ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
- Biomedical Research (2012) Volume 23, Issue 4
Persistent hyperglycemia generating reactive oxygen species in renal cells, a probable cause of inflammation in type2 diabetic nephropathy subjects
1Department of Biochemistry, GR Medical College, Gwalior (M.P), India
- *Corresponding Author:
- Neelima Singh
Department of Biochemistry
GR Medical College, Gwalior (M.P)
India
Accepted date: April 11 2012
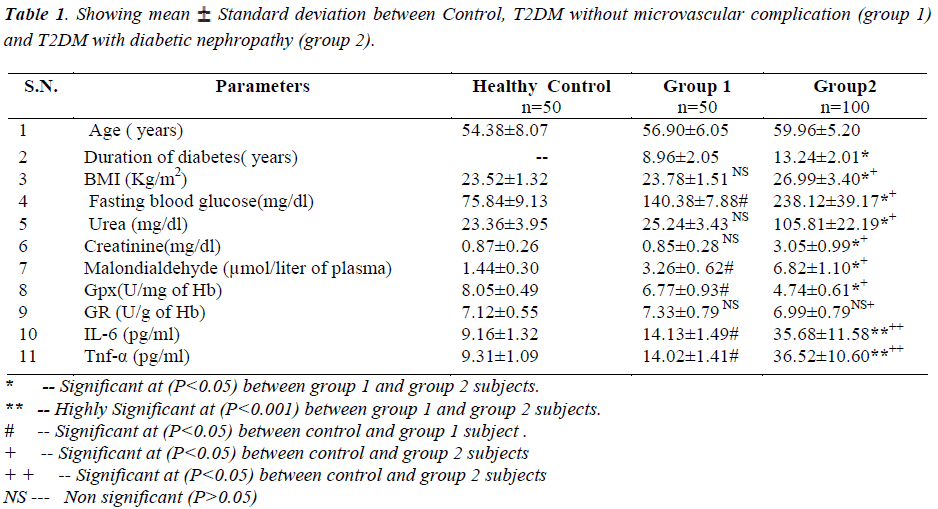
Oxidative stress is increased in diabetes and the overproduction of the ROS (full form ?) in diabetes is a direct consequence of persistent hyperglycemia for the production of inflammatory cytokines such as tumor necrosis factor (TNF)-α and interleukin (IL-6) in renal cells, which are the factors responsible for diabetic complications i.e. diabetic nephropathy. The study aimed to predict the development of type 2 diabetic nephropathy due to persistent hyperglycemia induced oxidative stress producing inflammation. Serum levels of inflammatory markers (IL-6 and Tnf-α), antioxidants, [Glutathione reductase (GR) and Glutathione peroxidase (GPx)], plasma malondialdehyde (MDA), fasting blood sugar, urea and creatinine levels were estimated in controls (n=50), controlled diabetes without diabetic nephropathy, T2DM without microvascular complication (n=50, groupI) and T2DM with diabetic nephropathy (n=100, group II). Sample was collected from J.A group of hospitals, GRMC, Gwalior (M.P) and ethical committee has approved this research work. Statistical method was done by using one way Anova utlizing Dunnet T3 test. Serum levels of inflammatory markers (IL-6 and Tnf-α), malondialdehyde, fasting blood sugar, were high in group II as compared to both group I and controls, (P<0.001), with group II having more significant than group I (P<0. 001). Antioxidant enzyme glutathione peroxidase was found to be decreased in both groups of diabetic patients as compared to controls, (P<<0.05), with group II showing significant than group I at (P<0.001). Glutathione reductase was found to be normal range in all the groups. Serum urea and creatinine levels were increased in group II, (P<0.001), but serum levels were within normal range in group I and healthy controls. Results of the present study indicates that inflammatory markers and oxidative stress are increased with decreased antioxidant defense levels in patients with diabetic nephropathy due to hyperglycemia induced oxidative stress.
Keywords
Oxidative stress, inflammation, diabetic nephropathy
Abbreviations
ROS: Reactive oxygen species, IL-6: Interleukin-6, Tnf-α: Tumor necrosis factor alpha, nF-κB: nuclear factor-κB, PKC: Protein kinase C, RAGE: Recent advanced glycation end products, T2DM: Type2 diabetes mellitus.
Introduction
Diabetic nephropathy is the most common cause of microvascular chronic complication of type-2 diabetes mellitus which is associated with considerable morbidity and mortality, finally leading to end-stage renal disease [1]1. Diabetic nephropathy is a progressive disease that takes several years to develop. It involves various functional clinical abnormalities of the kidney such as elevated creatinine, urea, albuminuria, decline glomerular filtration rate, elevated arterial blood pressure, and fluid retention [2,3]. The pathogenesis of diabetic nephropathy is likely to be multifactorial: it strongly dependent on the duration of diabetes; other risk factors include oxidative stress induced poor glycemic control, hypertension, hypertriglyceridemia, which increases production of cytokines IL-6 and Tnf-α from endothelial cells casing inflammation in type-2 diabetes [4].
Oxidative stress has been defined as a loss of balance between reactive oxygen species (ROS) and protective antioxidant defense system[5]. Increased oxidative stress induced by hyperglycemia may be due to multiple mechanisms (eg, the activation of polyol pathway, inhibition of pentose phosphate pathway, mitochondria dysfunction, activation of NAD(P)H oxidase, and uncoupling of endothelial NO synthase [eNOS], as well as impairment of antioxidant defense system [6, 7]. The oxidative stress generated by hyperglycemia increases reactive oxygen species (ROS), which leads to the activation of various redox-sensitive cell signaling molecules and the production of cytotoxic materials. This is followed by cellular dysfunction and damage, and ultimately results in diabetic micro and macrovascular complications [8, 9].
There is a strong belief that renal glomeruli are particularly sensitive to oxidative stress, suggesting the involvement and participation of ROS in the pathogenesis of diabetic nephropathy. Possible mechanisms for the induction of inflammation in vascular tissues may include activation of PKC pathway and oxidative stress upregulation of RAGE and activation of innate immunity [10]. For instance, carboxymethyllysine- protein adducts due to malondialdehyde, AGEs, can increase the expression of a variety of proinflammatory molecules and NF-κB through the interaction with RAGE in renal cells causing diabetic nephropathy in T2DM [11]
Material and Methods
The study was carried out in Department of Biochemistry in collaboration with Department of Nephrology J.A group of Hospitals, G.R Medical College, Gwalior. The ethical committee of GRMC has approved this research work. The diabetic nephropathy patients attending Department of Nephrology in GRMC, J.A group of hospitals were included in this research work by their consent. The standard screening procedures such as fasting blood sugar, creatinine and radiological findings etc. were the criteria for selection.
Details of study are as follows:
Experimental designs of study are as follows:
Total number of subjects (experimental): 200
150 – Diabetic subjects.
100 – T2DM with diabetic nephropathy (Group 2).
50–T2DM without microvascular complication (Group1).
50 – Healthy Control (age matched)
10 ml of blood sample was withdrawn from the anticubital vein following overnight fasting. The blood sample was collected in plain, fluoride and EDTA vacutainers. The blood sample was centrifuged for 10 min. at 3000 rpm at room temp. The serum was stored at 4 ºC for biochemical and immunological investigations.
Results
Fasting blood sugar level was estimated by GOD-POD method by Biosystems S.A. Barcelona (spain) Catalogue No: M1503i-09 [12]. Urea and Creatinine was estimated in auto analyzer by kit methods of Biosystems S.A. Barcelona (spain) Catalogue No: M12516i-08 and M12502i-12 respectively [13, 14] Glutathione peroxides (Gpx) was estimated by the method of Bergmeyer [15]. Glutathione Reductase (GR) was estimated by the method of Goldberg DM & Spooner RI [16]. Plasma Malondialdehyde (MDA) was estimated by Jean CD [17]. Inflammatory markers Tnf-α and Il-6 was estimated by kits available from immunotech company from patients serum by sandwitch ELISA method [18, 19].
Statistical analysis was done by using One Way Anova utilizing Dunnet T3 test. One Way Anova was used to estimate differences between control, T2DM without microvascular complication and in T2DM with diabetic nephropathy. All parameters were given as mean± standard deviation. The criterion for significance was P < 0.05.
Diabetic nephropathy patients (group 2) have significant increase in fasting blood sugar, serum urea, creatinine, malondialdehyde and inflammatory markers (IL-6 and Tnf-α) levels but decrease in glutathione peroxidase as compared to both T2DM without microvascular complication (Group 1) and healthy controls. All the parameters are showing highly significant at (P<0.05).
Fasting blood sugar, serum urea and creatinine was increased in group 2 as compared to group 1 and healthy controls and was significant at 5% (P<0.05) .
Antioxidant enzyme glutathione peroxidase being decreased in group 2 as compared to both group 1 and healthy controls and it was highly significant at (P< 0.05) whereas Glutathione reductase was found moderately decreased in group 2 as compared to group 1 subjects but it was not statistically significant (P>0.05). But the comparison of Glutathione Reductase within group 1 and healthy controls was not statistically significant. Malondialdehyde (MDA) was increased in group 2 as compared to group 1 and healthy controls and was significant at 5% (P< 0.001).
The body mass index (BMI) was increased in group 2 as compared to group 1 and healthy controls and was significant at 5% (P<0.05) .
Inflammatory markers (IL-6 and Tnf-α) was increased in group 2 as compared to group 1 and healthy controls and was significant at 5% (P<0.001).
Discussion
Oxidative stress depicts the existence of products called free radicals and reactive oxygen species (ROS) which are formed under normal physiological conditions, become deleterious when not being quenched by the antioxidant systems. The body mass index (BMI) increased in group 2 as compared to group 1 and healthy controls might be due to fluid retention in diabetic nephropathy patients. Peroxidation of cell membrane lipids, oxidation of proteins, renal vasoconstriction and damage to DNA are the negative biological effects of reactive oxygen species.. Our study showed moderate decrease of Glutathione reductase in both group 2 as compared to group 1 T2DM subjects but glutathione peroxidase level was significantly decreased in both group 1 and group 2 T2DM subjects as compared to healthy controls which might be due to hyperglycemia induced oxidative stress, glycation of antioxidant enzymes, low hemoglobin concentration and due to excess utilization of NADPH in renal mesangial cells via polyol pathway in type -2 diabetic nephropathy subjects (group2). Our study results were also consistent with (vaishali srivastava et. al 2004)[20].
ROS produced in hyperglycemia increases peroxidation of cellular membrane lipids as well as increasing the oxidation of proteins that yield protein carbonyl derivatives, producing high level of MDA in the diabetic nephropathy subjects which is a suggestive feature of oxidative stress in long standing type-2 diabetes. Our results are also consistent with the study reported by Cvetkovae et al, 2009[21].
End-stage renal failure (ESRD) due to diabetes mellitus has been recently described as a worldwide medical catastrophe. Development of diabetic nephropathy, a condition characterized by renal hypertrophy, is associated with pathophysiological changes in the entire kidney: glomeruli, vessels and the tubulointerstitium. A cellular nodular lesion is the main feature of the disease in the glomerulus. In the renal vessels, the presence of arteriosclerosis often compromises the tubulointerstitium, promotes fibrosis, and eventually leads to ESRD. The appearance of microalbuminuria (30 to 300 mg/dl) is an early indicator of renal involvement in this disease. Once overt proteinuria (>300 mg/dl) is present, confirming diabetic nephropathy, a steady decline in GFR occurs, so that about 50% of these patients can reach ESRD within 7-10 years. Our study shows increase in IL-6 and Tnf-α levels in group 2 subjects as compared to group 1 and healthy controls which might be due to hyperglycemia induced oxidative stress and advanced glycation end products which finally regulates the transcription of nuclear factor-Kappa binding (nf-Kb) in the nucleus producing cytokines, the mediators of inflammation in type 2 diabetic nephropathy subjects.
The released cytokines may have direct effect on the protein permeability barrier of the glomerulus causing alterations in hemodynamic factors of renal cells like glomerular basement membrane thickening, renal mesangial cell expansion and hyperplasia of extracellular matrix, a crucial lesion of diabetic nephropathy and a strong predictor of renal progression in long standing type-2 diabetic patients. Our study results are also consistent with (Alexandraki, 2006; Navarro-Gonzalez et al, 2008) [22, 23] showing increasing levels of cytokines (TNF-α and IL-6) in type2 diabetic nephropathy subjects.
So, finally our study concludes that inflammation is a cardinal process in the appearance of diabetes due to persistent hyperglycemia. The inflammatory milieu i.e. reactive oxygen species production in diabetic kidney contributes significantly to the development of diabetic nephropathy in T2DM.
References
- Raine AEG. Epidemiology, development and treatment of end-stage renal failure in type 2 (non-insulin-dependent) diabetic patients in Europe. Diabetologia 1993; 36: 1099-1104.
- Wolf. G, Ziyadeh. FN. Cellular and molecular mechanisms of proteinuria in diabetic nephropathy.Nephron Physiol 2007; 106: 26-31.
- Balakumar P,Chakkarwar VA ,Kishan P. Vascular endothelial dysfunction: a tug of war in diabetic nephropathy? Biomed Pharmacother 2009; 63: 171-179.
- Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993; 329: 977-986.
- Dursun E, Ozben T, Suleymanlar G, Dursun B,Yakupoglu G. Effect of hemodialysis on the oxidative stress and antioxidants. Clinical Chemistry of Laboratory Medicine 2002; 40: 1009-1013.
- He Z, Rask-Madesen C, King GL. Pathogenesis of diabetic microvascular complications. In International Textbook of Diabetes Mellitus, Edition 3, Vol 2. Edited by De Fronzo R, et al. John Wiley & Sons 2004; 1135-1159.
- He Z, King GL. Microvascular complications of diabetes. Endocrinol Metab Clin North Am 2004; 33: 215-xii.
- Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes,Endocr Rev 2002; 23: 599-622.
- Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes (1991); 40: 405–412.
- Pickup JC. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care 2004; 27: 813-823.
- Kislinger T, Fu C, Huber BN (epsilon)-(carboxymethyl) lysine adducts of proteins are ligands for receptor for advanced glycation end products that activate cell signaling pathways and modulate gene expression. J Biol Chem 1999; 274: 31740-31749.
- Trinder P. Ann Clin Biochem 1969; 6: 24.
- Bermeyer HU and Gutmann I. Methods of enzymatic Analysis, ed Bermeyer HU, Academic Press, NY 1974; 4: 1794-1798.
- Fabinyl DL, Ertingshausen G. Automated reaction rate method for determination of serum creatinine with Centrifi Chem Clin Chem 1971; 17: 696-700.
- Bergmeyer HV. Method in Enzymatic Analysis, New York Academic Press 1963; pp875-879.
- Goldberg DM, Spooner RJ. Methods of Enzymatic Analysis (Bergmeyen HV Ed.) 1983; 3: (3) pp258-265,Verlog Chemie, Deerfield Beach, Fl. Jean CD, Maryse T, Marie JF. Plasma Malondialdehyde levels during Myocardial infarction. Clinica
- Chimica Acta 1983; 129: 319-322.
- Enzyme immunoassay for the in vitro determination of Tnf-α in serum. Kit for determination of Tnf-α, 96 wells (cat. # im1121)
- Enzyme immunoassay for the in vitro determination of IL-6 in serum. Kit for determination of IL-6, 96 wells (cat. # im1120)
- Vaishali S. Correlation of trace elements with status of antioxidant enzymes in normal pregnancy, pregnancy induced hypertension and menopausal state of women.Ph.D. thesis 2004.
- Cvetkoviae T, Mitiae B, Lazareviae G, Vlahoviae P, Antiae S, Stefanoviae V. Oxidative stress parameters as possible urine markers in patients with diabetic nephropathy. J Diab and Its Complications 2009; 23: 337-342.
- Alexandraki K. Inflammatory process in type 2 diabetes. The role of cytokines. Ann NY Acad Sci2006; 1084: 89-117.
- Navarro-Gonzalez JF, Mora-Fernandez C. The role of inflammatory cytokines in diabetic nephropathy. J Am Soc Nephrol 2008;19: 433-442.