ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 3
Pharmacological evaluation of oral fast disintegrating films containing local anaesthetic agent lignocaine
1Department of Clinical Pharmacy, The Second People’s Hospital of Liaocheng, Liaochen, China
2Department of Pharmacy, the Affiliated Hospital of Shandong Traditional Chinese Medical University, Jinan, China
- *Corresponding Author:
- Jing Li
Department of Pharmacy
The Affiliated Hospital of Shandong Traditional Chinese
Medical University, P. R. China
Accepted on July 27, 2016
The aim of the present research was formulate and develop Lignocaine containing oral fast disintegrating films to treat mouth ulcers as well as to evaluate the local anaesthetic activity of the formulation using tail flick test in rat model. 32 full factorial design was used to formulate oral fast disintegrating films by solvent casting evaporation technique. Chitosan, Croscarmellose Sodium (CCS) and Dibutyl Phthalate (DBT) were used as polymer, superdisintegrant and plasticizer respectively. Chitosan, CCS and DBT were considered as independent variables while disintegration time and in vitro drug release were considered as dependent variables. Effect of independent variables on dependent variables was studied using design expert software. In vitro drug release, disintegration time, physical appearance, folding endurance, physical appearance, thickness, weight variation, drug content uniformity of the films were characterized. The local anaesthetic efficacy of the films was evaluated on healthy male rats by tail flick test. The optimized film showed disintegration within 10 sec and 98.85% drug release over the period of 1 h. All independent variables selected for the study were statistically significant (p<0.05). The local anaesthetic efficacy of films with CCS was found to be highest (2932.87 ± 78.30 AUEC (sec/min)) as compare to control film and films without CCS (p<0.05). The films complies the requirements for oral drug delivery. The study concludes that oral fast disintegrating films of Lignocaine serves as potential drug delivery systems for mouth ulcer management.
Keywords
Local anaesthesia, Fast-disintegrating films, Lignocaine, Oral films, Tail flick test.
Introduction
An ulcer also known as oral ulcer or mucosal ulcer may be a deeper breach or damage of epithelial tissue and/or lamina propria that leads to the loss of surface tissue, necrosis [1]. The major causes being the aphthous stomatitis, local trauma, some viruses (e.g. Herpex simplex, Varicella Zoster, HIV), fungi (e.g. Candida albicans), bacteria (e.g. Mycobacterium tuberculosis) causing infections are also responsible for the ulceration [2,3]. The treatment for the mouth ulcer may be symptomatic or cause related. Smoothing or removing a local cause of trauma is also the way of treatment of oral ulcer. Maintaining good oral hygiene and use of antiseptic mouthwashes (e.g. chlorhexidine) are preventive measures while topical analgesics (e.g. Benzydamine) may reduce the pain when used in form of topical gels, creams or systemic steroids. Among all these treatments the use of local anaesthetics is the choice of medication for mouth ulcers.
Local anaesthetics cause reversible loss of sensation by decreasing rate of depolarization and repolarisation of the excited membranes [4]. Lignocaine also known as Lidocaine or Xylocaine, antiarrhythmatic drug commonly used as local anaesthetic in topical as well as dental surgeries [5] due to its rapid onset of action (between 20 seconds to 1 minute) and intermediate duration of efficacy (5-30 min). It is absorbed in mucous membranes following topical administration where the rate and extent of the absorption depends on various factors such as concentration, viscosity of the agent, duration of exposure, specific site of application. Lignocaine has been used to treat the mouth ulcers due to its excellent local anaesthetic effect that leads to relieve the pain of the mouth ulcer.
Oral fast-disintegrating film of Lignocaine is novel approach to treat the mouth ulcers and could serve as alternative to the conventional dosage forms like gels, ointments, mouth washes, syrups and injectable [6]. The fast disintegrating films makes availability of the drug very rapidly at the site of application as compare to gels and ointments. Also these films are very easy to administer and can be useful for paediatric as well as geriatric, emetic, bedridden patients. An oral fast disintegrating film is usually placed on the tongue and gets dissolved at salivary pH to release the medication for rapid absorption through oral mucosa [7]. Such buccal route bypasses the gut absorption and access into the hepatic portal system avoiding the first pass metabolism and increasing the bioavailability [8].
Materials and Methods
Lignocaine was obtained as gift sample from Shouguang Fukang Pharmacy Factory (Shandong, China). Chitosan, Croscarmelose Sodium (CCS) and Dibutyl Phthalate (DBT) were purchased from sigma Aldrich; USA. All other chemical agents and materials were of analytical grade and used as received.
Statistical design of experiment
All the experiments were designed using Design Expert Software® 6.0.8. Portable Stat- Ease, Inc. software. 3 factor 2 level full statistical designs were used for the optimization and to derive polynomial equation for each variable [9]. Chitosan (A), CCS concentration (B) and concentration of DBT (C) were considered as independent variables while disintegration time (Y1) and in vitro drug release (Y2) were considered as dependent variables. Variables and three levels are presented in Table 1.
| Variable | (-1) Low level | (+1) High level |
|---|---|---|
| Independent | ||
| A=Chitosan Conc. | 30 (mg) | 40 (mg) |
| B=CCS Conc. | 2 (%) | 4 (%) |
| C=DBT Conc. | 10 (%) | 15 (%) |
| Dependent | ||
| Y1= DT | ||
| Y2=%DR |
Table 1. Dependent and independent variables at two different levels.
Preparation of fast disintegrating films of lignocaine
Solvent casting evaporation method was used for the oral fast disintegrating films of Lignocaine [10]. The formula compositions of various formulations were presented in Table 2. The required quantity (30 mg to 40 mg) of polymer (Chitosan) and plasticizer (DBT) was dissolved in 3% acetic acid (20 ml). The dispersion containing Chitosan and DBT was allowed to stir for 1 h on magnetic stirrer at 1000 rpm for uniform distribution and kept aside to remove entrapped air bubbles and called as Phase ‘A’. Aqueous solution (10 ml) was prepared by dissolving (2-4%) of CCS, 50 mg of Lignocaine and 50 mg of mannitol and called as Phase ‘B’. Phase ‘A’ was added to Phase ‘B’ and stirred for 3 h to remove the air bubble. This entire phase was casted on glass petridish of specified dimension (25 cm2) and dried in the vacuum oven at 350?C for 24 h. The film was carefully removed from the petri dish, observed for any imperfections and cut into circular pieces (5 cm2).
| Batch | Factor | ||
|---|---|---|---|
| A | B | C | |
| F1 | 1 | 1 | 1 |
| F2 | 1 | -1 | 1 |
| F3 | 1 | -1 | -1 |
| F4 | -1 | 1 | 1 |
| F5 | -1 | 1 | -1 |
| F6 | 1 | 1 | -1 |
| F7 | -1 | -1 | 1 |
| F8 | -1 | -1 | -1 |
Table 2. Formulation of lignocaine fast disintegrating films using 23 factorial design.
Evaluation of fast disintegrating films Lignocaine
Physical evaluation of films: Physical parameters of the films were evaluated. Visual inspection of the films was carried out for roughness, smoothness, color, clarity, transparency of films.
Thickness: Thickness of the 10 films was measured using micrometer screw gauze and mean was determined.
pH: The film was soaked in distilled water until the films get dissolved at room temperature and the pH was determined by placing the electrode near the surface of the film (n=3). The surface pH of the film measured by placing the film in petridish and wet it with distilled water.
Folding endurance: This test was performed to determine the strength of the film. The folding endurance test was performed by repeatedly folding the film at the same place until it broke. The number of times the film could be folded at the same place without breaking or cracking gave the value of folding endurance.
Content uniformity test: Lignocaine from the film was determined by soaking in 15 ml of methanol. The polymeric dispersion containing Lignocaine was filtered to remove undissolved residue [11]. Aliquots were prepared and absorbance’s was measured spectrophotometrically at 275 nm to calculate the drug content.
In vitro disintegration time (DT) test: Disintegration time of the film (1 cm2) was determined in pH 6.8 phosphate buffer kept in petri dish. The time taken by film to complete disintegrate was considered as disintegration time [12].
Ex vivo permeation studies: Shandong Traditional Chinese Medical University (approval no Shan/FCZ1A66/CHN) approved the animal study and all animal handling were performed according to ethical guidelines. Lignocaine permeation study was performed through porcine oral mucosa. The porcine oral mucosa (previously soaked for 24 h in pH 6.8 phosphate buffer) was stretched around one side of the specially designed glass diffusion cell [13]. The donor compartment was kept in to glass beaker containing pH 6.8 phosphate buffer (receptor compartment) as release media. Proper care was taken to touch the oral mucosa to the receptor compartment. 1 cm2 buccal film was placed on the oral mucosa. The release media was maintained at 37 ± 0.5?C and stirred at 50 rpm. The sampling (5 ml) was done at predetermined time interval and replaced with equal volume of fresh buffer to maintain the sink condition. The amount of drug released was determined by using UV-spectrophotometer at 275 nm.
Tail flick Test: Healthy male rats of 8-9 weeks old (approx. 250-320 g), were selected for the test. Before application of the formulations to the tail of rats, films were dissolved in water to form the drug-polymeric dispersion. This dispersion could be easy for the application as compare to the films on the tail of the rats. The rats were divided in to three groups (each group containing three rats) such as control group, films without CCS group and films with CCS group.
The individual rat was mounted test apparatus with the tail length of 12 cm from its tip, exposed to heat from a projector lamp. A dose of 30 mg of drug polymer dispersion was applied to the root of the tail on the midline. The tail flick anaesthetic test was started after the administration and the test was done every 5 min until the duration time fell to control value. The area under the effective curve from the beginning till the end of 120 min (AUEC0 → 120 min) of the rat tail flick test curve was calculated using the linear trapezoidal rule. The efficacy factor in local anaesthetic effect of Lignocaine film after topical application of film containing CCS was compared with the control film devoid of any additives [14].
Field emission scanning electron microscopy FESEM): Surface properties of the films were determined using field emission scanning electron microscopy (FESEM-S4800, Hitachi, Japan).
Results
Physical characteristics of films
Fast disintegrating films of Lignocaine were smooth, soft, transparent and colorless in nature. Films have good aesthetic properties. High Lignocaine content (97.10 to 99.90%) was observed in films. The films were found to be very thin in nature as desired. The range of thickness was between 0.15 to 0.35 mm. Folding endurance of 120 to 180 indicated greater strength of the films. The pH of the film was 6.60 to 7.10. All the physical parameters are summarized in Table 3.
| Batch | Drug content (%) |
Folding endurance | Thickness (mm) |
pH | Film nature |
|---|---|---|---|---|---|
| F1 | 98.9 | 137 | 0.15 | 6.65 | Smooth |
| F2 | 99.45 | 175 | 0.22 | 7.1 | Transparent |
| F3 | 98.76 | 177 | 0.23 | 6.8 | Transparent |
| F4 | 99.43 | 165 | 0.27 | 6.84 | Smooth |
| F5 | 97.12 | 180 | 0.26 | 7.1 | Smooth |
| F6 | 99.9 | 160 | 0.35 | 6.62 | Smooth |
| F7 | 98.54 | 173 | 0.2 | 6.68 | Soft |
| F8 | 97.1 | 120 | 0.28 | 6.9 | Soft |
Table 3. Physical evaluation of films.
Statistical analysis of disintegration time (DT) of the films
Statistical analysis of the Lignocaine films was done using design expert software. DT of all films was determined in pH 6.8 phosphate buffer (pH of saliva ranges from pH 6.2 to 7.4). The DT of all formulations are presented in Table 4. It could be concluded that all formulations have fast disintegrating property at salivary pH. The polynomial equation for DT can be given by
| Batch | DT (s) | DR (%) |
|---|---|---|
| F1 | 10 | 98.85 |
| F2 | 51 | 83.25 |
| F3 | 42 | 79.88 |
| F4 | 22 | 84.3 |
| F5 | 15 | 91.15 |
| F6 | 21 | 89.13 |
| F7 | 53 | 80.43 |
| F8 | 63 | 73.12 |
Table 4. DT and %DR@t=1 h.
Y1 = +36.25+3.25A-16.75 B+0.50 C+0.000 BC → (1)
Where Y1 is the DT, A, B and C are Chitosan concentration, CCS concentration and DBT concentration respectively. The statistical model for Y1 (2 FI) was found to be significant (p<0.05) with model F-value 0.0150 as shown in Table 5. Synergistic and antagonistic effect of dependent variables could be explained by positive and negative values from the polynomial equation. All independent variables A, B and C showed statistical significant effect (p<0.05) as shown in Table 6.
| Model | R2 | AdjustedR2 | Predicted R2 | Std. Dev | Press | Remarks |
|---|---|---|---|---|---|---|
| Response Y1 | ||||||
| Linear | 0.8260 | 0.8035 | 0.6514 | 7.36 | 510.20 | ……… |
| 2FI | 0.9745 | 0.9184 | 0.7512 | 5.31 | 600.89 | Suggested |
| Quadratic | 0.9265 | 0.9072 | 0.7825 | 0.45 | 550.30 | |
| Cubic | 0.9120 | 0.8966 | 0.5912 | 0.49 | 462.20 | ……… |
| Response Y2 | ||||||
| Linear | 0.6599 | 0.6090 | 0.3563 | 3.75 | 120.73 | ……… |
| 2FI | 0.9353 | 0.8491 | 0.5402 | 2.50 | 133.65 | Suggested |
| Quadratic | 0.9020 | 0.8871 | 0.8598 | 4.90 | 112.30 | ……… |
| Cubic | 0.9114 | 0.9025 | 0.8790 | 6.50 | 125.36 | ……… |
| Regression equations of the fitted models | ||||||
| Y1=+36.25-3.25A-16.75B+0.50C+0.000BC | ||||||
| Y2=+86.87+1.57A+5.20B+1.51C+1.48AC | ||||||
Table 5. Regression analysis of responses Y1 and Y2.
| Source | DF | Sum of squares | Mean Square | F Value | P value |
|---|---|---|---|---|---|
| Model for Y1 | 4 | 2331 | 582.75 | 20.69 | 0.0160 |
| A | 1 | 84.50 | 84.50 | 3.00 | 0.0046 |
| B | 1 | 2244.50 | 2244.50 | 79.69 | 0.0028 |
| C | 1 | 2.00 | 2.00 | 0.071 | 0.0030 |
| BC | 1 | 0.000 | 0.000 | 0.000 | 1.000 |
| Model for Y2 | 4 | 271.89 | 67.97 | 10.85 | 0.0395 |
| A | 1 | 19.59 | 19.59 | 3.13 | 0.0351 |
| B | 1 | 216.53 | 216.53 | 34.56 | 0.0098 |
| C | 1 | 18.24 | 18.24 | 2.91 | 0.02865 |
| AC | 1 | 17.52 | 17.52 | 2.80 | 0.1930 |
Table 6. ANOVA of models for Y1 and Y2.
Statistical analysis of in vitro drug release
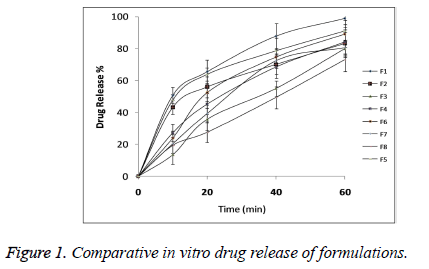
The second independent variable was drug release (Y2). So the in vitro drug release study was performed at salivary pH 6.8. Table 4 shows the percentage drug release from all the formulations. From the results it could be concluded that all films had immediate drug release profile which is desirable for fast disintegrating formulations. The polynomial equation for DR (Y2) was given by
Y2= +86.87+1.57 A+5.20 B+1.51 C+1.48 AC → (2)
The statistical model for Y2 (2 FI) was found to be significant (p<0.05) with model F-value 0.0395 as shown in Table 5. All the three independent variables showed statistical significant effect on DR with p<0.05 as shown in Table 6. Comparative DR Profile is given in Figure 1.
Surface morphology of the films
Surface morphology of the films when studied by using scanning electron microscopy as shown in Figure 2 it confirmed the smooth as well as rough surface nature of the formulated films. The wrinkles observed on the surface of the films were due to the over drying of the films. The films were without the cracks and pores on the surface owing to the well acceptance by the patients.
Tail flick test for local anaesthetic action
Basal nociception level, the analgesic activity of pharmacological agent and tolerance formation can be used to determine from tail flick test. Table 7 Shows the AUECO → 120 min of the rat tail flick test for various formulations containing Lignocaine.
| Formulation | AUEC (sec/min) | Efficacy factor |
|---|---|---|
| Control film dispersion | 900.50 ± 89.27 | 1 |
| Film without CCS dispersion | 1524.67 ± 59.45 | 1.69 |
| Film with CCS dispersion | 2932.87 ± 78.30 | 3.25 |
Table 7. Local anaesthetic action of various formulations.
The AUEC value of the control film dispersion was 900.50 ± 89.27 sec/min, while that of film without CCS dispersion and film with CCS dispersion were 1524.67 ± 59.45 and 2932.87 ± 78.30 sec/min respectively. It could be clear from the results that the films without CCS dispersion had greater local anaesthetic effect as compare to the control film dispersion. In this case local anaesthetic efficacy was 1.69 fold as compare to the control film dispersion. The films with CCS dispersion had showed highest local anaesthetic efficacy (3.25 fold) as compare to the control film dispersion and film without CCS dispersion. The disintegrants property of the CCS played important role for the enhancing local anaesthetic efficacy of the films. Due to rapid disintegration of the film, the Lignocaine was easily available for the pharmacological action on the applied area.
Discussion
There are wide numbers of marketed oral fast disintegrating films available which include Doneprezil rapid dissolving films, Ondansatraon rapid dissolving films manufactured by Labtec Pharma. Altoid cinnamon strips, Boosts vitamin C strips, Cool shock peppermint strips, Benzocaine films, Caffeine films manufactured by Dow chemical company [15]. Ondensetron (Zuplenz®) was the first FDA approved oral disintegrating film marketed since 2010 [10]. All these drug delivery system of oral fast disintegrating films are having no adverse side effect.
Oral fast disintegrating films of the Lignocaine were developed with the intention to enhance the local anaesthetic action by making instant availability of the drug at the site of application without causing any side effects as compare to the other drug delivery systems of Lignocaine. Oral fast disintegrating films serves as a new drug delivery system which was developed based on the transdermal patch technology [16]. The oral disintegrating films were in the form of very thin strips which should be placed on oral mucosa or tongue of the patient. These films rapidly wet by saliva and gets hydrated and adhered on to the site of application [17]. This novel approach for the treatment of mouth ulcer could be useful to increase consumer acceptance by virtue of rapid dissolution, self-administration without water or chewing. The F1 formulation showed DT of 10 sec so these films rapidly disintegrated and dissolved to release the medication for the local anaesthetic effect on ulcers.
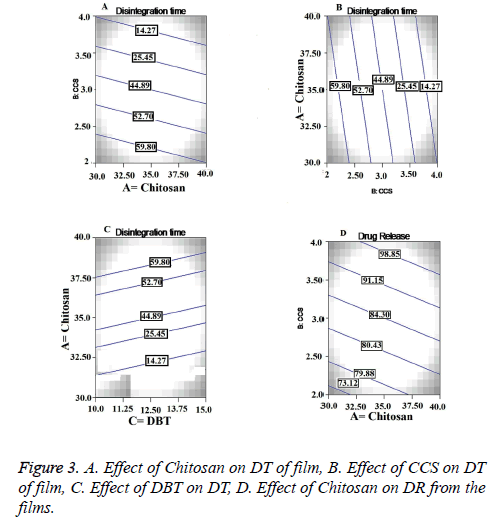
Chitosan is hydrophilic polymer it is used in different formulations with different functions. It is water insoluble polymer and forms gel when comes in contact with the water. Before formation of the gel, it swells in to the aqueous medium. This swelling and gelling property played synergistic effect on the DT of the films [18]. From the counter plot in Figure 3A we can conclude that there is inverse relationship between concentration of chitosan and DT of the films. In these formulations the chitosan acted as a disintegrant. Also the chitosan is good film forming agent with good wetting properties, non-toxic and non-irritant which imparted the necessary mechanical properties to the films [19]. CCS being a crossed link polymer of carboxymethyl cellulose sodium is insoluble, hydrophilic and absorbent in nature. Widely it has been used as superdisintegrant in various pharmaceutical dosage forms. [20]. A direct and statistical significant effect (p=0.0030) was observed between concentration of CCS and DT from contour plot in Figure 3B. DBT as a plasticizer was used to impart the strength and flexibility to the films. Also it has been utilized in films to prevent the brittleness and breakage of the films. The negative effect of the DBT was found on DT with increasing concentration as shown in Figure 3C. But direct relation was observed with increasing concentration of DBT and physical strength of the films. Greater physical strength at higher levels of DBT led to higher impart resistance to break the film, resulting in increased DT.
Due to the gelling property of the chitosan in presence of aqueous medium, it forms the rate controlling layer for the patch system. So gelling of chitosan has retarded the release of Lignocaine from the films. Also chitosan is pseudo plastic material which is excellent viscosity enhancing agent and viscosity increases with increase in chitosan concentration. The combined effect of gelling property and viscosity enhancing effect of chitosan has controlled the release of Lignocaine from the films [21].
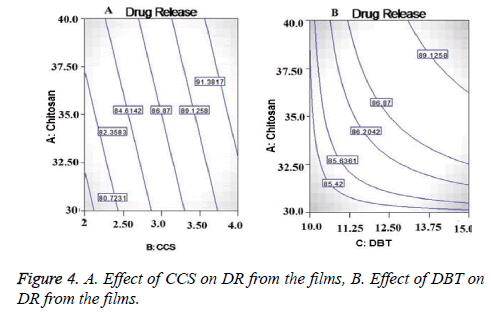
From the contor plot Figure 3D, it could be clear that retardation of drug release was found with increasing the polymer concentration from 35 to 45%. Hydrophilic polymers in aqueous environment swell and create the pore and channels which helps to release the drug from the polymer matrix. The effect of polymer was found to be statistically significant (p<0.05) on drug release as shown in Figure 4A. The disintegrant property of the CCS helped to break the film that resulted in to release of the Lignocaine. Plasticizer also had significant and positive effect on DR as shown in Figure 4.
Also from Tail flick Test it was confirmed that due to instant availability of the Lignocaine from the films, the local anaesthetic effect was increased 3.25 fold as compare to the control film. The disintegrants property of the CCS played important role for the enhancing local anaesthetic efficacy of the films.
Conclusion
The study concludes that oral fast disintegrating films of Lignocaine serves as potential drug delivery systems for mouth ulcer management with enhanced local anaesthetic action.
References
- Sandy H, Michelle M, Chasari T, Katherine L, Andrew D, Franz B. Topical lidocaine to improve oral intake inchildrenwithpainful infectiousmouthulcers- blinded,randomized, placebo-controlled trial. Annals Emerg Med 2014;63: 292-299.
- Hsuan C, Shu CM, Dino T, Yuh YC, Li FW, Ling JW. Oralulcersas an initial presentation of juvenile pemphigus-a case report. Pediatr Neonatol 2013; 52: 105-127.
- Mahmoud KO, Karasneh J, Lynch E. Psychological profiles in patients with recurrent aphthousulcers. International J Oral Maxillofac Surg 2012; 41: 384-388.
- Patricio A, Zapata MFJ, Sierra-Valdez FJ, Ruiz-Suarez JC. The interaction oflocalanestheticswith lipid membranes. J Molec Graphics Model 2014; 53: 200-205.
- Ahmad J, Andrabi SIH, Rathore MA. Comparison of topical glyceryl trinitrate withlignocaine ointment for treatment of anal fissure: A randomised controlled trial. Int J Surg 2007; 5: 429-432.
- Chaudhary H, Gauri S, Rathee P, Kumar V. Development and optimization offastdissolving oro-dispersiblefilmsof granisetron HCl using Box-Behnken statistical design. Bull Facul Pharm Cairo Uni 2013; 51: 193-201.
- Dinge A, Nagarsenker M. Formulation and evaluation of fast dissolving films for delivery of triclosan to the oral cavity. AAPS Pharm Sci Tech 2008; 9: 349-356.
- Muzib YI, Kumari KS. Mucoadhesive buccal films of glibenclamide-Development and evaluation. Int J Pharma Investig 2011; 1: 42-47.
- Pan W, Zhiwei W, Zhichao W. Insights into the effect of preparation variables on morphology and performance of polyacrylonitrile membranes using Plackett-Burman design experiments. Chem Eng J 2012; 194: 50-58.
- Nagaraju T, Gowthami R, Rajashekar M. Comprehensive review on oral disintegrating films. Curr Drug Deliv 2012; 3: 30-45.
- Demiana I, Nesseem SF, Eid SS. Development of novel transdermal self-adhesive films for tenoxicam, an anti-inflammatory. Drug Life Sci 2011; 89: 430-438.
- Wang Z,Itoh Y,Hosaka Y,Kobayashi I,Nakano Y,Maeda I,Umeda F,Yamakawa J,Kawase M,Yag K. Novel transdermal drug delivery system with polyhydroxyalkanoate and starburst polyamidoamine dendrimer. J Biosci Bioeng 2003; 95: 541-543.
- Chauhan AS, Sridevia S, Chalasania KB, Jain AK, Jain SK, Jain NK. Dendrimer-mediated transdermal delivery-enhanced bioavailability of Indomethacin. J Control Rel 2003; 90: 335-343.
- Cheong WC, Deok BK, Sang CS. Development of bio adhesive transdermal bupivacaine gels for enhanced local anaesthetic action. Iranian J Pharm l Res 2012; 11: 423-431.
- Jayjock E, Schmitt R, Chein C. Determination of fast dissolve oral film dissolution rate via conductivity. Dow Chem Comp 2005; 1-4.
- Prabhu P, Malli R, Koland M. Formulation and evaluation of fast dissolving films of levocitirizine di hydrochloride. Int J Pharm Investig 2011; 1: 99-104.
- Choudhary DR, Patel VA, kundawala AJ. Formulation and evaluation of quick dissolving film of levocetirizine dihydrochloride. Int J Pharma Tech 2011; 3: 1740-1749.
- Elzatahry AA, Mohy Eldin MS. Preparation and characterization of metronidazole loaded chitosan nanoparticles for drug delivery application. Polym Adv Technol 2008; 19: 1787-1791.
- Papadimitriou S, Bikiaris D, Avgoustakis K, Karavas E, Georgarakis M. Chitosan nanoparticles loaded with dorzolamide and pramipexole. Carbohydrpolym 2008; 73: 44-54.
- Dinge A, Nagarsenker M. Formulation and evaluation of fast dissolving films for delivery of triclosan to the oral cavity. AAPS Pharm Sci Tech 2008; 9: 349-356.
- Verma P, Ahuja M. Optimization, characterization and evaluation ofchitosan-tailored cubic nanoparticles of clotrimazole. Int J Bio Macro 2015; 73: 138-145.