ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
- Biomedical Research (2015) Volume 26, Issue 2
Organization of cytoskeleton and chromatin is related to the timing of the first zygotic cleavage and early developmental competence.
1Institute of Medical Molecular Biotechnology, Faculty of Medicine, Universiti Teknologi MARA, Sungai Buloh Campus, 47000 Sungai Buloh, Selangor, Malaysia.
2Faculty of Pharmacy and Health Sciences, Universiti Kuala Lumpur Royal College of Medicine Perak, 30100 Ipoh, Perak, Malaysia.
3Drug Discovery and Health Community of Research, Universiti Teknologi MARA (UiTM), 40450 Shah Alam, Selangor Darul Ehsan, Malaysia.
4Faculty of Medicine, Universiti Teknologi MARA, Sungai Buloh Campus, 47000 Sungai Buloh, Selangor, Malaysia
5Faculty of Health Sciences, Universiti Teknologi MARA, Puncak Alam Campus, 42300 Puncak Alam Selangor, Malaysia
- Corresponding Author:
- Mohamed Noor Khan Nor-Ashikin
Institute of Medical Molecular Biotechnology (IMMB)
Faculty of Medicine, Universiti Teknologi MARA
Sungai Buloh Campus, 47000 Sungai Buloh
Selangor, Malaysia
Accepted Date: December 12 2014
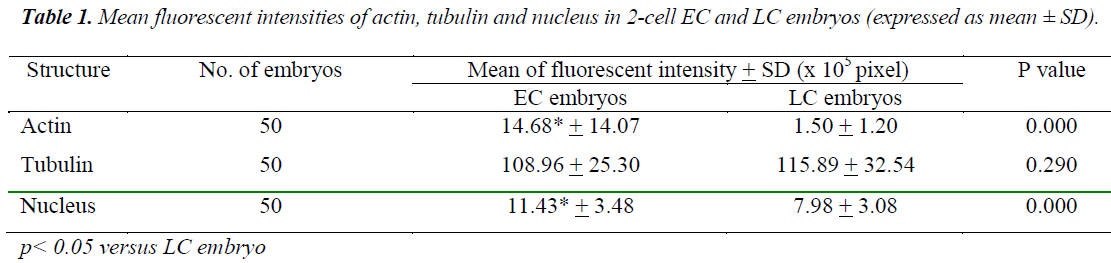
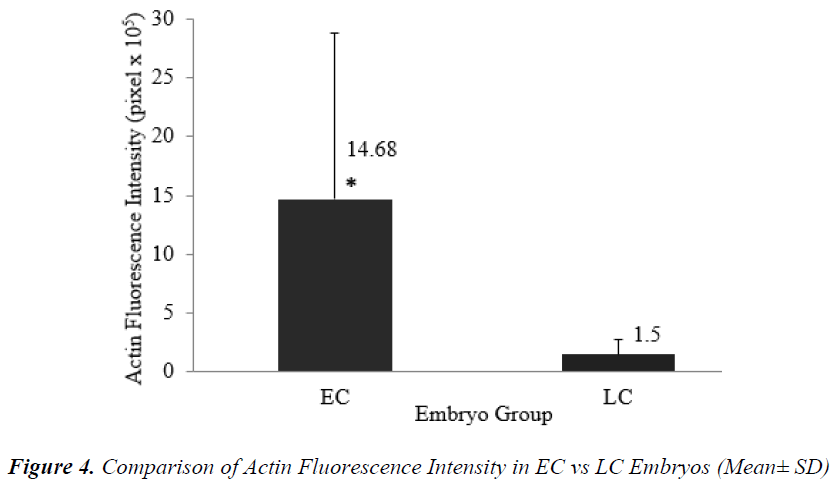
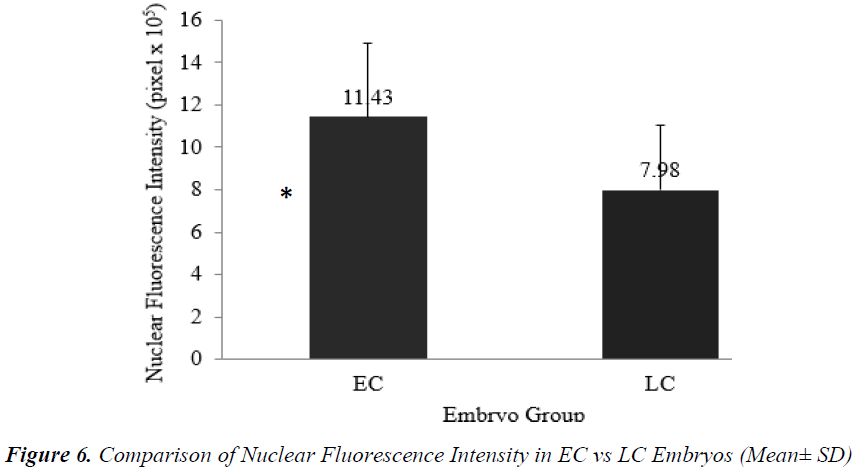
Timing of the first zygotic cleavage is a reliable predictor of embryo quality. Embryos that cleave early have higher developmental viability compared to their late counterparts. It is hypothesized that differences in viability is attributed to cytoskeletal and chromatin organizations. This study investigated cytoskeletal and chromatin structures, and distributions in earlycleaving (EC) versus late-cleaving (LC) embryos. Embryos were retrieved from superovulated ICR mice, 28 hours after hCG injection. Two-cell stage embryos were categorized as EC, while zygotes with two pronuclei as LC embryos. After overnight culture in M16 medium, embryos were fixed and immunostained to visualize cytoskeletal and chromatin distributions, and intensities. EC embryos were observed to have significantly higher actin and chromatin fluorescence intensities compared to LC embryos [(14.68 + 14.07) x 105 versus (1.50 + 1.20) x 105 pixels (p<0.05) and (11.43 + 3.48) x 105 versus (7.98 + 3.08) x 105 pixels (p<0.05)], respectively. There was no significant difference in tubulin intensity between EC and LC embryos. This suggests that higher densities of actin and chromatin in EC embryos appreciably contributed to more efficient cell division and therefore, greater developmental competence.
Keywords
Early cleavage; mouse embryo; cytoskeleton; chromatin; fluorescence intensity
Introduction
Timing of the first zygotic cleavage is a reliable indicator for embryo quality [1-3]. It is a better assessment method compared to traditional morphological grading. Embryos that cleaved early were proven to be of better quality compared to their later counterparts. Many animal and clinical studies have shown that early cleaving embryos have not only higher developmental viability and higher blastocyst formation, but also produced higher pregnancy and live birth rates [3-6].
Although the selection of embryos based on the timing of the first zygotic cleavage has now become a common selection practice in many in vitro fertilization (IVF) laboratories, factors that contribute to the superiority of early cleavers are still not clear. It has been hypothesized that it may be related to cytoskeletal ultrastructure of the embryos. This is because the cytoskeleton plays an important role in organelle transport, cell division, motility, and signalling [7].
There are three subclasses of cytoskeleton - microfilaments including actin, intermediate filaments, and microtubules, including tubulin [7]. Actin and tubulin have been demonstrated to play an important role during embryo cleavage. Tubulin assists migration of pronuclei during the fertilization process [8]. It is involved in mitosis and chromosomal spindle formation and movement of organelles such as mitochondria [8]. Actin is also involved in many important cellular processes, such as cell motility, cytokinesis and division, organelle movement, spindle migration, distribution of mito chondria [9,10], polarization of embryos, pronuclear apposition, cell signalling and maintenance of cell shape [11,12]. Many of these processes are mediated by the interactions of actin with cell membranes.
Data on the distribution patterns and intensities of actin, tubulin and nuclear chromatin during early stages of embryo development is scarce. Understanding the distribution patterns and intensity of those structures will provide important knowledge of their contribution and association with the viability of early and late cleaving embryos.
Material and Methods
Source of embryos
All procedures involving animals were approved by Animal Care and Use Committee (ACUC), UiTM (ACUC- 7/11). Embryos were obtained from female ICR mice, aged 6 – 8 weeks. Mice were superovulated by intraperitoneal (i.p.) injection of 5 IU of Pregnant Mare Serum Gonadotrophin (PMSG; Folligon, Intervet), followed 48 hours later by 5 IU Human Chorionic Gonadotrophin (i.p.) (hCG; Chorulon, Intervet). The females were subsequently mated with male mice of the same strain, at a ratio of 1:1. The females were checked for the presence of a vaginal plug the morning after mating. After 28 hours of hCG administration, the oviducts of the pregnant female mice were excised and embryos were flushed out into M2 medium (Sigma, USA: M7167). The embryos were observed under an inverted microscope (Leica DMIRB, Germany). One-cell embryos with 2 pronuclei and embryos at the 2-cell stage were considered fertilized.
Timing of the first zygotic cleavage
Embryos were divided into two groups; early-cleaving and late-cleaving embryos according to the timing of their first zygotic cleavage. Embryos at the 2-cell stage at 28 – 30 hours post hCG administration were categorized as early cleaving (EC) embryos, whereas zygotes with the presence of the second polar body and two pronuclei were categoryzed as late cleaving (LC) embryos. The embryos were cultured overnight in a CO2 incubator in 50 μl drops of M16 culture medium (Sigma, USA: M7292) with 3% Bovine Serum Albumin (BSA) (Sigma, USA: A9418). Droplets were overlaid with mineral oil (Sigma, USA: M8410).
Experimental Design
A total of 50 EC embryos and 50 LC embryos were fixed at 48 hours post-hCG to study the distributions and densities of the actin, tubulin and chromatin at the 2-cell stage. Following immunolabeling with monoclonal anti-α- tubulin, Alexa Fluor and DAPI to identify tubulin, actin and nuclear chromatin respectively, embryos were examined under the Confocal Laser Scanning Microscope (CLSM) (Leica TCS SP5 AOBS, Germany). The intensities and distribution of the cytoskeletal components and nuclear chromatin were recorded.
Embryo fixation and immunofluorescent staining
Embryos were fixed in 4% paraformaldehyde at 37°C until further processing (for a maximum of three weeks). Following fixation, the embryos were washed in PBS for 10 min, to reduce free aldehydes and to block nonspecific reactions. Embryos were further processed for immunostaining, through serial incubations in dye.
Nuclear staining was performed by incubation with 4’, 6’-diamino-2-phenylindole dihydrochloride (DAPI) (Molecular Probes, Life Technologies, USA: D3571) for 30 min before being permeabilized with 1% Triton® X- 100 (Sigma, USA: X100) in Phosphate Buffer Saline (PBS) (Sigma, USA: P4417). After 10 min incubation in 1% Triton® X-100, actin structures were labelled with Alexa Fluor 635 Phalloidin (Molecular Probes, Life Technologies, USA: A34054) while tubulin structures were labelled with Monoclonal anti-α-Tubulin conjugate with FITC (Sigma, USA: F2168) for 1 hour. The embryos were then washed twice with PBS for 10 min and counterstained with DAPI for 30 min. Finally, the labelled samples were mounted on a glass microscope slide in a droplet of antifade medium (Pro Long Gold Antifading Agent) (Molecular Probes, Life Technologies, USA: P36934) to retard photobleaching. All samples were stored in the dark at 4°C prior to processing and imaging.
Slides were viewed under the Confocal Laser Scanning Microscope (CLSM) (Leica TCS SP5 AOBS, Germany), and the images taken were converted to JPEG format. Four laser sources from Diode 405nm, Argon 488nm, HeNe 633nm and HeNe 543nm were used to simultaneously excite the fluorescent signals from DAPI, Alexa Fluor 635 and anti α-Tubulin. Thresholds settings for intensity and saturation were maintained constant across all experimental groups. The actin, tubulin and chromatin intensities in each confocal image of EC embryos and LC embryos were calculated using the QWin Software.
Statistical analysis
Statistical analysis was performed using the SPSS software for Windows version 19.0.1 (Statistical Package for Social Sciences, Inc., USA). Assessment of actin, tubulin and chromatin distributions was based on the intensity of fluorescent probes after immunofluorescence staining. Independent T-test was performed to analyze differences in intensities among EC and LC embryos. P value of less than 0.05 was considered statistically significant.
Results
A total of 100 embryos at the 2-cell stage (50 EC embryos; 50 LC embryos) were stained by immunofluoresence staining for cytoskeleton study. The embryos were analyzed for distributions and fluorescence intensities of actin, tubulin and nuclear chromatin using Confo cal Laser Scanning Microscope (CLSM). The confocal images of embryos were analyzed by using QWin Software V3.
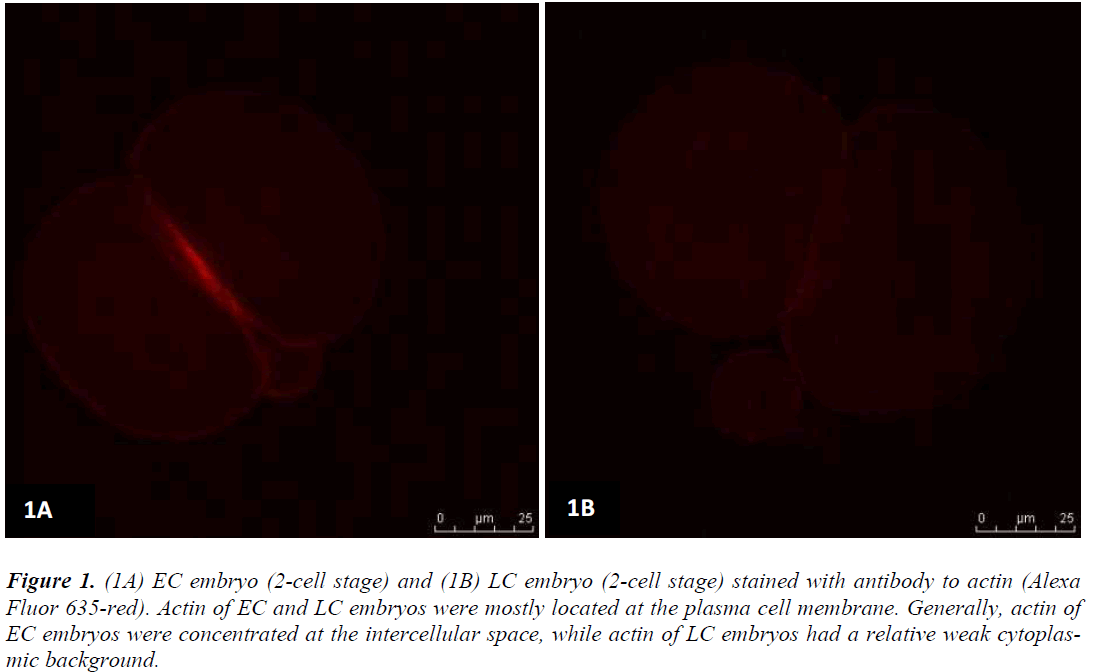
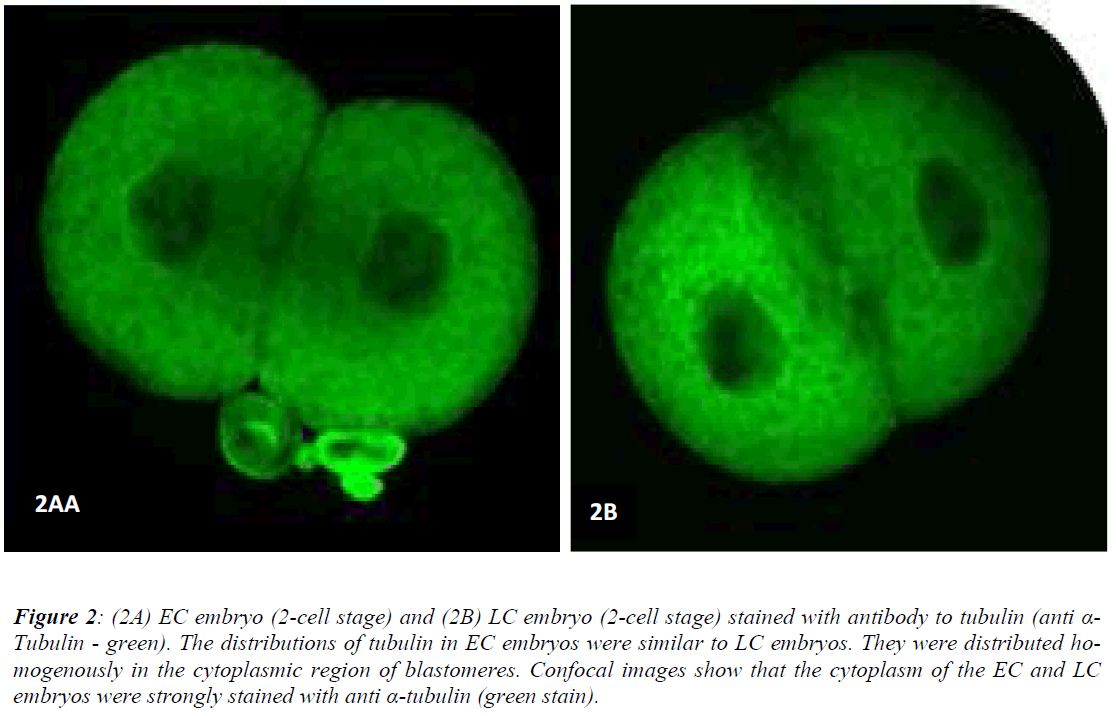
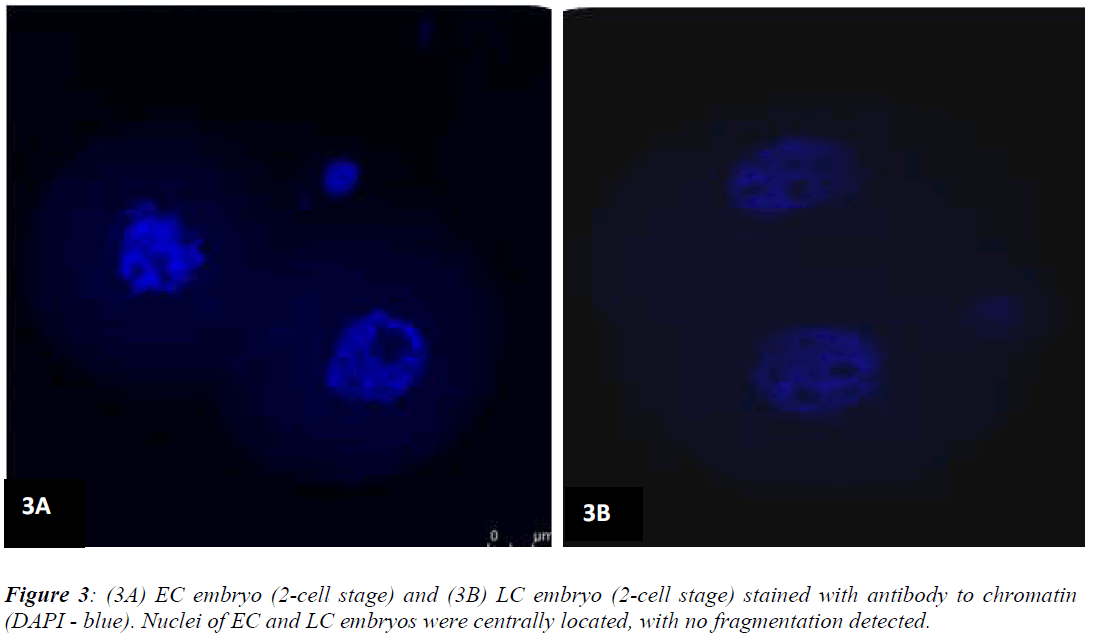
Figures 1 (A) and (B) show actin distributions in EC and LC embryos. The fluorescence images showed that actin of EC and LC embryos were mostly located at the plasma cell membrane. Generally, actin of EC embryos were concentrated at the intercellular space. Actin of LC embryos were seen to have a relatively weak cytoplasmic background. Figures 2 (A) and (B) show tubulin distributions in EC and LC embryos. Tubulin of EC as well as LC embryos were homogenously distributed in the cytoplasmic region of blastomeres. The cytoplasm of EC and LC embryos were strongly stained with anti α-tubulin (greenstain). Figures 3 (A) and (B) show nuclear chromatin distributions in EC and LC embryos. Apart from visual observations, this study also compared the integral intensity of fluorescence in confocal images. Table 1 shows the fluorescence intensities of actin, tubulin and chromatin of EC and LC embryos. Analysis by QWin Software version 3 showed that EC embryos had significantly higher actin fluorescence intensity [(14.68 + 14.07) x 105 pixel] compared to LC embryos [(1.50 + 1.20) x 105 pixel] (p<0.05) (Figure 4).
Figure 1: (1A) EC embryo (2-cell stage) and (1B) LC embryo (2-cell stage) stained with antibody to actin (Alexa Fluor 635-red). Actin of EC and LC embryos were mostly located at the plasma cell membrane. Generally, actin of EC embryos were concentrated at the intercellular space, while actin of LC embryos had a relative weak cytoplasmic background.
Figure 2: (2A) EC embryo (2-cell stage) and (2B) LC embryo (2-cell stage) stained with antibody to tubulin (anti α-Tubulin - green). The distributions of tubulin in EC embryos were similar to LC embryos. They were distributed homogenously in the cytoplasmic region of blastomeres. Confocal images show that the cytoplasm of the EC and LC embryos were strongly stained with anti α-tubulin (green stain).
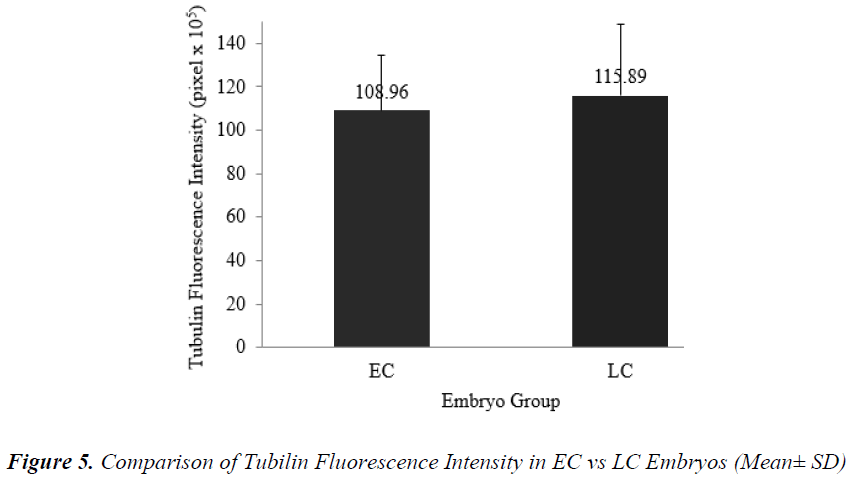
Figure 5 shows that the mean + SD of tubulin fluorescence intensity in EC and LC embryos were (108.96 + 25.30) x 105 pixel and (115.89 + 32.54) x 105 pixel respectively (Figure 5). Tubulin intensities of EC and LC embryos were not significantly different.
Analysis of nuclear chromatin intensity showed that EC embryos had significantly higher mean nuclear intensity [(11.43 + 3.48) x 105 pixel] compared to LC embryos [(7.98 + 3.08) x 105 pixel] (Figure 6).
Discussion
Recent studies in Assisted Reproduction Technology (ART) focused on the selection of the best quality embryo to improve the success of live birth from single embryo transfers (SETs). Many studies showed that embryos which cleaved early are of better quality compared to their later counterparts [3-6]. However, factors contributing to this difference are still not clear. This study examined the cytoskeletal organization and chromatin configurations of EC and LC mouse embryos at the 2-cell stage. The visualization of cytoskeletal morphology and quantification of visual data would provide greater understanding of the differences controlling development competency in EC and LC embryos.
Results from the present study showed that actin of EC and LC embryos were mostly located at the plasma membrane. This is in accordance with results from a previous study on mouse embryos, which found that actin was located beneath the cell membrane and was accentuated at the intercellular contact areas [13]. In the present study, EC embryos had their actin concentrated at the pericellular space. A study by Matsumoto et al. [14] examined the distribution of actin in non-blocked and blocked 2-cell stage rat embryos. The authors found that actin was distributed adjacent to nuclei and along the inside of the plasma membrane in non-blocked embryos at the two-cell stage. In embryos blocked at the two-cell stage, however, actin formed granules and dispersed in the cytoplasm.
The distribution of actin is related to its function in providing support and structure to the plasma membrane. Apart from that, actin is also known to functionally interact with integrin proteins in the plasma membrane. Actin-integrin protein interactions are important for maintaining cell growth, survival [7] and intracellular organelle organisation [15].
Quantitative analyses of confocal images from the present study showed that EC embryos had significantly higher actin intensities compared to LC embryos. Increased actin provides benefits to the EC embryos in maintaining cell growth and survivability until the blastocyst stage. The differences in actin abundance, which was reflected by greater intensities, could provide the explanation as to why EC embryos have higher viability compared to LC embryos [3-6]. In a study on equine embryos, Tremoleda et al. [16] noted that cell cleavages during early embryonic development were accompanied by complex arrangements of the cytoskeleton. Failure in the series of integrated cytoskeleton- mediated events resulted in developmental arrest. Levy et al. [17] also found that human embryos which were arrested early in their development showed actin abnormalities. Another study by Zijlstra et al. [18] found a correlation between actin quality with embryo viability. Embryos which had typical actin content and distribution underwent normal cell division. The present study suggests that higher intensities of actin in EC embryos were correlated to better actin quality, thus resulting in improved development competence over LC embryos.
Another component of the cytoskeleton which plays an important role in the development of preimplantation embryos is tubulin. Tubulin is involved in the regulation of cell shape and organization during compaction [11]. The present study found that tubulin of EC and LC embryos were distributed homogenously in the cytoplasmic region of blastomeres. In congruence, previous studies on mouse embryos also found that tubulin were distributed homogeneously in the cytoplasm in non-blocked embryos at the two-cell stage [14]. However, in embryos exhibiting the two-cell block, thicker fibrous microtubules were formed and distributed as rude meshwork structures in the cytoplasm. The distribution of tubulin in the cytoplasmic region is related to their function in a variety of cellular processes such as cell motility, cell division, intracellular transport and organelle distribution maintenance [19,20]. In the present study, tubulin intensity was higher than actin. A possible reason is that tubulin are more rigid and have larger diameters (25 nm) compared to actin (5 to 9 nm).
The main function of the nucleus is to control gene expression and mediate the replication of DNA during the cell cycle. Bavister et al. [21] stated that the ultrastructural study of embryo through fluorescence staining might provide a better understanding of the energetic and dynamic relationships between the nucleus and mitochondria. The fluorescence intensity of nucleus in confocal images is directly proportional to the amount of DNA present. The present study showed that nuclear chromatin intensity of EC embryos was significantly higher than LC embryos (p<0.05). This finding implies increased DNA replication in the nucleus of EC embryos. In tandem with this, our previous study also showed that nuclear intensity increased as the number of the cells increased [22]. Although Levy et al. [17] found that most arrested embryos displayed nuclear abnormalities such as chromatin condensation and fragmentation, results from the present study showed that nuclei morphology were normal in both EC and LC embryos, with no fragmentation observed.
Conclusion
It can be concluded that the event of first zygotic cleavage are highly dependent upon the organization of actin and tubulin. Higher densities of actin and chromatin in EC embryos suggest improved functionality of these structures, which appeared to result in more efficient cell division and therefore greater developmental competence.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Acknowledgements
This study was supported by the Research Acculturation Grant Scheme [600-RMI/RAGS 5/3 (89/2013)], Fundamental Research Grant Scheme [600-RMI/FRGS 5/3 (18/2014)] from the Ministry of Education, Malaysia.
References
- Francsovits P, Toth L, Takacs ZF, et al. Early pronuclear breakdown is a good indicator of embryo quality and viability. Fertil Steril 2005; 84(4): 881-887.
- Fu J, Wang X-J, Wang Y-W, et al. The influence of early cleavage on embryo developmental potential and IVF/ICSI outcome. J Assist Reprod Gen 2009; 26(8): 437?441.
- Nielsen HI, Ali J. Embryo culture media, culture techniques and embryo selection: A tribute to Wesley Kingston Whitten. J Reprod Stem Cell Biotechnol 2010; 1(1): 1?29.
- Lundin K, Bergh C, Hardarson T. Early embryo cleavage is a strong indicator of embryo quality in human IVF. Hum Reprod 2001; 16(12): 2652-2657.
- Lechniak D, Pers-Kamcyc E, Pawlak P. Timing of first zygotic cleavage as a marker of developmental potential of mammalian embryo. Reprod Biol 2008; 8(1): 23?42.
- Isom SC, Li RF, Whitworth KM, Prather RS. Timing of first embryonic cleavage is a positive indicator of the in vitro developmental potential of porcine embryos derived from in vitro fertilization, somatic cell nuclear transfer and parthenogenesis. Mol Reprod Dev 2012; 79(3): 197-207.
- McKayed K, Simpson J. Actin in Action: Imaging Approaches to Study Cytoskeleton Structure and Function. Cells 2013; 2(4): 715-731.
- Chankitisakul V, Tharasanit T, Tasripoo K, Techakumphu M. Chronological Reorganization of Microtubules, Actin Microfilaments, and Chromatin during the First Cell Cycle in Swamp Buffalo (Bubalus bubalis) Embryos. Vet Med Int 2010; 382989.
- Albertini DF, Overstrom EW, Ebert KM. Changes in the organization of the actin cytoskeleton during preimplantation development of the pig embryo. Biol Reprod 1987; 37(2): 441?451.
- Barnett DK, Kimura J, Bavister BD. Translocation of active mitochondria during hamster preimplantation embryo development studied by confocal laser scanning microscopy. Dev Dyn 1996; 205(1): 64?72.
- Maro B, Pickering SJ. Microtubules influence compaction in preimplantation mouse embryos. J Embryol Exp Morph 1984; 84(1): 217-232.
- Connors SA, Kanatsu-Shinohara M, Schultz, RM, Kopf GS. Involvement of the cytoskeleton in the movement of cortical granules during oocyte maturation, and cortical granule anchoring in mouse eggs. Dev Biol 1998; 200: 103?115.
- Lehtonen E, Badley RA. Localization of cytoskeletal proteins in preimplantation mouse embryos. J Embryol Exp Morph 1980; 55: 211-225.
- Matsumoto H, Shoji N, Umezu M, Sato E. Microtubule and microfilament dynamics in rat embryos during the two-cell block in vitro. J Exp Zool 1998; 281: 149-153.
- Boldogh I, Vojtov N, Karmon S, Pon LA. Interaction between mitochondria and the actin cytoskeleton in budding yeast requires two integral mitochondrial outer membrane proteins, Mmm1p and Mdm10p. J Cell Biol 1998; 141: 1371?1381.
- Tremoleda JL, Van Haeften T, Stout TA, Colenbrander B, Bevers MM. Cytoskeleton and Chromatin Reorganization in Horse Oocytes Following Intracytoplasmic Sperm Injection: Patterns Associated with Normal and Defective Fertilization. Biol Reprod 2003; 69(1): 186-194.
- Levy R, Benchaib M, Cordonier H, Souchier C, Guerin JF. Laser Scanning Confocal Imaging of Abnormal or Arrested Human Preimplantation Embryos. J Assist Reprod Gen 1998; 15(8): 485-495.
- Zijlstra C, Kidson A, Schoevers EJ, Daemen AJJM, Tharasanit T, Kuijk EW, Hazeleger W, Ducro-Steverink DWB, Colenbrander B, Roelen BAJ. Blastocyst morphology, actin cytoskeleton quality and chromosome content are correlated with embryo quality in the pig. Theriogenology 2008; 70: 923?935.
- Jaffe AB, Hall A. Rho gtpases: Biochemistry and biology. Ann Rev Cell Dev Biol 2005; 21: 247?269.
- Kavallaris M. Microtubules and resistance to tubulinbinding agents. Nat Rev Cancer 2010; 10: 194?204.
- Bavister BD, Squirrell JM. Mitochondrial distribution and function in oocytes and early embryos. Hum Reprod 2000; 15(2): 189-198.
- Razif D, Nor-Shahida AR, Salina O, Mohd-Fazirul M, Norhazlin J, Wan-Hafizah WJ, Rajikin MH, Froemming GRA, Nor-Ashikin MNK. Cytoskeletal alterations in different developmental stages of in vivo cryopreserved preimplantation murine embryos. Med Sci Monit Basic Res 2013; 19: 258-266.