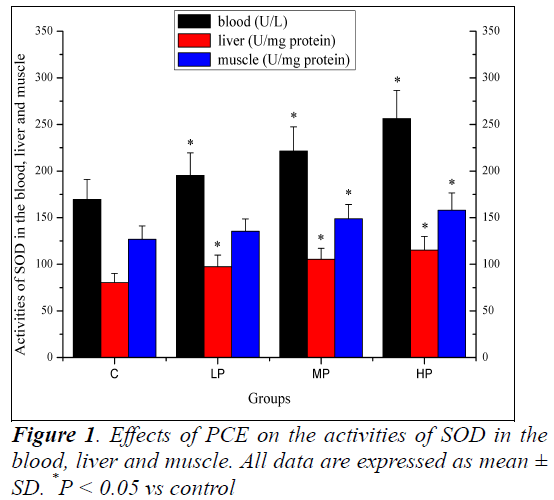
Effects of PCE on the activities of SOD in the blood, liver and muscle
Effects of PCE on the activities of SOD in the blood, liver and muscle are shown in Figure 1. The activities of SOD in the blood and liver of mice in the LP, MP and HP groups were significantly higher (P < 0.05) than those of mice in the C group. The activity of SOD in the muscle of mice in the MP and HP groups was significantly higher (P < 0.05) than that of mice in the C group. Although the activity of SOD increased in the muscle of mice in the LP group, the increase was not significant (P > 0.05).
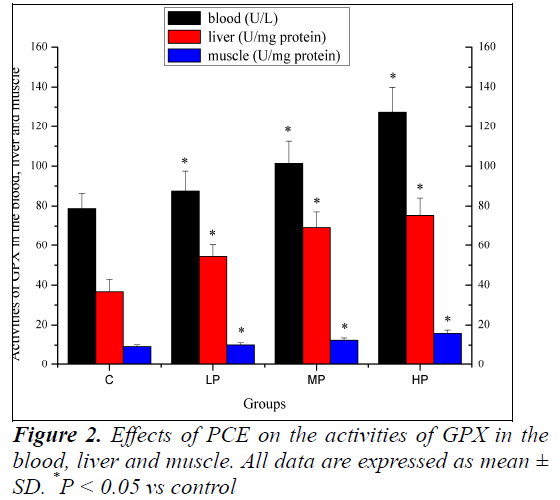
Effects of PCE on the activities of GPX in the blood, liver and muscle
Effects of PCE on the activities of GPX in the blood, liver and muscle are shown in Figure 2. The activities of GPX in the blood, liver and muscle of mice in the LP, MP and HP groups were significantly higher (P < 0.05) than those of the mice in the C group.
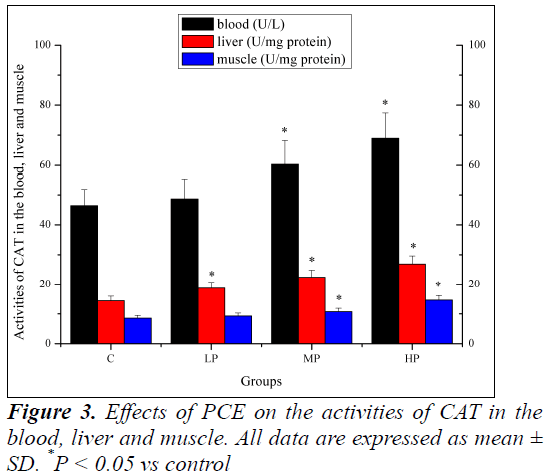
Effects of PCE on the activities of CAT in the blood, liver and muscle
Effects of PCE on the activities of CAT in the blood, liver and muscle are shown in Figure 3. The activity of CAT in the liver of mice in the LP, MP and HP groups was significantly higher (P < 0.05) than that of mice in the C group. The activities of CAT in the blood and muscle of mice in the MP and HP groups were significantly higher (P < 0.05) than those of mice in the C group. Although the activities of CAT increased in the blood and muscle of mice in the LP group, the increase was not significant (P > 0.05).
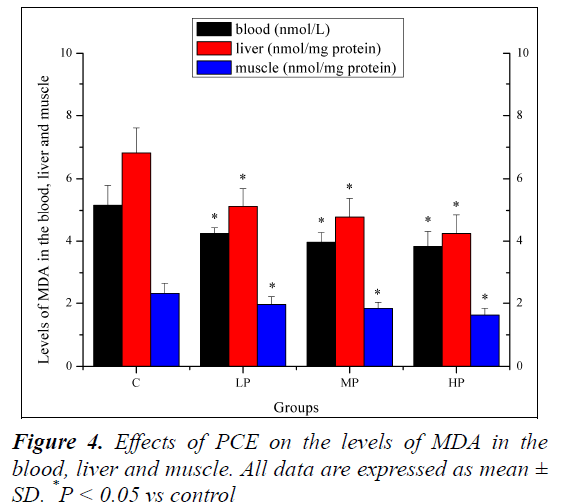
Effects of PCE on the levels of MDA in the blood, liver and muscle
Effects of PCE on the levels of MDA in the blood, liver and muscle are shown in Figure 4. The levels of MDA in the blood, liver and muscle of mice in the LP, MP and HP groups were significantly lower (P < 0.05) than those of mice in the C group.
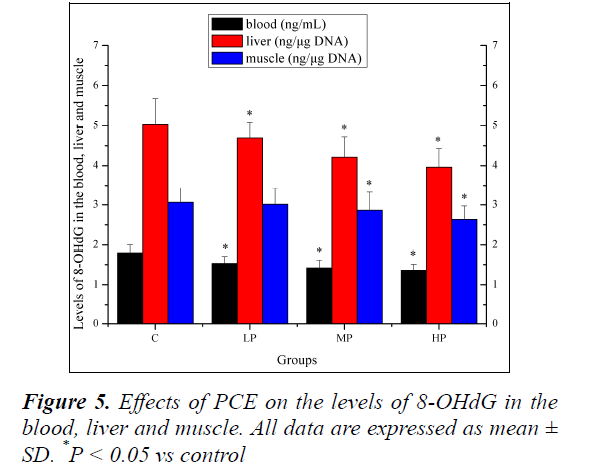
Effects of PCE on the levels of 8-OHdG in the blood, liver and muscle
Effects of PCE on the levels of 8-OHdG in the blood, liver and muscle are shown in Figure 5. The levels of 8-OHdG in the blood and liver of mice in the LP, MP and HP groups were significantly lower (P < 0.05) than those mice in the C group. The level of 8-OHdG in the muscle of mice in the MP and HP groups was significantly lower (P < 0.05) than that of mice in the C group. Although the level of 8-OHdG decreased in the muscle of mice in the LP group, the decrease was not significant (P > 0.05).
Discussion
It is well-established that physical exercise is associated with increased free radical generation, primarily due to a dramatic increase in oxygen uptake both at the whole body level and at local tissue levels [12]. Production of these deleterious free radicals differs based on the intensity, frequency and duration of various exercises [13]. There is evidence that acute, intense physical exercise induces oxidative stress due to increased generation of reactive oxygen species (ROS) and/or reduced antioxidant capacity [14]. These excessive ROS then attack vital biomolecules such as plasma membrane lipids and proteins, thus deteriorating normal cellular functions. Moreover, exercise-induced oxidative stress may be associated with muscle fatigue, muscle damage and decreased physical performance [15,16]. More than three decades have elapsed since the first findings related to exercise-induced oxidative stress, and the topic is still of interest to researchers in different scientific fields [17].
Previous studies have shown that exercise-induced oxidative stress can be counteracted by functional food ingredients such as polysaccharides, flavonoids, saponins and phenolics [11,18-20]. Therefore, the present study was designed to verify the possible protective effects of PCE on exhaustive swimming exercise-induced oxidative stress by measuring the activities of antioxidant enzymes and levels of MDA and 8-OHdG in mice receiving PCE intervention and those not receiving PCE intervention (control group).
The antioxidant defence systems (AOS) of a living body include antioxidant enzymes and nutrients, which may be involved in reducing oxidative stress. Because antioxidant enzymes play an important role in providing protection against free radical damage, a decrease in the activity or expression of these enzymes may predispose tissues to free radical damage [21]. SOD, CAT and GPX are important components of the AOS. CAT catalyses the detoxification of H2O2 to H2O, GPX activates GSH-scavenging reactions of free radicals (˖OH) and singlet oxygen species (1O2), and SOD catalyses the dismutation of two superoxide anions to form H2O2 and O2 [22]. The present study showed that the activities of SOD, CAT and GPX in the blood, liver and muscle of mice in the MP and HP groups were significantly higher (P < 0.05) than those of mice in the C group. The increase in the activities of antioxidant enzymes in mice receiving PCE intervention may be because of the antioxidant activities of PCE itself.ustive exercise-induced oxidative stress is characterised by ROS-induced lipid peroxidation, DNA damage and protein degradation [23]. MDA is a secondary product generated during the oxidation of polyunsaturated fatty acids and is frequently used as an indicator of lipid peroxidation and oxidative stress in vivo [24]. Increased MDA levels confirm the increase in exhaustive exercise- induced oxidative stress. Increased MDA levels can be normalised using functional food ingredients [15,19,24]. In the present study, levels of MDA in the blood, liver and muscle of mice in the LP, MP and HP groups were significantly lower (P < 0.05) than those of mice in the C group, indicating that PCE could effectively reduce lipid peroxidation.
Oxidative damage of DNA usually involves damage to single bases. It is estimated that ROS alter at least 35 different bases. Base damage differs depending on the ROS that damages the DNA [25]. Because 8-OHdG is not an intermediate of normal nucleotide metabolism, it is used as an important indicator of DNA damage and repair. The present study showed that the levels of 8-OHdG in the blood, liver and muscle of mice in the MP and HP groups were significantly lower (P < 0.05) than those of mice in the C group, indicating that PCE could prevent DNA damage and attenuate oxidative stress. However, the detailed mechanism of PCE action is unclear. This may be because PCE also increases DNA repair.
Conclusions
The results of this study indicate that PCE has a protective effect on exhaustive exercise-induced oxidative stress in mice because it increases the activities of antioxidant enzymes and decreases the levels of MDA and 8-OHdG in the blood, liver and muscle. Although the detailed mechanism of the protective effect of PCE remains to be elucidated, this study provides evidence that supports the use of PCE as an effective ergogenic aid for athletes.
Acknowledgment
This research was financially supported by the Science Foundation of Henan Province of China.
References
- Li ZY, Deng XL, Huang WH, Li L, Li H, Jing X, Tian YY, Lv PY, Yang TL, Zhou HH, Ouyang DS. Lignans from the bark of Eucommia ulmoides inhibited Ang II-stimulated extracellular matrix biosynthesis in mesangial cells. Chin Med 2014; 9: 8-10.
- Li Y, Wang MJ, Li S, Zhang YM, Zhao Y, Xie RM, Sun WJ. Effect of total glycosides from Eucommia ulmoides seed on bone microarchitecture in rats. Phytother Res 2011; 25: 1895-1897.
- Liu E, Han L, Wang J, He W, Shang H, Gao X, Wang T. Eucommia ulmoides bark protects against renal injury in cadmium-challenged rats. J Med Food 2012; 15: 307-314.
- Park SA, Choi MS, Kim MJ, Jung UJ, Kim HJ, Park KK, Noh HJ, Park HM, Park YB, Lee JS, Lee MK.Hypoglycemic and hypolipidemic action of Du-zhong (Eucommia ulmoides Oliver) leaves water extract in C57BL/KsJ-db/db mice. J Ethnopharmacol 2006; 107:412-417.
- Kim MC, Kim DS, Kim SJ, Park J, Kim HL, Kim SY, Ahn KS, Jang HJ, Lee SG, Lee KM, Hong SH, Um JY.Eucommiae cortex inhibits TNF-α and IL-6 through the suppression of caspase-1 in lipopolysaccharide-stimulated mouse peritoneal macrophages. Am J Chin Med 2012; 40: 135-149.
- Dai X, Huang Q, Zhou B, Gong Z, Liu Z, Shi S. Preparative isolation and purification of seven main antioxidants from Eucommia ulmoides Oliv.(Du-zhong) leaves using HSCCC guided by DPPH-HPLC experiment. Food Chem 2013; 139:563-570.
- Hong YK, Liu WJ, Li T, She SY. Optimization of extraction of Eucommia ulmoides polysaccharides by response surface methodology. Carbohyd Polym 2013; 92: 1761-1766.
- Zhu H, Zhang Y, Zhang J, Chen D. Isolation and characterization of an anti-complementary protein-bound polysaccharide from the stem barks of Eucommia ulmoides. Int Immunopharmacol 2008; 8:1222-1230.
- Chen XM, Jin Jing, Tang J, Wang ZF, Wang JJ, Jin LQ, Lu JX. Extraction, purification, characterization and hypoglycemic activity of a polysaccharide isolated from the root of Ophiopogon japonicus. Carbohyd Polym 2011; 83: 749-754.
- Chen R, Li Y, Dong H, Liu Z, Li S, Yang S, Li X. Optimization of ultrasonic extraction process of polysaccharides from Ornithogalum Caudatum Ait and evaluation of its biological activities. Ultrason Sonochem 2012; 19: 1160-1168.
- Yan F, Wang B, Zhang Y. Polysaccharides from Cordyceps sinensis mycelium ameliorate exhaustive swimming exercise-induced oxidative stress. Pharm Biol 2014; 52: 157-161.
- Ji LL. Oxidative stress during exercise: implication of antioxidant nutrients. Free Radic Biol Med 1995; 18:1079-1086.
- Sachifumi K, Etsuko T. Vitamin E Supplementation Attenuates Strenuous Exercise Induced DNA Damage and Lipid Peroxidation of the Liver in Rats. Kawasaki J med welfare 2008; 14: 1-7.
- Miyazaki H1, Oh-ishi S, Ookawara T, Kizaki T, Toshinai K, Ha S, Haga S, Ji LL, Ohno H. Strenuous endurance training in humans reduces oxidative stress following exhausting exercise. Eur J Appl Physiol 2001; 84: 1-6.
- Yu SH, Huang HY, Korivi M, Hsu MF, Huang CY, Hou CW, Chen CY, Kao CL, Lee RP, Lee SD, Kuo CH. Oral Rg1 supplementation strengthens antioxidant defense system against exercise-induced oxidative stress in rat skeletal muscles. J Int Soc Sports Nutr 2012; 18: 23-25.
- Jówko E, Sacharuk J, Balasińska B, Ostaszewski P, Charmas M, Charmas R. Green tea extract supplementation gives protection against exercise-induced oxidative damage in healthy men. Nutr Res 2011; 31:813-821.
- Popovic LM, Mitic NR, Radic I, Miric D, Kisic B, Krdzic B, Djokic T. The effect of exhaustive exercise on oxidative stress generation and antioxidant defense in guinea pigs. Adv Clin Exp Med 2012; 31: 313-320.
- Belviranlı M, Gökbel H, Okudan N, Büyükbaş S. Effects of grape seed polyphenols on oxidative damage in liver tissue of acutely and chronically exercised rats. Phytother Res 2013; 27: 672-677.
- Xu HC, Wang MY. Effect of flavonoids from Lotus (Nelumbo nuficera Gaertn) leaf on biochemical parameters related to oxidative stress induced by exhaustive swimming exercise of mice. Biomed Res 2014; 25: 1-5.
- Zhonghui Z, Xiaowei Z, Fang F. Ganoderma lucidum polysaccharides supplementation attenuates exercise-induced oxidative stress in skeletal muscle of mice. Saudi J Biol Sci 2014; 21: 119-123.
- Lee SP, Mar GY, Ng LT. Effects of tocotrienol-rich fraction on exercise endurance capa5city and oxidative stress in forced swimming rats. Eur J Appl Physiol 2009; 107: 587-95.
- Guizani N, Waly MI, Ali A, Al-Saidi G, Singh V, Bhatt N, Rahman MS. Papaya epicarp extract protects against hydrogen peroxide-induced oxidative stress in human SH-SY5Y neuronal cells. Exp Biol Med (Maywood) 2011; 236: 1205-1210.
- Misra DS, Maiti R, Ghosh D. Protection of swimming-induced oxidative stress in some vital organs by the treatment of composite extract of Withania somnifera, Ocimum sanctum and Zingiber officinalis in male rat. Afr J Tradit Complement Altern Med 2009; 3: 534-543.
- Chen Z, Li S, Wang X, Zhang CL. Protective effects of Radix Pseudostellariae polysaccharides against exercise- induced oxidative stress in male rats. Exp Ther Med 2013; 5: 1089-1092.
- Pajović SB, Saicić ZS, Spasić MB, Petrović VM. The effect of ovarian hormones on antioxidant enzyme activities in the brain of male rats. Physiol Res 2003; 52: 189-194.