ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2018) Volume 29, Issue 12
Phytochemical analysis and antioxidant activity of Persia americana and Actinidia deliciosa fruit extracts by DPPH method
Sadia Rafique* and Naveed Akhtar
Faculty of Pharmacy and Alternative Medicine, the Islamia University of Bahawalpur, Punjab, Pakistan
- *Corresponding Author:
- Sadia Rafique
Faculty of Pharmacy and Alternative Medicine
The Islamia University of Bahawalpur
Punjab, Pakistan
Accepted May 08, 2017
DOI: 10.4066/biomedicalresearch.29-16-2209
Visit for more related articles at Biomedical ResearchExtracts having antioxidant properties show promising effect on human skin. The main objective of this study was to perform phytochemical analysis to identify active constituents and determine the antioxidant activity through DPPH (2, 2 diphenyl-1-picryl hydrazyl) method in sampled fruit. In this work, extract of Persia americana variety ‘Hass’ and Actinidia deliciosa fruit were selected. Various qualitative and quantitative tests were performed for phytochemical analysis. For antioxidant activity, different parts of fruit samples of Persia americana were prepared in methanol and in acetone solution while in case of Actinidia deliciosa whole fruit was soaked in methanol and in ethanol solution. Phytochemical analysis showed the higher concentration of flavonoids and phenolics contents in both fruit extracts. The analysis also showed the presence of vitamin C, alkaloids, glycosides, amino acids, carbohydrates, proteins, steroids and triterpenoids. The DPPH results using ascorbic acid as standard showed that acetone and methanol extract of Persia americana possess higher antioxidant activity (84%) than methanol extract alone (60%) while Actinidia deliciosa whole fruit showed better antioxidant activity in ethanol extract (79%) as compared to methanol extract (57%). The extracts possess antioxidant activity can be incorporated in different skin formulations and their In-vitro and In- vivo studies may be performed for cosmetic market.
Keywords
Persia americana, Actinidia deliciosa, phytochemical, DPPH, antioxidant, flavonoids and phenolic contents.
Introduction
Persia americana variety ‘Hass’ belongs to family lauraceae and its fruit is berry shaped [1]. ‘Hass’ variety changes its colour from green to black upon maturation [2]. It’s pulp is rich in unsaturated fatty acids including oleic, linoleic, palmitoleic acid which tend to oxidize during storage [3]. It contain greatest amount of vitamin C, alkaloids, glycosides, flavonoids, carbohydrates, protein, minerals, steroids and triterpenoids [4,5]. It is also the rich source of phytochemical constituents that reduces the risk of cardiovascular disease [6]. These phytochemicals such as flavonoids, carotenoids and antioxidative vitamins found in this fruit reduces the potential risk of cancer disease [7]. Actinidia deliciosa belongs to Actinidiaceae family, oval globular shaped fleshy fruit filled with black, small edible seeds [8]. It is a good source of vitamin C, but excellent sources of folate, potassium, vitamin K and vitamin E [9]. The phenolic and flavonoid contents present in highest ratio in fruit juice [10]. Actinidia deliciosa fruit is also beneficial in cardiovascular disease [11]. Due to its highest antioxidant activity it may prevent the skin diseases caused by oxidative stress [12].
There are different methods used to test the antioxidant activity. These methods include Total radical-trapping antioxidant parameter assay (TRAP assay), Trolox equivalent antioxidant capacity assay (TEAC I-III assay), N,N-dimethylp- phenylendiamine assay (DMPD assay), 2,2 diphenyl-lpicrylhydrazyl assay (DPPH assay), Ferric reducing ability of plasma assay (FRAP assay), β-carotene bleaching test (BCB), Thiobarbituric acid reactive species assay (TBARS assay) and Photochemiluminescence assay (PCL assay) [13,14]. Due to radical scavenging activity, the DPPH is very simple, famous, most sensitive and very convenient method for screening the antioxidant activity [15]. In this method, hydrogen is a donor atom that measures radical scavenger atoms and give antioxidant activity. The changes in colour occur from purple to yellow when antioxidant absorption occurred. The strong absorption seemed at 517 nm. This is stichiometric reaction based upon the absorption of hydrogen atom. When UV absorption falls below 517 nm, the antioxidant effect can be observed [16].
Persia americana antioxidant activity is high and our main study focus was also upon it’s antioxidant activity [17]. Every part (peel, pulp and seed) of Persia americana has different in antioxidant activity and it changes with the change of solvent [18]. Due to the presence of high contents of oil, it can be used in pharmaceutical and cosmetics industry [19]. Actinidia deliciosa also posses higher antioxidant activity because it’s ORAC value is greater when whole fruit was taken for extraction [20]. Our main study objective was to evaluate the total phenolic contents, total flavonoid contents, vitamin C, antioxidant activity and phytochemical analysis of Persia americana and Actinidia deliciosa fruit extracts.
Materials and Methods
Chemicals and apparatus
Methanol, ethanol (Merck KGaA Darmstadt, Germany), acetone (BDH, England), distilled water (Department of pharmacy, IUB, Pakistan), fruits (Metro, Faisalabad, Pakistan), DPPH (Sigma, USA), rotary evaporator (Eyela, Co. Ltd. Japan), UV-VIS spectrophotometer (U vikon XL, Bio-Tek Instruments, Bad Friedrichshall, Germany), microplate reader ( Synergy HT Bio Tek®, USA).
Collection of fruit material
The fruits of Persia americana and Actinidia deliciosa were collected from local market, Metro, Faisalabad, Pakistan and the specimens were identified from herbarium botany department, University of Agriculture, Faisalabad, Pakistan under voucher no 411-1-16 for Actinidia deliciosa and 411-2-16 for Persia americana.
Preparation of fruit extract
Different types of fruit extracts were prepared in different solvents. Persia americana pulp was grinded into fine particles and then soaked in 70% methanol solution. Similarly, Persia americana peel and pulp was also soaked after grinding in 70% methanol solution. Persia americana seed alone was grinded and then soaked in 70% methanol solution. Persia americana peel and pulp was grinded together and then soaked in 60% acetone and 10% methanol solution. Persia americana whole fruit was grinded and soaked in 70% acetone solution.
Similarly, Actinidia deliciosa whole fruit was taken and grinded into fine particles by using mixer grinder and then this material was soaked in a solvent mixture containing 70% ethanol solution and another sample was prepared by grinding fruit and then soaking it in 70% methanol solution. Soaking of all samples was done for 48 h then filtered through muslin cloth and finally through whatman No 1 filters paper. The obtained filterate was concentrated in rotary evaporator. The extracts were then kept in refrigerator at 8°C for antioxidant activity and for phytochemical analysis.
DPPH method
The free radical scavenging activity of different extracts of Persia americana and Actinidia deliciosa was observed. A stable free radical of 2,2-diphenyl-1-picryl-hydrazyl (DPPH) was used with slight modification to determine the antioxidant activity [21]. 100 μM concentration of DPPH in methanol was used. The total volume of assay was 100 μl, in which test solution was 10 μl and DPPH solution was 90 μl in 96 wellplate. Both solutions were mixed in vortex mixer for 30 min and incubated at 37°C. Microplate reader Synergy HT BioTek® USA was used to measure any decrease in absorbance at 517 nm in a UV spectrophotometer (double beam spectrophotometer Uvikon XL, Bio-Tek instruments, Bad friedrichshall, Germany). The reference standard of asorbic acid was used. All results were repeated for three times. The following formula was used to calculate the percentage inhibition.
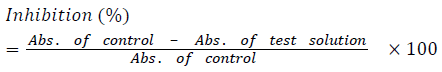
Where:
Absorbance of control = Total radical activity without inhibitor.
Absorbance of Test = Activity in the presence of test compound.
Phytochemical analysis
Both fruit extracts were subjected to testing for active principles of vitamin C, alkaloids, glycosides, phenolics, flavonoids, carbohydrates, steroids, triterpenoids, tannins and saponins. Total phenolic contents and total flavonoid contents were find out quantitatively. To find out total phenolic contents, take 50 μl of sample and 50 μl of Folin-Coicalteu reagent. Add it in 750 μl of distilled water and mixture was kept at room temperature for 10 min. Then add 150 μl of 20% Na2CO3 in whole mixture. Heat this mixture in water bath at 40ºC for 20 minutes and then cooled in ice bath. Absorbance was measured at 755 nm using spectrophotometer [22]. To test the flavonoid contents, take 0.5 ml of each fruit extract and then it was mixed with 0.5 ml of 2% AlCl3 methanol solution. The mixture was incubated at room temperature for 10 min. Absorbance was measured at 368 nm [23]. Different tests have been performed for qualitative analysis of both fruits. These tests include test for flavonoids, vitamin C, alkaloids, glycosides, amino acids, carbohydrates, proteins, steroids and triterpenoids, tannins and saponins. For qualitative analysis of flavonoids, ferric chloride test has been performed. The appearance of blackish red or green precipitate, upon addition of 2-3 drops of ferric chloride solution in a test sample would indicate the presence of flavonoids [24]. For vitamin C, DNPH test has been performed. Dissolve 2,4 dinitro phenyl hydrazine in concentrated sulfuric acid. Both test solutions were treated separately with this reference mixture and vigorously shake it. The appearance of yellow precipitate would indicate the presence of vitamin C [25].
Hager’s test has been performed to find out the presence of alkaloids. In this test, treat both the test solution separately with 3-4 drops of Hager’s reagent (Saturated picric acid solution). If yellow precipitate would appear upon shaking then it means alkaloids are present [25,26]. Bromine test has been performed for glycosides. Equal amount of both test solution and bromine water was dissolved in test tubes separately and shake it well. Again the appearance of yellow precipitate would give the positive response [26]. Another test used for glycosides is keller killiani test. Ferric chloride solution was mixed with 2-3 drops of glacial acetic acid in 2 test tubes and then mix them in a single test tube. When 2-3 drops of concentrated sulfuric acid was added in it, the formation of 2 layers were observed. Reddish brown layer would appear in bottom, while acetic acid layer would appear in top which changes to bluish green indicate the presence of glycosides [27].
Ninhydrin test was performed for amino acids. Boil the test solution with 0.2% Ninhydrin solution. If purple colour appear upon boiling would suggest that free amino acids are present [28]. Benedict’s test was performed for carbohydrates. Benedict’s solution is the mixture of alkaline solution and complex of cupric citrate. When 2 ml of this reagent is added in a test solution and boil it on water bath for few minutes. If reddish brown precipitate would appear shows the presence of carbohydrates [29]. For proteins detection, biuret test is performed. Treat the test sample with a solution containing two drops of copper sulphate (0.1%) and sodium hydroxide solution (10%). The presence of violet or pink colour would suggest that protein is present [25]. For steroids and triterpenoids, liebermann burchard test was performed. Mix the test sample with 3-4 drops of acetic anhydride and heat the mixture till boiled. Then cool it. Then add concentrated sulphuric acid from the sides of test tube. If brown ring appear at the junction of 2 layers. The upper layer would be of green colour and the lower layer would be of deep red colour would suggest the presence of steroids and triterpenoids [26]. Gelatin test is the conformation of tannins. If small amount of gelatin solution is added on a test sample and white precipitate would appear at the bottom of test tube would give a positive result for tannins [30]. For saponins, foam test is performed. When test sample is mixed with distilled water and shaken contineously for 5 min. If froth appear and remain stable for 15 minutes then it means saponins are present [31].
Results
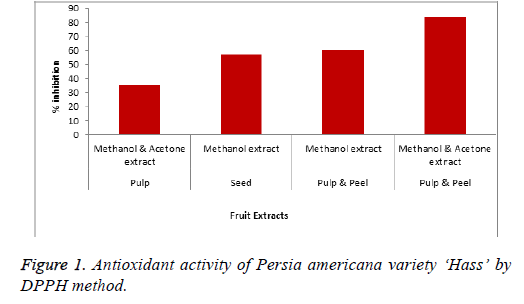
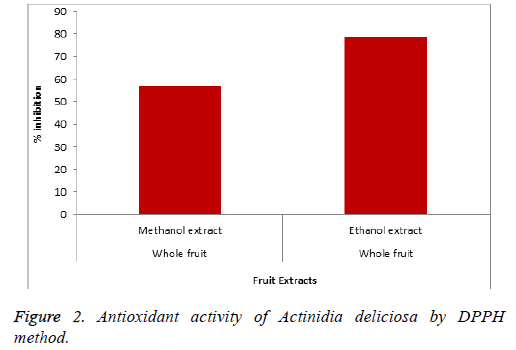
The antioxidant activity of different parts of Persia americana variety ‘Hass’ is given in Figure 1 and Actinidia deliciosa in Figure 2. The quantitative and qualitative analysis of Persia americana and Actinidia deliciosa is given in Tables 1 and 2.
| Chemical tests | Persia americana extract | Actinidia deliciosa extract | ||
|---|---|---|---|---|
| Test for TPC (mg GAE/g extract) | Absorbance | 141.14 | 116.43 | |
| Persia americana | 0.994 | |||
| Actinidia deliciosa | 0.821 | |||
| Test for TFC (mg QE/g) | 67.87 | 61.91 | ||
Table 1. Quantitative analysis of Persia americana variety ‘Hass’ and Actinidia deliciosa fruit extracts.
| Chemical tests | Persia americana extract | Actinidia deliciosa extract | |
|---|---|---|---|
| Test for Flavonoids | Ferric chloride test | +ve | +ve |
| Test for Vitamin C | DNPH test | +ve | +ve |
| Test for Alkaloids | Hager’s test | +ve | +ve |
| Test for Glycosides | Bromine test | -ve | +ve |
| Keller Killiani test | +ve | +ve | |
| Test for Amino acids | Ninhydrin test | +ve | +ve |
| Test for Carbohydrates | Benedict’s test | +ve | +ve |
| Test for Proteins | Biuret test | +ve | +ve |
| Test for Steroids & Triterpenoids | Liebermann Burchard test | +ve | +ve |
| Test for Tannins | Gelatin test | -ve | +ve |
| Test for Saponins | Foam test | -ve | -ve |
Table 2. Qualitative anslysis of Persia americana variety ‘Hass’ and Actinidia deliciosa fruit extracts
The results of Persia americana variety ‘Hass’ showed maximum antioxidant activity when peel and pulp of fruit were extracted with different ratio of methanol and acetone solvent. It is reported that 70% of aqueous acetone is more effective for maximum amount of condensed tannins [32]. If 50% of acetone extract is used than it will achieve the greatest level of phenolics as compared to any other solvent [33]. The DPPH results using ascorbic acid as reference standard showed that acetone and methanol extract of Persia americana possess 84% antioxidant activity while methanol extract showed 60% antioxidant activity and Actinidia deliciosa whole fruit showed 79% of antioxidant activity in ethanol extract as compared to methanol extract which give antioxidant activity of 57%.
The quantitative analysis of both fruits Persia americana and Actinidia deliciosa showed that both fruits contain excellent total phenolic contents and total flavonoid contents. The TPC (mg GAE/g) of Persia americana and Actinidia deliciosa are 141.14 and 116.43 while TFC (mg QE/g) of Persia americana and Actinidia deliciosa are 67.87 and 61.91 respectively. The ferric chloride test confirms the presence of flavonoids in both fruit. Moreover, the presence of vitamin C and phenolic contents also lead to increase the antioxidant activity [6]. The presence of polyphenols and phytochemicals also correlate with antioxidant activity [34].
Discussion
Consumption of such fruits with high antioxidant activity and high value of bioactive compounds give best neutrition results [35].Therefore, most of the scientists recommend to consume such fruits [36]. Two things of Phenolic largely determine the efficiency of extraction process. One is its chemical structure and other is it’s polarity and due to this reason, Persia americana achieved the highest level of total phenolic contents in acetone solution as compared to methanol solution [37]. While in case of Actinidia deliciosa, when whole fruit was extracted in ethanol solution, it showed maximum antioxidant activity than whole fruit is extracted in methanol solution as shown in Figure 2. The hydrophobic property is dominant in ethanol solution because of more number of carbons present in ethanol. The ethanol is also the best extraction solvent for both hydrophilic and hydrophobic extract as compared to methanol solution, so antioxidant activity is higher in it [38].
Naturally, the fruits have higher phenol antioxidant than vegetables and they can reduce the lipoprotein and protect them from oxidantion [39]. The phytochemical contents present in both fruits may be attributable to increase the antioxidant activity of both fruits [40]. The quantitative analysis of both fruits shows that total phenolic contents and total flavonoid contents present in Persia americana are greater than Actinidia deliciosa, so the antioxidant activity of Persia americana is 84% as compared to Actinidia deliciosa that have 79% antioxidant activity. But gelatin test shows that tannins are only present in Actinidia deliciosa. It was found that total polyphenols and flavonoids contents were significantly higher with the change of extraction solvent. Although the total phenolic contents and total flavonoid contents not only increases the antioxidant activity but there is a linear correlation with phytochemicals such as phenolics, flavonoids and steroids [41]. The fatty acids present in Persia americana acts with phytochemicals reduce the risk of cancer disease [19]. These are polyphenols which gives antimicrobial, antioxidant and healing activity to the skin [42]. Several other studies shows that polyphenolic and flavonoid contents may lead to increase antioxidant effect medicinally [43]. Actinidia deliciosa healthful atributes related to high contents of ascorbic acid, polyphenols and the presence of flavonoids [44]. The results of Actinidia deliciosa showed that ethanol extract have large percent of inhibition in DPPH as compared to other extracts [45]. The antioxidant activity of Persia americana and Actinidia deliciosa can be incorporated in conventional and sustained release skin formulations and the formulations can be tested in healthy and unhealthy volunteers to explore their effects on different parameters of human skin i.e melanin, erythema, moisture content and transepidermal water loss.
Conclusion
Antioxidant activity of different parts of Persia americana variety ‘Hass’ was taken into consideration by changing the different solvent ratio. The same solvent ratio and method was used for pulp and seed separately and peel plus pulp combined. The pulp showed lowest antioxidant activity then seed but highest antioxidant activity was observed for peel and pulp when combined together. In case of Actinidia deliciosa, the fruit shows highest antioxidant activity of 79% in ethanol solution as compared to methanol solution by DPPH method. Moreover, the skin part of Actinidia deliciosa exhibits the higher antioxidant activity as compared to fleshly part, so, the consumption of whole fruit is not only convenient but also beneficial for health promoting effect. This antioxidant activity is very useful and effective which can be used to make various cosmeceuticals formulations that show better anti-acne, skin soothing, anti-aging and skin fairness effects.
It can also be concluded from the present study that total phenolic contents and total flavonoid contents present in Persia americana are higher than Actinidia deliciosa while vitamin C, alkaloids, glycosides, amino acids, carbohydrates, proteins, steroids and triterpenoids are present in both fruits. The presence of tannins in Actinidia deliciosa made it more beneficial for preparation of cosmeceutical preparations.
Acknowledgement
The authors would like to say thank to respected Prof. Dr. Mahmood Ahmad, Dean of Faculty of Pharmacy, IUB, Bahawalpur, Pakistan for providing cosmetic laboratory services.
References
- Bergh B, Ellstrand N. Taxonomy of the avocado. California Avocado Society Yearbook. 1986; 70: 135-145.
- Cox KA, McGhie TK, White A, Woolf AB. Skin colour and pigment changes during ripening of ‘Hass’ avocado fruit. Postharvest Biol Technol 2004; 31: 287-294.
- Prabath Pathirana U, Sekozawa Y, Sugaya S, Gemma H. Changes in lipid oxidation stability and antioxidant properties of avocado in response to 1-MCP and low oxygen treatment under low-temperature storage. Int Food Res J 2013.
- Vinha AF, Moreira J, Barreira SV. Physicochemical parameters, phytochemical composition and antioxidant activity of the Algarvian avocado (Persea americana Mill.). J Agri Sci 2013; 5: 100.
- Adeyemi O, Okpo S, Ogunti O. Analgesic and anti-inflammatory effects of the aqueous extract of leaves of Persea americana Mill (Lauraceae). Fitoterapia 2002; 73: 375-380.
- Villa-Rodríguez JA, Molina-Corral FJ, Ayala-Zavala JF, Olivas GI, González-Aguilar GA. Effect of maturity stage on the content of fatty acids and antioxidant activity of ‘Hass’ avocado. Food Res Int 2011; 44: 1231-1237.
- Lu Q-Y, Arteaga JR, Zhang Q, Huerta S, Go VLW, Heber D. Inhibition of prostate cancer cell growth by an avocado extract: role of lipid-soluble bioactive substances. J Nutritional Biochem 2005; 16: 23-30.
- Takeoka GR, Guntert M, Flath RA, Wurz RE, Jennings W. Volatile constituents of kiwi fruit (Actinidia chinensis Planch.). J Agri Food Chem 1986; 34: 576-578.
- Ferguson A, Ferguson L, editors. Are kiwifruit really good for you? International Symposium on Kiwifruit 2002.
- Dawes HM, Keene JB. Phenolic composition of kiwifruit juice. J Agri Food Chem 1999; 47: 2398-2403.
- Duttaroy AK, Jørgensen A. Effects of kiwi fruit consumption on platelet aggregation and plasma lipids in healthy human volunteers. Platelets 2004; 15: 287-292.
- Iwasawa H, Morita E, Yui S, Yamazaki M. Anti-oxidant effects of kiwi fruit in vitro and in vivo. Biol Pharm Bull 2011; 34: 128-134.
- Schlesier K, Harwat M, Böhm V, Bitsch R. Assessment of antioxidant activity by using different in vitro methods. Free Radical Res 2002; 36: 177-187.
- Kulisic T, Radonic A, Katalinic V, Milos M. Use of different methods for testing antioxidative activity of oregano essential oil. Food Chem 2004; 85: 633-640.
- Koleva II, van Beek TA, Linssen JP, Groot Ad, Evstatieva LN. Screening of plant extracts for antioxidant activity: a comparative study on three testing methods. Phytochemical Anal 2002; 13: 8-17.
- https://www.scribd.com/document/271054618/15-3b-Natural-Product-Screening-Anti-Oxidant-Screen-DPPH-of-Extract-Crude-Extract1
- Nabavi SF, Nabavi SM, Setzer WN, Nabavi SA, Nabavi SA, Ebrahimzadeh MA. Antioxidant and antihemolytic activity of lipid-soluble bioactive substances in avocado fruits. Fruits 2013; 68: 185-193.
- Bertling I, Tesfay S, Bower J. Antioxidants in ‘Hass’ avocado. South African Avocado Growers’ Association Yearbook 2007; 30: 17-19.
- Duarte PF, Chaves MA, Borges CD, Mendonça CRB. Avocado: characteristics, health benefits and uses. Ciência Rural 2016; 46: 747-754.
- Wang H, Cao G, Prior RL. Total antioxidant capacity of fruits. J Agri Food Chem 1996; 44: 701-705.
- Ratshilivha N, Awouafack M, du Toit E, Eloff J. The variation in antimicrobial and antioxidant activities of acetone leaf extracts of 12 Moringa oleifera (Moringaceae) trees enables the selection of trees with additional uses. South African J Botany 2014; 92: 59-64.
- Nazarni R, Purnama D, Umar S, Eni H. The effect of fermentation on total phenolic, flavonoid and tannin content and its relation to antibacterial activity in jaruk tigarun (Crataeva nurvala, Buch HAM). Int Food Res J 2016.
- Patel I, Padse O, Ingole Y, editors. Comparative analysis of antioxidant and antidiabetic activity for apple (Malus domestica), banana (Musa paradisiaca) & kiwi (Actinidia deliciosa). National Conference “ACGT; 2015.
- Audu SA, Mohammed I, Kaita HA. Phytochemical screening of the leaves of Lophira lanceolata (Ochanaceae). Life Sci J 2007; 4: 75-79.
- Bhandary SK, Kumari S, Bhat VS, Sharmila K, Bekal MP. Preliminary phytochemical screening of various extracts of Punica granatum peel, whole fruit and seeds. J Health Sci 2012; 2: 35-38.
- De S, Dey Y, Ghosh A. Phytochemical investigation and chromatographic evaluation of the different extracts of tuber of Amorphaphallus paeoniifolius (Araceae). Int J Pharm Biomed Res 2010; 1: 150-157.
- Edeoga H, Okwu D, Mbaebie B. Phytochemical constituents of some Nigerian medicinal plants. African J Biotechnol 2005; 4: 685-688.
- Lee YP, Takahashi T. An improved colorimetric determination of amino acids with the use of ninhydrin. Anal Biochem 1966; 14: 71-77.
- Yadav R, Agarwala M. Phytochemical analysis of some medicinal plants. J Phytol 2011.
- Samatha T, Srinivas P, Shyamsundarachary R, Rajinikanth M, Rama Swamy N. Phytochemical analysis of seeds, stem bark and root of an endangered medicinal forest tree Oroxylum indicum (L) Kurz. Int J Pharm Bio Sci 2012; 3: 1063-1075.
- Ayoola G, Coker H, Adesegun S, Adepoju-Bello A, Obaweya K, Ezennia E. Phytochemical screening and antioxidant activities of some selected medicinal plants used for malaria therapy in Southwestern Nigeria. Trop J Pharm Res 2008; 7: 1019-1024.
- Chavan U, Shahidi F, Naczk M. Extraction of condensed tannins from beach pea (Lathyrus maritimus L.) as affected by different solvents. Food Chemistry 2001; 75: 509-512.
- Zhou K, Yu L. Effects of extraction solvent on wheat bran antioxidant activity estimation. LWT-Food Sci Technol 2004; 37: 717-721.
- Ding H, Chin YW, Kinghorn AD, D’Ambrosio SM, editors. Chemopreventive characteristics of avocado fruit. Seminars Cancer Biol 2007.
- Park YS, Leontowicz H, Leontowicz M, Namiesnik J, Suhaj M, Cvikrová M. Comparison of the contents of bioactive compounds and the level of antioxidant activity in different kiwifruit cultivars. J Food Composit Anal 2011; 24: 963-970.
- Nampoothiri SV, Praseetha E, Venugopalan V, Nirmala Menon A. Process development for the enrichment of curcuminoids in turmeric spent oleoresin and its inhibitory potential against LDL oxidation and angiotensin-converting enzyme. Int J Food Sci Nutrition 2012; 63: 696-702.
- Rodríguez-Carpena JG, Morcuende D, Andrade MJ, Kylli P, Estévez M. Avocado (Persea americana Mill.) phenolics, in vitro antioxidant and antimicrobial activities, and inhibition of lipid and protein oxidation in porcine patties. J Agric Food Chem 2011; 59: 5625-5635.
- Halimoon N, Mardhiah Hayati A. Determination and evaluation of antioxidative activity in red dragon fruit (hylocereus undatus) and green kiwi fruit (actinidia deliciosa). Am J Appl Sci 2010; 7: 1432-1438.
- Vinson JA, Su X, Zubik L, Bose P. Phenol antioxidant quantity and quality in foods: fruits. J Agric Food Chem 2001; 49: 5315-5321.
- Tavarini S, Degl’Innocenti E, Remorini D, Massai R, Guidi L. Antioxidant capacity, ascorbic acid, total phenols and carotenoids changes during harvest and after storage of Hayward kiwifruit. Food Chemistry 2008; 107: 282-288.
- Andriani Y, Ramli NM, Syamsumir DF, Kassim MNI, Jaafar J, Aziz NA. Phytochemical analysis, antioxidant, antibacterial and cytotoxicity properties of keys and cores part of Pandanus tectorius fruits. Arabian J Chemistry 2015.
- de Sousa Leal A, de Carvalho Leal LH, da Silva D, Nunes LCC, Lopes JAD. Incorporation of tannic acid in formulations for topical use in wound healing: A technological prospecting. African J Pharmacy Pharmacol 2015; 9: 662-674.
- Jung KA, Song TC, Han D, Kim IH, Kim YE, Lee CH. Cardiovascular protective properties of kiwifruit extracts in vitro. Biol Pharmaceutical Bulletin 2005; 28: 1782-1785.
- Pal RS, Kumar VA, Arora S, Sharma A, Kumar V, Agrawal S. Physicochemical and antioxidant properties of kiwifruit as a function of cultivar and fruit harvested month. Brazilian Arch Biol Technol 2015; 58: 262-271.
- Al-Kawaz HS, AL-Mashhady LA. Evaluation of the phytochemical constituents and oxidant–antioxidant status for actinidia deliciosa extracts. Int J Pharmay Ther 2016; 7: 31-41.