ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2022) Volume 33, Issue 3
Proficiency of topical platelet rich plasma with vacuum assisted closure over platelet rich plasma alone in diabetic foot ulcers: A clinical prospective comparative study.
Kamal Kumar Arora1, Apurba Patra2, Priti Chaudhary2*
1Department of Orthopaedics, Government Medical College, Amritsar, Punjab, India
2Department of Anatomy, All India Institute of Medical Sciences, Bathinda, Punjab, India
- Corresponding Author:
- Priti Chaudhary
Department of Anatomy
All India Institute of Medical Sciences
Bathinda
Punjab
India
Accepted date: March 09, 2022
Introduction: Type 2 Diabetes Mellitus is usually associated with peripheral neuropathy, peripheral vascular disease with consequential limb ischemia and eventually diabetic foot ulcers. The healing process is slow due to microangiopathy and wound is easily infected with microbials leading to superficial infection, progressing to deep infection and eventually landing in amputation most oft he times.
Plate Rich Plasma (PRP) is very cost effective, readily available blood derivative and has the capability to stimulate cell proliferation and differentiation. It improves tissue healing and regeneration and exhibit potent activities against a number of pathogens.
Vacuum Assisted Closure (VAC), on the other hand, is a new novel way to treat Diabetic Foot Ulcers (DFU) by having Negative Pressure Wound Healing (NPWH). The present study focused on the advantage of (PRP+VAC) dressing over (topical PRP application with its peripheral injection) alone for aiding and enhancing the process of wound healing in DFU.
Materials and methods: This was a prospective comparative study of 100 cases to compare the outcomes of wound healing by topical PRP application with its peripheral injection.
Results: Mean time taken for appearance of granulation tissue, 100% granulation tissue, average reduction in wo und surface area, showed significant (P ≥ 0.005) differences between the (PRP+VAC) and the (topical PRP application with its peripheral injection) dressing groups.
Conclusion: (PRP+VAC) dressings are more effective than conventional (topical PRP application with its peripheral injection) dressings in wound healing of diabetic foot ulcers.
Keywords
Type 2 diabetes mellitus, Plate rich plasma, Vacuum assisted closure.
Introduction
The healing process of Diabetic Foot Ulcer (DFU) is a complex mechanism involving the integrated interface between molecular signals and different cells together with Platelets. These platelets in the presence of a diabetic wound get activated with thrombin and this aPRP releases growth factors like Platelet-Derived Growth Factor (PDGF), Vascular Endothelial Growth Factor (VEGF), Epidermal Growth Factor (EGF), Insulin-like Growth Factor (IGF), and Transforming Growth Factor beta (TGF-β). These growth factors trigger angiogenesis, extracellular matrix production and cytokine release, and help in wound healing [1].
Activated Plate rich plasma, aPRP with 4× of normal concentration platelets (1000000 platelets/microlitre) is very cost effective, readily available blood derivative, with a capacity to stimulate cell proliferation and differentiation. Cellular mitogenesis and angiogenesis are both upregulated by activated platelets [2]. Its high leukocyte concentration, autologous property helps in local debridement and antibacterial activity without an immune reaction [3]. Autologous aPRP not only enhances wound healing but also helps to regenerate skin tissue [4]. It is much advantageous in fracture wound healing, is remedy for skin defects or dental mucosal wounds [5,6].
Proper preparation and centrifuge technique is decisive to obtaining high quality active PRP [7,8]. Lack of biological effect may be due to poor PRP processing or inadequate standard laboratory centrifuges that cannot properly prepare PRP rather than the specialized FDA cleared equipment with validated processes [9].
As activated Platelet Rich Plasma (aPRP) is autologous, immune rejections are a non-issue [10]. It contains the same materials present in the blood that induce clotting, except in higher concentration [11]. It secretes growth factors which function by activating a cytoplasmic signal that further promotes normal gene expression.
Platelets are composed of a cytoskeleton and intracellular structures such as glycogen, lysosomes, and two granules, the dense granule and the alpha-granule.
The alphagranule contains clotting factors, growth factors, proteins and works via degranulation process [12]. (PRP is collected in an anticoagulated form in a tube containing sodium citrate for the these growth factors to remain inactive) [13,14]. These alpha granules bind to the trans membrane receptor of target cells such as mesenchymal stem cells, fibroblasts, endothelial cells and epidermal cells, activating these [15]. This further activates intracellular signal proteins that express a gene sequence directing cellular proliferation, collagen synthesis, extracellular matrix formation, and numerous other pathways to promote healing and repair processes [16].
Damaged platelets with degraded/non-viable cellular components are incapable of inducing this response [17].
Negative Pressure Wound Therapy (NPWT), on the other hand, is a novel way to treat Diabetic Foot Ulcers (DFU) by creating an intermittent negative pressure across these ulcers. NPWT is effective in managing wound infections, soft tissue loss, vascular insufficiency, and traumatic wounds [18,19]. It is a non-invasive method based on well defined, controlled negative pressure application via medical grade reticulated polyurethane ether or polyvinyl foam dressing to wound surfaces. The technique characteristically removes exudates from wounds and hence reduces extravascular, interstitial fluid; subsequently leading to enhanced microcirculation [20]. Taking away of wound fluid removes factors that suppress fibroblasts, vascular endothelial cells and keratinocytes, all of which promote wound healing.
Experimental studies have revealed a positive influence on both local microcirculation and granulation tissue formation. Local mechanical physical factors, yet not completely understood, similar to tissue expansion, and seem to promote cell growth. These studies have revealed that cells, allowed to stretch, tend to divide and proliferate in the presence of soluble mitogens.
One explanation for the high acceptance on part of the therapists and the widespread use of the VAC method in these are the excellent clinical results.
The present was a clinical, prospective comparative study to check the proficiency of topical platelet rich plasma with vacuum assisted closure over only topical platelet rich plasma application in diabetic foot ulcers.
Materials and Methods
This was a clinical prospective comparative study, done on 100 patients of either sex, in their middle years of life, from the demographic profile, having type 2 diabetes mellitus and on oral hypoglycemic drugs (Biguanide/ Thiazolidinediones/Alpha-glucosidase inhibitors) with Diabetic Foot Ulcer (DFU) admitted in the Department of orthopedics at a tertiary care hospital in Punjab, INDIA, to compare the outcomes of wound healing with topical aPRP application, to topical aPRP combined with VAC from January 2019 to December 2020. An informed consent was taken from each participant. Thereafter ethical clearance was taken from IEC of the institution to conduct the study.
Inclusion criteria
➢ Patients within age group of 30-60 years
➢ Patients with a Diabetic Foot Ulcer (DFU) of greater than 4-week duration not healed with conventional conservative methods of treatment.
Exclusion criteria
➢ Patient with systemic disorder (COPD, tuberculosis) etc.
➢ Psychological disorders, unable to maintain personal hygiene.
➢ Patient with coagulopathies.
➢ Advanced peripheral vascular disease secondary to type 2 Diabetes Mellitus.
➢ Other confounding factors (old age, compliance, arteriosclerosis, varicose ulcers, alcoholism, and smoking).
Patients allocated to one of the treatment arms through sampling without replacement method for their random allocation. Patients included in the study underwent routine investigations and initial debridement. Wound size (cm2) measured on day ‘0’(zero) with two largest perpendicular diameters and calibrated on a graph paper, depth of the wounds was not a consideration in the present series as the included patients had sloughing of epidermis and dermis only. On the same day, deep tissue swab was taken and appropriate antibiotics initiated as per culture and sensitivity report. After achieving adequate haemostasis after debridement, a foam band dressing applied over the wounds under aseptic condition with application of intermittent negative pressure after 4 hours of application of dressing; removed and replaced with a new one every four days. All the patients had punch biopsies taken as a part of protocol during follow up at 3, 6 and 12 weeks and at regular intervals thereafter. This was done to confirm the appearance, presence and progression of the granulation tissue, revascularization and to crosscheck any adverse change/malignant transformation of wound, if any. Decrease in size of wound documented at each visit until permanent healing of the wound was there.
Preparation
Autologous PRP (1000000 platelets per microlitre of blood) obtained from freshly drawn blood (30 ml of venous blood) of the patient with an added anticoagulant (sodium citrate). It drawn blood experienced two centrifugation (spin) steps.
The first spin, known as HARD SPIN, (more than 3000 rpm for 15 minutes) separated the Red Blood Cells (RBC) from the plasma containing the platelets, White Blood Cells (WBC), and clotting factors. Three layers resulted from the hard spin: an upper layer containing platelets and White Blood Cells (WBC), a middle layer known as the Buffy coat containing maximum number of platelets, and a bottom layer containing Red Blood Cells (RBC). The red blood cell layer removed and discarded.
The second spin also called SOFT SPIN (more than 2000 rpm for 5 minutes)], separated the Platelet Rich Plasma (PRP) in the bottom of the tube of the Platelet Poor Plasma (PPP) in the top of the tube by removal of more red blood cell. This created a bottom layer rich in platelets and leucocytes used for aPRP dressing.
In (aPRP+VAC), the dressing sealed at periphery of the wound and connected it to VAC unit through a drainage tube to produce a desired pressure of 125 mm of hg, intermittently for two minutes every five minutes for half an hour, six times a day, after four hours of PRP dressing to allow the maximum imbibition of topically placed aPRP over the ulcer bed. Once the vacuum was on, it sucked air out of the dressing causing its collapse and drawing the edges of the wound inwards. It also took away exudates from the wound in tandem, through evacuation tube embedded in the dressing on one side and connected to a fluid collection canister contained within the vacuum/ suction machine on other side, to collect these.
Method of application of dressings
In the first group, the sterile foam dressing cut to the approximate size of the wound placed gently into position after the application of freshly prepared aPRP.
In the second group, as per VAC application guidelines described by Lee et al., [14], a drainage tube was placed on top of the foam and a second piece of foam was placed over it. (For shallower wounds, a single piece of foam used and the drainage tube inserted inside it). The foam, together with first few inches of the drainage tube and the surrounding area of healthy skin, checked to ensure that the dressing formed an airtight seal both with the skin and drainage tube. The distal end of the drain connected to a vacuum unit, programmed to produce the desired negative pressure of 125 mm of hg, intermittently for two minutes every five minutes for half an hour, six times a day, after four hours of PRP dressing. It sucked air out of the dressing causing its collapse and drawing the edges of the wound inwards. It also took away exudates from the wound in tandem through evacuation tube connected to a fluid collection canister contained within the vacuum/ suction machine.
Change of dressing
Performed every four days in both the groups with wound inspection at that time. The resident doctor conducted manual measurement of size and granulated area in cm2. Findings calibrated on a graph paper.
Parameters for evaluation
Patients evaluated clinically for appearance of granulation tissue, 100% appearance of granulation tissue, full coverage of the ulcer, reduction in wound surface area and duration of hospital stay.
Statistical analysis
The results of observations of individual patients pooled for each intervention group. Data analysis performed using SPSS version 20 (SPSS Inc., Chicago, Illinois, USA). Numerical data expressed as mean ± Standard Deviation (SD) or percent as proportionate to the sample size. The significance of difference between two groups was determined using “P” value.
A “P” value less than 0.05 considered significant.
Period of follow up
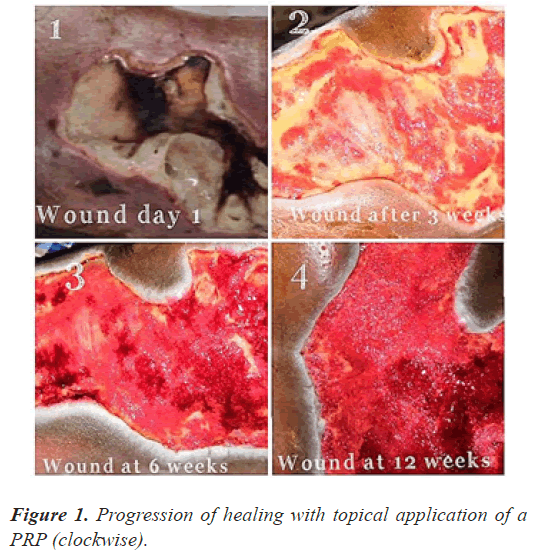
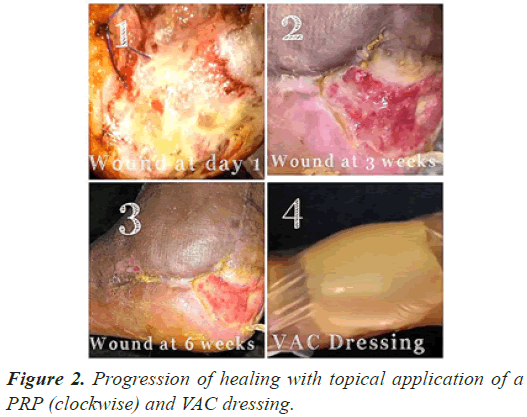
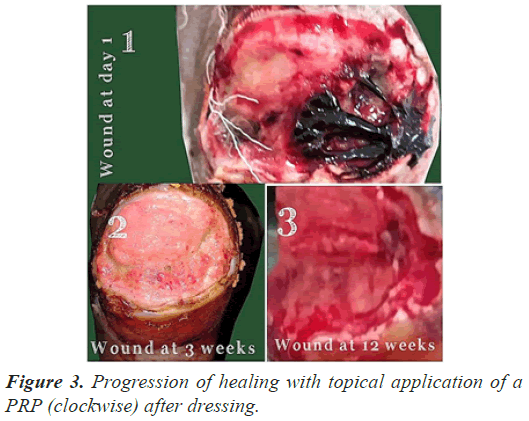
The follow up done at three, six and 12 weeks (Figures 1-3) and thereafter at regular intervals until definitive wound coverage was there.
Results
All the patients were having type 2 diabetes mellitus (80% were on oral hypoglycemic agents and rest 20% were on insulin therapy) with an ulcer of greater than 4 weeks duration ulcer on sole/dorsum of foot. The size of the wound ranged from 5.1 cms to 8.7 cms (mean of 7.6 ± 0.8 cms). Foot angiopathy (assessed by color Doppler) was the most associated co-morbidity. The mean time taken for appearance of granulation tissue, 100% granulation tissue, permanent wound coverage, reduction in wound surface area and hospital stay, was lesser in the (aPRP+VAC) dressing group, with a significant p value (P≤0.005), than in the (topical aPRP application) dressing group. Successive punch biopsies at regular follow up visits showed no adverse cellular change/malignant change in the present series. Complications such as infection, presence of exudates and a persistant pain were there in both modalities of treatment but were less in the (aPRP+VAC) dressing group. Only 86% of the patients needed the split skin grafting in (aPRP+VAC) dressing group when compared to topical aPRP application dressing group.
Discussion
We all know that that despite many advances in the treatment of Diabetic Foot Ulcer (DFU), patients are yet living with amputation as destructive complication. Topical application of activated platelet rich plasma and Negative Pressure Wound Therapy (NPWT) are two familiar modalities in the management of chronic Diabetic Foot Ulcers (DFU). Although the former could significantly increase the rate of healing of DFUs, latter leads to a faster granulation tissue formation and reduction in wound size in comparison to the former. Autologous aPRP is economical and affordable as it is prepared with a small volume (7cc of aPRP prepared from 30cc of freshly drawn venous blood) of patient’s blood and the risk of transmission of blood borne diseases or immunological reaction is not an apprehension.
Autologous aPRP provides the growth factor needed for natural healing process in diabetic patients. Seven fundamental protein growth factors, actively secreted by platelet initiate wound healing process. aPRP also has three proteins known to act as cell adhesion molecules i.e. fibrin, fibronectin and vitronectin. Platelets also secrete Transforming Growth Factor-Beta (TGF-Beta) and Monocyte Chemo attractant Protein-1 (MCP-1) that would attract monocytes and neutrophils to the wound site.
Conclusion
Present study found that PRP can facilitate healing of DFUs and therefore can reduce the risk of amputation but more so when it used in alliance with negative pressure wound therapy. We conclude that (aPRP+VAC) dressings are superior to topical aPRP application dressings in wound healing of diabetic foot ulcers.
Limitations of the Study
Lack of control over some confounding factors such as patients’ nutrition, activities, and their level of adherence to their medical treatments are some of the limitations of the present study. Moreover, a very small sample is another limitation of the study. Therefore, replication of study with larger sample sizes is recommended.
Conflicts of Interest
There were no conflicts of interest in the present study.
References
- Eppley BL, Pietzak WS, Blanton M. Platelet rich plasma: A review of biology and applications in plastic surgery. Plast Reconstr Surg 2006; 118: 147e‐159e.
[Crossref] [Google Scholar] [PubMed]
- Sclafani AP, Azzi J. Platelet preparations for use in facial rejuvenation and wound healing: A critical review of current literature. Aesthec Plast Surg 2015; 39: 495‐505.
[Crossref] [Google Scholar] [PubMed]
- Kim DH, Je YJ, Kim CD. Can platelet rich plasma be used for skin rejuvenation? Evaluation of effects of platelet rich plasma on human dermal fibroblast. Ann Dermatol 2011; 23:424‐431.
[Crossref] [Google Scholar] [PubMed]
- Carter CA, Jolly DG, Worden Sr CE, Hendren DG, Kane CJ. Platelet rich plasma gel promotes differentiation and regeneration during equine wound healing. Exp Mol Pathol 2003; 74: 244-255.
[Crossref] [Google Scholar] [PubMed]
- Lindeboom JA, Mathura KR, Aartman IH, Kroon FH, Milstein DM, Ince C. Influence of the application of platelet‐enriched plasma in oral mucosal wound healing. Clin Oral Implants Res 2007; 18: 133-139.
[Crossref] [Google Scholar] [PubMed]
- Artitua E, Andia I, Ardanza B, Nurden P, Nurden A. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost 2004; 91:4.
[Crossref] [Google Scholar] [PubMed]
- Dhurat R, Sukesh MS. Principles and methods of preparation of platelet‐rich plasma: A review and author′s perspective. J CutanAesthet Surg 2014; 7: 189‐197.
[Crossref] [Google Scholar] [PubMed]
- Boswell SG, Cole BJ, Sundman EA, Karas V, Fortier LA. Platelet‐rich plasma: A milieu of bioactive factors. Arthroscopy 2012; 28: 429‐439.
[Crossref] [Google Scholar] [PubMed]
- Everts PA, Knape JT, Weibrich G. Platelet rich plasma and platelet gel: A review. J Extra Corpor Technol 2006; 38: 174‐187. [Crossref]
[Google Scholar] [PubMed]
- Marx RE. Platelet‐rich plasma: Evidence to support its use. J Oral Maxillofac Surg 2004; 62: 489‐496.
[Crossref] [Google Scholar] [PubMed]
- Alves R, GrimaltR. A review of platelet‐rich plasma: History, biology, mechanism of action, and classification. Skin Appendage Disord 2018; 4: 18‐24.
[Crossref] [Google Scholar] [PubMed]
- Chan RK, Liu P, Lew DH. Expired liquid preserved platelet releases retain proliferative activity. J Surg Res 2005; 126: 55‐58.
[Crossref] [Google Scholar] [PubMed]
- Sclafani AP, McCormick SA. Induction of dermal collagenesis, angiogenesis, and adipogenesis in human skin by injection of platelet rich fibrin matrix. Arch Facial Plast Surg.2012; 14:132‐136.
[Crossref] [Google Scholar] [PubMed]
- Andia I, Abate M. Platelet rich plasma: Underlying biology and clinical correlates. Regen Med 2013; 8: 645‐658.
[Crossref] [Google Scholar] [PubMed]
- Venturi ML, Attinger CE, Mesbahi AN, Hess CL, Graw KS. Mechanisms and clinical applications of the Vacuum-Assisted Closure (VAC) device. Am J Clin Dermatolo.2005; 6: 185-194.
[Crossref] [Google Scholar] [PubMed]
- Morykwas MJ, Argenta LC, Shelton-Brown EI, McGuirt W. Vacuum-assisted closure: A new method for wound control and treatment: Animal studies and basic foundation. Ann Plast Surg 1997; 38: 553-562.
[Crossref] [Google Scholar] [PubMed]
- Horch RE. Basics foundation and results of the vacuum therapy in the reconstructive surgery. Zentralbl Chir 2004; 129: S2-S5.
[Crossref] [Google Scholar] [PubMed]
- Saxena V, Hwang CW, Huang S, Eichbaum Q, Ingber D, Orgill DP. Vacuum-assisted closure: Microdeformations of wounds and cell proliferation. Plast Reconstr Surg 2004; 114: 1086-1096.
[Crossref] [Google Scholar] [PubMed]
- Renner R, Rogalski C, Friedlein H, Simon JC. Vacuum therapy in dermatology: A review. J Dtsch Dermatol Ges 2006; 4: 468-476.
[Crossref] [Google Scholar] [PubMed]
- Driver VR, Hanft J, Fylling CP, Beriou JM. A prospective, randomized, controlled trial of autologous platelet-rich plasma gel for the treatment of diabetic foot ulcers. Ostomy Wound Manage 2006; 52: 68-70. [Crossref]
[Google Scholar] [PubMed]