ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
- Biomedical Research (2012) Volume 23, Issue 4
Peroxynitrite-induced apoptosis in human brain vascular smooth muscle cells is associated with induction of PDCD4 gene expression
1Center for Reproductive Medicine, Shandong Provincial Hospital Affiliated to Shandong University, Jinan, 250021, P.R. China
2Department of Otolaryngology-Head and Neck Surgery, Provincial Hospital affiliated to Shandong University, Jinan, 250021, P.R. China
3Central Lab, Provincial Hospital affiliated to Shandong University, Jinan 250021, P.R. China Yang Li and Lin Li equally participated in this study
- *Corresponding Author:
- Jianfeng Li or Zhiyong Yue
Center for Reproductive Medicine
Shandong Provincial Hospital Affiliated to Shandong University
Jinan, 250021, P.R. China
Accepted Date: July 27 2012
The present study investigated whether peroxynitrite (ONOO-)-elicited cytotoxicity on human brain vessel smooth muscle cells (HBVSMCs) was related to alteration in programmed cell death 4 (PDCD4) gene expression. Cell viability, morphological changes and apoptosis were determined by methylthiazoletetrazolium (MTT) assay, acridine orange staining and flow cytometry, respectively. Expressions of PDCD4 at both mRNA and protein levels were examined by RT-PCR and Western blot respectively. Protein expressions of caspase-3, Bcl-2 and Bax were detected by Western an increased apoptotic rate of HBVSMCs after treatment with ONOO-. ONOO- was able to upregulate mRNA and protein expressions of the PDCD4 gene. Moreover, protein expressions of caspase-3 and Bax were significantly increased, whereas the expression of Bcl-2 was markedly decreased in response to exposure of ONOO-. This study demonstrates, for the first time, that the PDCD4 gene is involved in ONOO-- induced apoptosis in HBVSMCs.
Keywords
peroxynitrite, PDCD4 gene, human brain vascular smooth muscle cell, apoptosis
Introduction
As a fundamental signaling molecule, nitric oxide (NO) not only regulates critical cellular functions, but mediates cellular damages as well. The latter is now realized to be mainly attributed to the production of ONOO- rather than NO itself [1]. ONOO-, the product of a reaction between nitric oxide and superoxide, is a potent and versatile oxidant [2]. In biological systems, the detection of ONOO- is extremely difficult due to its short biological half-life, multiple target reactions, participation of alternative oxidation/nitration pathways etc.[3]. Cytotoxicity of ONOO- is dependent on its accessibility. In fact, ONOO- possesses a unique trait that can diffuse freely across phospholipid membrane bilayers to react with a wide variety of bio-macromolecules, which commits cells to necrosis or apoptosis in different cell types [4,5] including vascular smooth muscle cells (VSMCs). Thus, it is conceivable that excessive generation of endogenous ONOO- plays key roles in the initiation and progress of vascular diseases.
Vascular diseases are characterized by alterations of blood vessel structure determined mainly by the status of VSMC, which is now viewed as the result of the opposing effects of cell proliferation and apoptosis. As VSMCs is the major component of blood vessel wall, apoptosis of VSMCs is a prominent feature of the vascular remodeling process that occurs in atherosclerosis, hypertension, restenosis and stroke [6]. It is well established that free radicals, which consist of two major types, i.e., reactive oxygen species (ROS) and reactive nitrogen species (RNS), such as OH˙, O2˙, NO˙, and ONOO-, are overproduced in the course of abnormal metabolic processes [5,6]. Thus, extensive study on apoptosis of VSMCs elicited by free radicals is of importance to decipher mechanisms underpinning these aforementioned vascular disorders.
Previously, we demonstrated that ONOO- can trigger apoptosis in canine VSMCs [7]. However, the precise mechanisms underlying ONOO--elicited apoptosis in VSMCs are still unknown. Recently, PDCD4 has emerged as a novel tumor suppressor gene, which is ubiquitously expressed in normal tissues, but, lost or suppressed expression of PDCD4 in several tumors that correlates with tumor progression and poor prognosis [8]. Its degradation is necessary for efficient protein translation in vivo, which is a prerequisite for cell proliferation [9]. Notably it has been found that PDCD4 is up-regulated during apoptosis in several cell types [8,9]. To date, however, no report in the literature has been appeared on its potential role of PDCD4 in ONOO-- induced apoptosis of VSMCs. Therefore, the present study was designed to investigate whether apoptosis of HBVSMCs occurs in response to ONOO-, with special attention given to the induction of PDCD4 expression in this process.
Materials and Methods
Materials
ONOO- was purchased from Cayman CHEMICAL (Ann, Arbor, MI, USA, Cat. 81565) and frozen at -80ºC before use, since the product is heat and light sensitive. Activity decreases approximately 2% per day at -20 ºC. The Human Brain Vascular Smooth Muscle Cells (HBVSMCs, #1100) were obtained from the ScienCell Research Laboratories (USA). Smooth muscle cell medium for definite purpose, fetal bovine serum and smooth muscle cells growth factors were from the ScienCell Research Laboratories (USA). Rabbit-anti-PDCD4 polyclonal antibody was purchased from BioWorld, Inc. (USA). All other agents were purchased from Sigma (St. Louis, MO, USA).
Cell Culture
HBVSMCs were cultured in smooth muscle cells medium containing 10% fetal bovine serum, 100 U/ml penicillin, and 100 μg /ml streptomycin at 37ºC in a humidified atmosphere composed of 95% air and 5% CO2. Cells were regularly sub-cultured with 0.25% trypsin, and then seeded into Petri dish (105/ dish), 24 or 96-well plate for different experiments.
Cell Viability Assessment
The 3-(4, 5-Dimethylthiazol-2-yl)-2, 5-Diphenyltetrazolium Bromide (MTT) assay was employed to assess the cell viability. MTT assay was based on the ability of the viable cells to reduce soluble yellow MTT to blue formazan crystals. In brief, cells (1×104/well) were seeded into 96-well plate containing 100 μl culture medium. Twenty-four hours before ONOO- treatment, medium was replaced with 1% serum medium. ONOO- was diluted in phosphate-buffered saline (PBS; pH 8.3) and was directly added to the culture medium at final concentrations of 10, 50, 100 μM for 24 h incubation. Control sample was treated with PBS (pH 8.3) only. The effect of decomposed ONOO- was also tested after the solution of ONOO- was kept in PBS (pH 7.2) at 37 ºC for 30 min as we described previously [7].
After 24 h of incubation with ONOO-, MTT was added to reach a final concentration of 0.5 mg/ml and incubation for a further 4 h at 37ºC. Medium was subsequently removed and the precipitate in each well was dissolved in dimethylsulfoxide. Plates were incubated for another 20 min in the dark. Optical density (OD) value, which represents the absorption of formazan dissolved by DMSO at 570 nm, was measured using an ELISA reader (Multiskan MK3, Finland).
Morphological Observation
Morphological changes of HBVSMCs in response to different concentrations of ONOO- for 24 h incubation were evaluated by acridine orange (AO). Briefly, cells (5 ×104 / well) were seeded into 24-well plate containing 1ml culture medium. After incubation for 24 h, the medium was removed and refilled with new serum-free medium containing ONOO- at concentrations of 10, 50, 100 μM for 24 h incubation. Cells were washed twice by phosphate buffered saline (PBS), fixed with 95% alcohol for 10 min and then stained by 0.01% acridine orange for 5 min. After washing twice with PBS, morphological changes were examined by fluorescence microscope (Leica, DMI3000). Cells which presented with nuclear fragmentation and/or marked condensation of chromatin with reduced nuclear size, or apoptotic body, were interpreted as apoptotic cells.
Flow Cytometry Assessment
Occurrence of apoptosis was quantitatively assessed by flow cytometry using Annexin V-FITC and propidium iodide (PI). Cells (5×104/ml) were seeded into 6-well plate and treated with ONOO- for 24 h. After treatment, cells were trypsinized, washed with PBS. After washing, cells (1×107/ml) were resuspended in binding buffer (200 μl) containing Annexin V-FITC (5 μl, 20 μg/ml) and PI (10 μl, 20 μg/ml) for at least 15 min at room temperature, and then added binding buffer (300 μl) before analysis with a FACScan (BectonDickinson, Mountain View, CA, USA) using CellQuest software (BD Biosciences, San Jose, CA, USA). A minimum of 104 cells were counted for each assay.
Reverse Transcription-Polymerase Chain Reaction (RTPCR)
Total RNA was extracted using TRIzol (Invitrogen) and was further purified with RNeasy purification kit (Qiagen). The reverse transcription reaction was performed using the ExScrioptRT reagent kit (Takara) in a final volume of 20 μl containing 1 μg total RNA, 4 μl 5×ExScriopt buffer, 1μl deoxynucleotide triphosphate (dNTP) mixture, 1 μl OligodT primer, 0.5 μl ExScriopt RTase, 0.5 μl RNase inhibitor and RNase-free water to a volume of 20 μl. RT was performed at 42ºC for 15 min, and terminated by heating at 95ºC for 2 min. PCR was performed following the instruction of TaKaRa TaqTM under the following conditions: predegeneration at 95ºC for 3 min, degeneration at 95ºC for 45 s, and renaturation at 60ºC for 40 s, elongation at 72ºC for 50 s. This process was 28 cycles in total. The products were identified by 1% agarose gel electrophoresis. All experiments were conducted three times. The primers were as follows:
PDCD4: forward: 5´-ATGTGGAGGAGGTGGATGTG-3´;
reverse: 5´- TGGTGTTAAAGTCTTCTCAAATGC -3´.
β-actin: forward: 5-CTCCTTAATGTCACGCACGATTT-3´;
reverse: 5´-GTGGGGCGCCCCAGGCACCA -3´.
Western Blot
HBVSMCs, which had been subjected to different concentrations of ONOO-, were harvested at indicated time points and lysed with a cold radio immune precipitation buffer (RIPA) protein lysis buffer for 30 min on ice. Lysates were transferred to Eppendorf tubes and clarified by centrifugation at 12 000g for 15 min at 4ºC. The supernatant was kept at -80ºC until use. The Bradford method was used to determine the protein concentration of the supernatant. Samples (40 μg of total protein each) were boiled at 95ºC for 5 min and loaded onto sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDSPAGE) containing 5% stacking gel and 15% separating gel, followed with a separation at 80 V for about 2 h and subsequent transferred to a polyvinylidene difluoride membrane. The membrane was blocked in 5% defatted milk for 1 h at room temperature, incubated with the first antibodies (anti-PDCD4: 1:1000, anti-caspase-9: 1:400, anti-caspase-3: 1:400, anti-Bcl-2: 1:400, and anti- Bax:1:400) and diluted in 5% defatted milk/Tris-buffered saline-Tween (TBST) overnight at 4ºC. After washing with TBST, the membrane was incubated with a secondary antibody against mouse IgG accordingly. Eventually, immunoblots were detected using an ECL kit (Santa Cruz, CA, USA) and visualized after X-ray film exposure. Blots were reprobed with β-actin antibody (1:2000) after stipping the membrane used for the aforementioned detection of specific signals and the results were used as loading controls. Densitometer readings were used to quantitate immunoreactive bands. The relative OD ratios of PDCD4, cleaved caspase-3, Bcl-2, and Bax were calculated with the Image J software by comparison to β-actin.
Statistic Analysis
Data were presented as mean ± SD. Statistical calculations were performed using SPSS11.5 software package from at least three separate experiments. (Explain the statistical procedure/principle in more detail). One-way analysis of variance (ANOVA) was applied by Fisher's protected least significant difference (PLSD)-test to compare the means of multiple groups. P-values of less than 0.05 were considered as significant.
Results
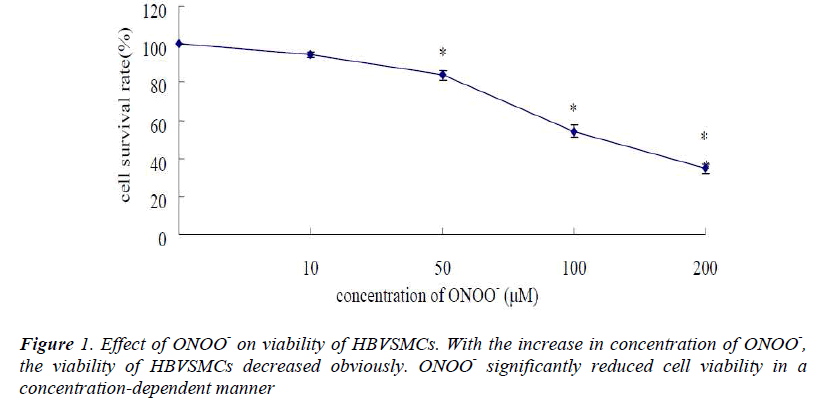
Effects of ONOO- on Cell Viability in HBVSMCs
Treatment with ONOO- resulted in a concentrationdependent reduction of survival of HBVSMCs as evidenced by MTT assay (Fig. 1). The greater the concentration of ONOO- was administrated, the less the number of surviving cells was remained. Use of decomposed ONOO- (see Materials and Methods) failed to exert any effects on cell viability or any of other parameters tested below.
HBVSMCs
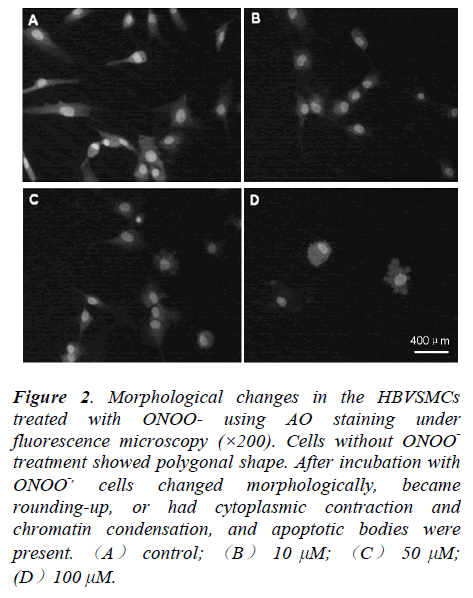
In order to assess whether cell death induced by ONOOwas due to necrosis or apoptosis, morphological changes of HBVSMCs after treatment with different concentrations of ONOO- for 24 h was determined by AO staining. Cells without ONOO- treatment showed polygonal shape, but, cells treated with ONOO- became rounding-up, or had cytoplasmic contraction and chromatin condensation. Apoptotic bodies, the main morphological characteristic of apoptosis, were present. Thus, our data indicted that ONOO- predominately induced apoptosis of the HBVSMCs (Fig.2).
Figure 2: Morphological changes in the HBVSMCs treated with ONOO- using AO staining under fluorescence microscopy (×200). Cells without ONOO- treatment showed polygonal shape. After incubation with ONOO-, cells changed morphologically, became rounding-up, or had cytoplasmic contraction and chromatin condensation, and apoptotic bodies were present. (A) control; (B) 10 μM; (C) 50 μM; (D)100 μM.
Flow Cytometry Analysis (FCM)
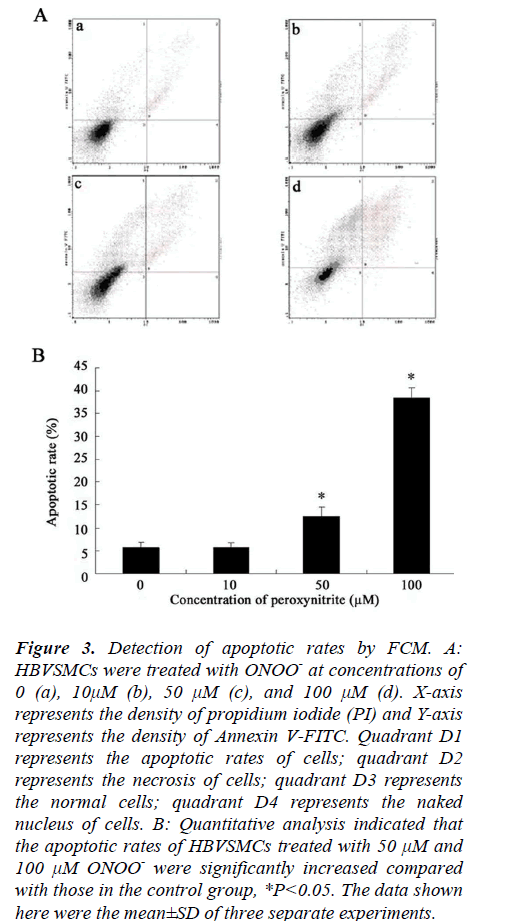
Quantitative analysis of apoptotic cells was determined by FCM. Compared to the apoptotic rate of control cells (5.84%), rates exposed to ONOO- at concentrations of 10, 50, 100 μM for 24 h incubation were approximately 5.8%, 12.6%, and 38.5%, respectively (Fig. 3). Statistical analysis showed that the apoptotic rates of HBVSMCs treated with 50 μM and 100 μM ONOO-, rather than treated with 10 μM ONOO-, were significantly increased compared with those in the control group (*P<0.05, Fig. 3), indicating that the percentage of apoptotic cells elevated with the increase in the concentration of ONOO-. These results further suggested that HBVSMCs underwent apoptosis in response to this oxidative insult.
Figure 3: Detection of apoptotic rates by FCM. A: HBVSMCs were treated with ONOO- at concentrations of 0 (a), 10μM (b), 50 μM (c), and 100 μM (d). X-axis represents the density of propidium iodide (PI) and Y-axis represents the density of Annexin V-FITC. Quadrant D1 represents the apoptotic rates of cells; quadrant D2 represents the necrosis of cells; quadrant D3 represents the normal cells; quadrant D4 represents the naked nucleus of cells. B: Quantitative analysis indicated that the apoptotic rates of HBVSMCs treated with 50 μM and 100 μM ONOO- were significantly increased compared with those in the control group, *P<0.05. The data shown here were the mean±SD of three separate experiments.
Effects of ONOO- on mRNA Expression of PDCD4 in HBVSMCs by RT-PCR
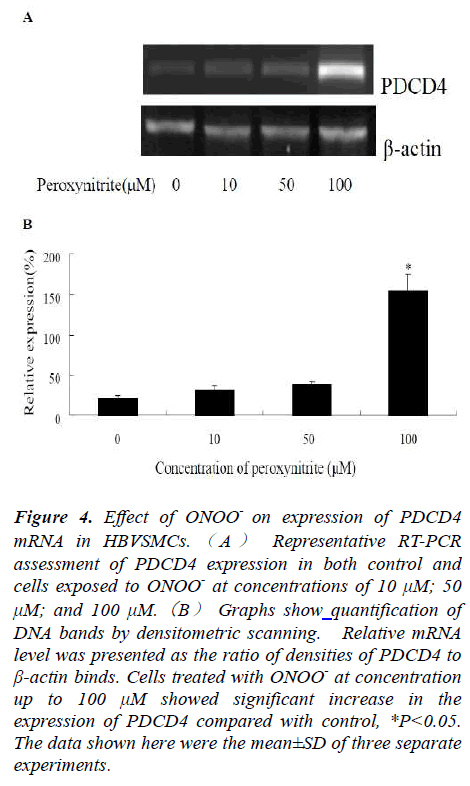
In order to test whether treatment with ONOO- could modulate the PDCD4 mRNA expressions, HBVSMCs were treated with ONOO- at different concentrations. Compared to control cells, PDCD4 mRNA expressions in all experimental groups were upregulated after 24 h of exposure. Image J software was used to analyze the relative photodensity, with β-actin as standard. Statistic analysis showed that PDCD4 mRNAexpression was significantly higher in experimental groups in comparison to that of the control group, *P<0.05 (Fig.4).
Figure 4: Effect of ONOO- on expression of PDCD4 mRNA in HBVSMCs. ( A ) Representative RT-PCR assessment of PDCD4 expression in both control and cells exposed to ONOO- at concentrations of 10 μM; 50 μM; and 100 μM.(B) Graphs show quantification of DNA bands by densitometric scanning. Relative mRNA level was presented as the ratio of densities of PDCD4 to β-actin binds. Cells treated with ONOO- at concentration up to 100 μM showed significant increase in the expression of PDCD4 compared with control, *P<0.05. The data shown here were the mean±SD of three separate experiments.
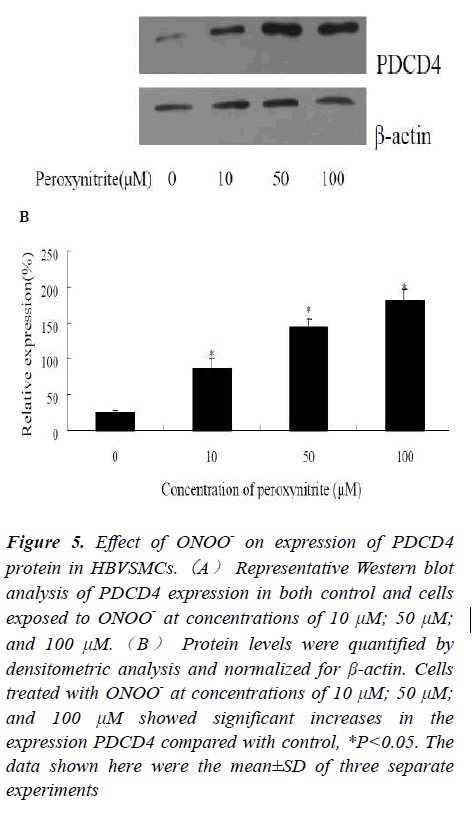
Effects of ONOO- on Protein Expression of PDCD4 in HBVSMCs by Western blot
To further investigate the alteration of PDCD4 expression at protein level, Western blot was employed in this work. Protein expression levels of PDCD4 in three experimental groups treated with ONOO- at concentrations of 10, 50, 100 μM for 24 h were upregulated at 24 h when compared to controls. Image J software was used to analyze the relative photodensity with β-actin as standard. Statistic analysis showed that PDCD4 protein expression was significantly higher in experimental groups in comparison to that of the control group, *P<0.05 (Fig.5). It should be pointed out that the PDCD4 expressions at indicated time points were different, in certain degrees, between the protein and mRNA levels. The explanation for the discrepancy of the mRNA and protein abundances might be a time lag between the responses of transcript and translation of this gene.
Figure 5: Effect of ONOO- on expression of PDCD4 protein in HBVSMCs.(A) Representative Western blot analysis of PDCD4 expression in both control and cells exposed to ONOO- at concentrations of 10 μM; 50 μM; and 100 μM.(B) Protein levels were quantified by densitometric analysis and normalized for β-actin. Cells treated with ONOO- at concentrations of 10 μM; 50 μM; and 100 μM showed significant increases in the expression PDCD4 compared with control, *P<0.05. The data shown here were the mean±SD of three separate experiments
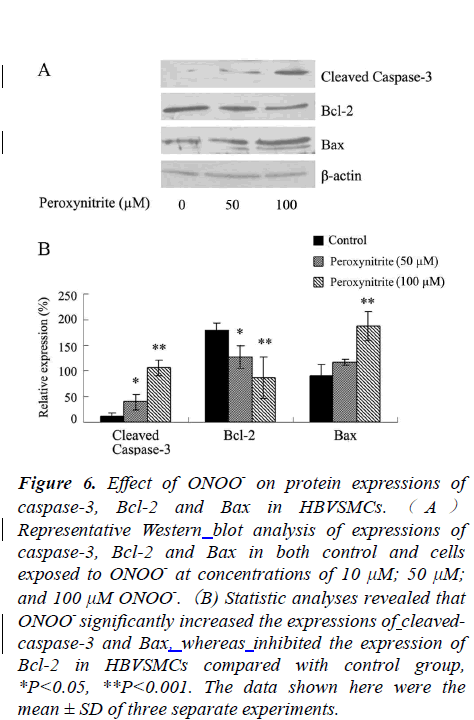
Effects of ONOO- on Protein Expressions of Caspase-3, Bax and Bcl-2 in HBVSMCs by Western blot
It is well recognized that the onset of apoptosis is initiated and executed via a caspase-dependent and/or a caspaseindependent pathway. To probe which pathway was involved in ONOO--induced apoptosis, protein expressions of caspase-3, Bcl-2 and Bax were examined.. Compared to the control group, expression of caspase-3 was increased significantly after exposure to ONOO- at concentrations of 50 and 100 μM for 24 h. Moreover, the expression of Bcl-2 (an anti-apoptotic gene) was decreased, whereas, the expression of Bax (a proapoptotic gene) was markedly increased, *P<0.05, or **P<0.01 (Fig.6).
Figure 6: Effect of ONOO- on protein expressions of caspase-3, Bcl-2 and Bax in HBVSMCs. ( A ) Representative Western blot analysis of expressions of caspase-3, Bcl-2 and Bax in both control and cells exposed to ONOO- at concentrations of 10 μM; 50 μM; and 100 μM ONOO-.(B) Statistic analyses revealed that ONOO- significantly increased the expressions of cleavedcaspase- 3 and Bax, whereas inhibited the expression of Bcl-2 in HBVSMCs compared with control group, *P<0.05, **P<0.001. The data shown here were the mean ± SD of three separate experiments.
Discussion
In the present investigation we demonstrated that ONOOdecreased the viability of HBVSMCs in a concentrationdependent manner as shown by MTT assay, indicating that ONOO- was able to suppress the growth of HVSMCs in vitro. Exposure of HVSMCs to ONOO- led to morphological alterations which were in accordance with typical morphological characteristics of cells that underwent apoptosis. Moreover, the apoptotic rates were increased when cells exposed to ONOO- as confirmed by flow cytometry. This further revealed the occurrence of apoptosis, rather than necrosis, induced by ONOO-. These data were similar with our previous findings in cultured VSMCs from animals [7].
Since Salgo et al. firstly reported that ONOO- can induce apoptosis in thymocytes [11]; a series of publications on ONOO--induced apoptosis in different cell types in vitro have appeared in literature [12]. The exact mechanisms by which ONOO- induces apoptosis in VSMCs, however, have been studied only sparingly in vascular smooth muscle cells in culture. It has been reported that the occurrences of apoptosis in different types of tumor is associated with the induction of PDCD4 tumor suppressor gene expression, providing evidence for its role in apoptosis [13]. However, little is known about the role of PDCD4 in vascular diseases. Since PDCD4 has a tight relation to cell apoptosis, we assume that the alteration of PDCD4 is likely to occur in the course of ONOO-- induced apoptosis in HBVSMCs. We demonstrated for the first time that expression of PDCD4 mRNA increased after exposure of HBVSMCs to ONOO-. Our findings imply that ONOO--induced apoptosis in HBVSMCs is related to induction of PDCD4 expression, which might play an important role in mediating apoptosis in HBVSMCs. These results were consistent with our previous reports found in tumor cells [14,15].
It is well established that at least two classical apoptotic pathways, i.e., the mitochondrial pathway and the death receptor pathway, both of which lead to activation of caspase cascades though a series of interactions [4]. We found that ONOO- led to significant upregulation of caspase-3 activation, suggesting that the mitochondrial apoptotic pathway plays important roles in ONOO-- induced apoptosis in HBVSMCs. Furthermore, we demonstrated that when apoptosis occurred, the expression of Bcl-2 protein was reduced significantly, whereas, the expression of Bax was increased. This indicates that Bcl-2 family protein may be involved in ONOO--induced damage in HBVSMCs.
In conclusion, the current study demonstrates that PDCD4 participates in ONOO--induced apoptosis in HBVSMCs. The precise role of PDCD4 underlying the action of ONOO- needs to be explored further. As ONOOgeneration in vivo is a crucial pathogenic mechanism responsible for vascular diseases, the intensive investigation aimed at removing ONOO- might offer a new powerful therapeutic approach for the treatment of vascular disorders in the future.
Acknowledgements
This work was supported in part by Department of Science & Technology of Shandong Province, P.R. China (2006GG2202). Also state the institutions that supported the rest of the work.
References
- Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev, 2007; 87(1): 315-424
- Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci USA, 1990; 87(4): 1620-624
- Poderoso JJ. The formation of peroxynitrite in the applied physiology of mitochondrial nitric oxide. Arch
- Biochem Biophys, 2009; 484(2): 214-220
- Liu W, Fan Z, Han Y, Lu S, Zhang D, Bai X, Xu W, Li J, Wang H. Curcumin attenuates peroxynitrite-induced neurotoxicity in spiral ganglion neurons. Neurotoxicology, 2011; 32(1):150-157
- Chen AF, Chen DD, Daiber A, Faraci FM, Li H, Rembold CM, Laher I. Free radical biology of the cardiovascular system. Clin Sci (Lond). 2012; 123(2): 73-91
- Schildknecht S, Ullrich V. Peroxynitrite as regulator of vascular prostanoid synthesis. Arch Biochem Biophys, 2009; 484(2): 183-189
- Li J, Su J, Li W, Liu W, Altura BT, Altura BM. Peroxynitrite induces apoptosis in canine cerebral vascular muscle cells: possible relation to neurodegenerative diseases and strokes. Neurosci Lett, 2003; 350(3): 173-177
- Göke R, Barth P, Schmidt A, Samans B, Lankat- Buttgereit B. Programmed cell death protein 4 suppresses CDK1/cdc2 via induction of p21(Waf1/- Cip1). Am J Physiol Cell Physiol, 2004; 287(6): C1541-6
- Suzuki C, Garces RG, Edmonds KA, Hiller S, Hyberts SG, Marintchev A, Wagner G. PDCD4 inhibits translation initiation by binding to eIF4A using both its MA3 domains. Proc Natl Acad Sci USA, 2008; 105(9): 3274-3279
- Ozpolat B, Akar U, Steiner M, Zorrilla-Calancha I, Tirado-Gomez M, Colburn N, Danilenko M, Kornblau S, Berestein GL. Programmed cell death-4 tumor suppressor protein contributes to retinoic acid-induced terminal granulocytic differentiation of human myeloid leukemia cells. Mol Cancer Res, 2007; 5(1): 95-108
- Salgo MG, Squadrito GL, Pryor WA. Peroxynitrite causes apoptosis in rat thymocytes. Biochem Biophys. Res Commun, 1995 ; 215(3):1111-1118
- Guner YS, Ochoa CJ, Wang J, Zhang X, Steinhauser S, Stephenson L, Grishin A, Upperman JS. Peroxynitriteinduced p38 MAPK pro-apoptotic signaling in enterocytes. Biochem Biophys Res Commun, 2009; 384(2): 221-5
- Lankat-Buttgereit B, Göke R. The tumour suppressor Pdcd4: recent advances in the elucidation of function and regulation. Biol Cell, 2009; 101(6): 309-317
- Gao F, Zhang P, Zhou C, Li J, Wang Q, Zhu F, Ma C, Sun W, Zhang L. Frequent loss of PDCD4 expression in human glioma: possible role in the tumorigenesis of glioma. Onco Rep, 2007; 17(1): 123-128.
- Zhang S, Li J, Jiang Y, Xu Y, Qin C. Programmed cell death 4 (PDCD4) suppresses metastastic potential of human hepatocellular carcinoma cells. J Exp Clin Cancer Res, 2009; 28:71-81