ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
- Biomedical Research (2010) Volume 21, Issue 3
Modifications in the basement membrane supramolecular structure of type IV collagen and laminin 5 organization facilitates skin derivative formation
Department of Anatomy, College of Medicine, King Saud University, Riyadh. Saudi Arabia.
Accepted Date: February 23 2010
In intimate contact with epithelial tissue is the extracellular matrix that forms a highly specialized condensed layer known as the basement membrane. The is supramolecular structure involves type IV collagen and laminin 5 is known to provide physical support for the epithelial tissue overlying it. In this study we examine other proposed role(s) of these molecular structures and their receptors during skin development using the mammary gland as a model. The pattern of expression of these molecules during skin formation was examined using immunohistochemistry, utilizing collagen IV, laminin 5 and β4 or α6 integrin antibodies. The dissected mammary glands were also examined by transmission electron microscopy. Our results suggest that these supramolecular structures play important roles in skin derivative development, more specifically mammary gland formation, of these roles; they ease their resistance to skin derivatives down growth (invasion) into the under laying tissue.
Keywords
Mammary gland, basement membrane, mammogenesis, developing skin.
Introduction
In close contact with epithelial tissue the extracellularmatrix forms a highly specialized condensed layer knownas the basement membrane. Basement membrane supramolecularorganization is determined principally butnot solely by type IV collagen, laminin, nidogen (entactin),fibronectin, tenascin and perlecan. Interactions of thebasement membrane molecules with their related cells aremediated by cell surface adhesion receptors, of whichintegrins are the main type.
Collagen IV is the principle type of collagen that formsthe insoluble scaffolding of the basement membrane network.It is synthesised by both stromal and epithelial cellsand seems to have both a stiffening as well as a flexibilityrole within the basement membrane [1]. Other types ofcollagen can be found at the epidermal basement membraneeither associating with fibril surfaces (includingtype VI, IX, XII, and type XIV collagen), or as transmembranousproteins (including types XIII and XVII collagen).Defects in, or absence of, collagen VII has beenshown to be the primary cause of dystrophic epidermolysisbullosa [2], an inherited skin blistering disorder. Similarly,a mutation in the collagen VII gene has recentlybeen found to cause another skin disorder known as epidermolysisbullosa pruriginosa [3-5]. Collagen VII was reported to be missing during human foetal developmentfrom the tips of the developing skin appendageal buds [6],although it is present in the basement membrane of theskin where no appendage development has taken place.Collagen IV, however, completely surrounds the developingappendage and is continuous within the epidermis [6].
In mammals, 12 laminin isoforms have been described sofar, however it appears that not all possible combinationsare achievable due to assembly restrictions [see reviewsby [7,8]]. For example, the γ2 chain has never been reportedto combine with the β1 chain [see [7]]. Lamininscan be further divided into 4 subfamilies according to thelength of their N-terminal domain and the number ofamino acids within it.
Laminin isoforms are tissue- as well as differentiationspecific; for example in mature mammalian skin thebasement membrane contains laminin 5 (α3β3γ2) [9],laminin 1 (α1β1γ1) [10], laminin 2 (α2β1γ1) [11], andlaminin 10 (α5β1γ1) [11]. During skin development theseisoforms appear and disappear according to the stage ofdevelopment.Defects in the three chains of laminin 5 havebeen identified as the cause of junctional epidermolysisbullosa [12-19], and mutation in genes encoding thelaminin 1 isoform produced a phenotype of junctionalepidermolysis bullosa, see review [8].
Hayashi and colleagues have further shown that duringhair development, laminin 1 is absent from the distal endof the growing hair follicle, but is present in the basementmembrane underlying the skin and around the hair follicleneck [10]. In a different study, laminin 5 was shown tohave a similar pattern [9]. The laminin 10-null mouse atE16.5 was reported to contain fewer hair germ cells thanthe control, and when fragments of skin from controlE16.5 embryos were transplanted in the dorsal side ofE16.5 laminin 10 null mice, the hair germ cells failed togrow and subsequently there was a complete regression ofthe hair follicle [20].
These studies suggest that laminin 1 and 5 probably contributeto the physical integrity of the skin and only “giveway” at some sites when needed, demonstrated duringappendage formation. In contrast, laminin 10 probablyplays an opposing role, i.e. encouraging and supportingappendage growth.
Integrins are the major receptors for extracellular matrixmolecules. They have been the subject of a large body ofscientific research since they were discovered more than20 years ago as a family of cell surface receptors [21].
The integrin α6β4 is the major integrin receptor in theskin at steady state and is found in the epidermal basallayer [22,23]. This integrin dimer connects the basal cells,through specialized cell-substrate attachment junctionsknown as hemidesmosomes, of which it is an integralpart, to the basement membrane molecules laminin 1 andlaminin 5 [1]. Absence of either α6 or β4 causes a severeskin blistering condition [24,25], that results from lack offunctional hemidesmosomes.
Postnatal mammary gland basement membrane containsmost of the laminin subunits: α1, α3, α5, β1, β2, β3, γ1and γ2 (26). This suggests that the mammary gland basementmembrane should contain; laminin 1 (α1β1γ1), 5(α3β3γ2), and 10/11 (α5β1γ1 / α5β2γ1) [26]. Also mammarygland basement membrane has collagen IV [27].The major integrin receptors in the mammary gland areα2β1, α3β1, α6β1, also α6β4 [see [28] and referencestherein).
Currently, there is considerable evidence for differentroles played by the extra cellular matrix (ECM) proteins(laminin and collagen) and their cell surface receptors theintegrins at the different mammary gland developmentalstages.
Most of what is known about the role of ECM proteins,and in particular the basement membrane during mammarygland development, has been obtained from experimentson post-embryonic mammary glands. One of thevery few experiments on expression of ECM proteins and their receptors at the basement membrane of embryonicmammary glands showed that basement membranelaminin 5 expression coincided with the basal keratinocyteexpression of β4 integrins [29]. Both were present atthe basement membrane around the mammary buds andepidermis at both E12 and E15. At E17, laminin 5 wasalmost absent from the developing mammary ducts andβ4 was very much reduced, while both were still presentat the basement membrane underlying the epidermis. Thisis analogous to the pattern of laminin 1 expression in developinghair follicles [10] and supports the suggestionthat laminins are involved in mammary duct development(30). They also demonstrate the importance of lamininsfor epithelial invasion, through which mammary glanddevelopment is affected.
The role of the laminin-binding integrins during embryonicmammary gland development appears to be complicatedand is currently incompletely understood. For example,mice lacking α3 or α6 or β4 integrins die at termor shortly after birth [24,25,31], so the potential effect onmammary gland development after birth is not known.However, examination of the E17 mammary gland of eitherα3-, α6- or β4-null mice revealed that they had normalmammary glands (similar to the wild type) (28). In apioneering experiment, mammary rudiments of α3- or α6-null mice at E17 were transplanted into mammary fatpads of syngeneic hosts [28] (the experiment was notdone with β4 nulls as lack of α6 also resulted in completeabsence of β4 expression). The results showed that transplantedmammary rudiments of either genotype developedand functioned almost as normal mammary gland.Transplanted mammary rudiments of α6-null miceshowed normal localisation of laminin 1 expression in thebasement membrane, however, from electron microscopeevidence as well as from the punctuate expression patternof laminin 5 seen by immunofluorescence, it seems thatthe mammary glands in these mice suffer from abnormalhemidesmosome formation [28]. However this also showsthat neither α3 nor α6 integrins are vital for mammarygland development.
From the evidence available it appears that laminins arecrucial for epithelial invasion, however it is still unclearwhich types of laminins are involved in such mechanisms.There is also some ambiguity about which type of integrinsinteract with the different laminin(s). For thisstudy the hypothesis was that laminin 5 could be involvedin the initiation, while laminin 10 is responsible for themaintenance, of the mammary bud downward growth intothe dermis. We also propose that α6β4 acts as the majorreceptor for laminin 5, and α3β1 is the major receptor forlaminin 10 during mammogenesis. The main aim of thisstudy was therefore to investigate this hypothesis by examiningthe expression pattern of selected basementmembrane components, to see if there were significant differences in the local ECM composition that might affectthe progression of mammary gland prenatal development.
Materials and Methods
Animals
For the experiments described in this study, a total of 546CD1 mouse embryos were studied: (E12=24, E12.5=56,E13=26, E13.5=31, E14=46, E14.5=88, E15.5= 37,E16=12, E16.5=43, E17.5=80 and E18.5=103).
Generating embryos of defined ages
As mouse embryonic development is a very rapid process,it was important to study embryos of as similar developmentalage as possible. Two different mating techniqueswere compared. One was based on a short (about 2hr) anddefined time for mating, and the second was based on themore widely used technique where the animals were lefttogether overnight to mate and pregnancies determined bythe presence of a vaginal plug at E 0.5.
Dissection
Pregnant mice were euthanased by CO2 suffocation followedby dislocation of the neck. Individual embryoswere collected and transferred into a glass Petri dishcoated with Sylgard® 184 Kit, silicone elastomer (Sigma,UK). This provides a stable surface for pinning down theembryos in order to secure them for dissection. Under thedissection microscope submerged under DMEM, the embryowas pinned outstretched using insect pins into theSylgard®. Gradually the pins were moved closer to thebody as the limbs and tail were trimmed. By adjusting theangle of the incident lights and tilting the embryo sideways,the mammary glands were identified according totheir location and shape.
About 2-4mm2 fragments of the skin (depending on theage of the embryo), containing one or a maximum twoadjacent mammary glands, e.g. No.4 & 5, were dissectedout.
Immunofluorescence
Skin fragments containing mammary glands were snapfrozen in liquid nitrogen immersed in Tissue-Tek® (Agar,UK) within an appropriate size foil cup. Serial frozen sections(10μm) were cut in a cryostat at -20º C and collectedon pre-coated slides (BDH, UK).
The DakoCytomation EnVision® Dual Link System Peroxidasekit (DAKO, UK) was used for immunohistochemistry,following the manufacturer’s recommendedprocedures. In this method, after washing in PBS theslides were incubated in the appropriate concentration ofeach antibody (collagen IV, laminin5 and β4 or α6 integrins;see Table 1 for details of antibodies used). overnight at -4° C, followed by two washes in PBS, each for 5min. After that, the peroxidase-labelled polymer (DAKO,UK) was applied for 30 min followed by 5 min in PBS.Sections were then covered with substrate chromogen for10 min, washed in running tap water, then counterstainedwith haematoxylin for 10-15 seconds. After counterstainingin both techniques, the slides were processed througha series of de-hydration steps and mounted.
Examination procedures
Samples were examined by fluorescent or bright fieldmicroscopy using a Zeiss Axioskop, fitted with a c olourAxioCam digital camera which was used for collectingimages (using objective lenses X4, X16, X25 and X40).
Results
Examination of ultra-thin sections under the electron microscopeshowed a continuous and well developed basementmembrane around the mammary gland at all mainembryonic stages i.e. the bud, peg and the sheath stages(data not shown). This was then followed by immunofluorescenceanalysis of the expression pattern of selectedbasement membrane components, i.e. collagen IV, integrinsα6 and β4 and laminin 5.
Expression pattern of Collagen IV
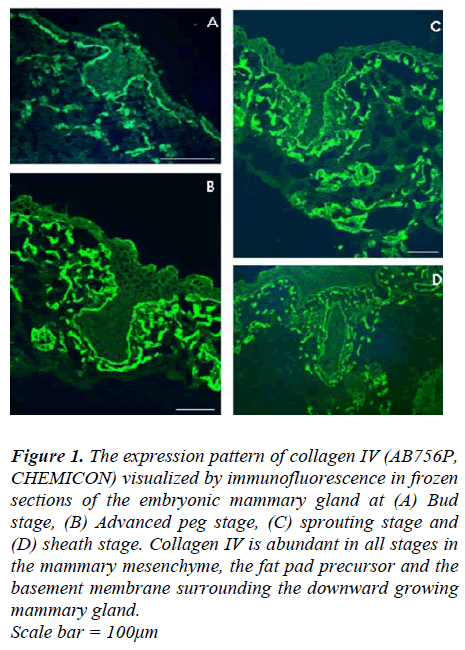
In frozen section of developing mammary gland, immunofluorescenceinvestigations show that collagen IV isexpressed in the skin basement membrane at E12.5,E15.5, E16.5 and E18.5. When the mammary gland wasstill at the bud stage (at E12.5), collagen IV was presentin the basement membrane underlying the ectoderm andaround the mammary bud; it was also within the mesenchymalcells underneath the ectoderm and those aroundthe mammary bud (Fig 1A). As mammary gland developmentprogressed, the advanced peg stage of mammarygland development (at E15.5) showed much more intensecollagen IV staining than the earlier bud stage, especially within the mammary mesenchyme and the fat pad precursor.This persisted through the subsequent sprouting(E16.5) and sheath stages (E18.5) of development (Fig 1C and D, respectively).
Figure 1: The expression pattern of collagen IV (AB756P, CHEMICON) visualized by immunofluorescence in frozen sections of the embryonic mammary gland at (A) Bud stage, (B) Advanced peg stage, (C) sprouting stage and (D) sheath stage. Collagen IV is abundant in all stages in the mammary mesenchyme, the fat pad precursor and the basement membrane surrounding the downward growing mammary gland. Scale bar = 100μm
Expression pattern of α6 and β4 integrins
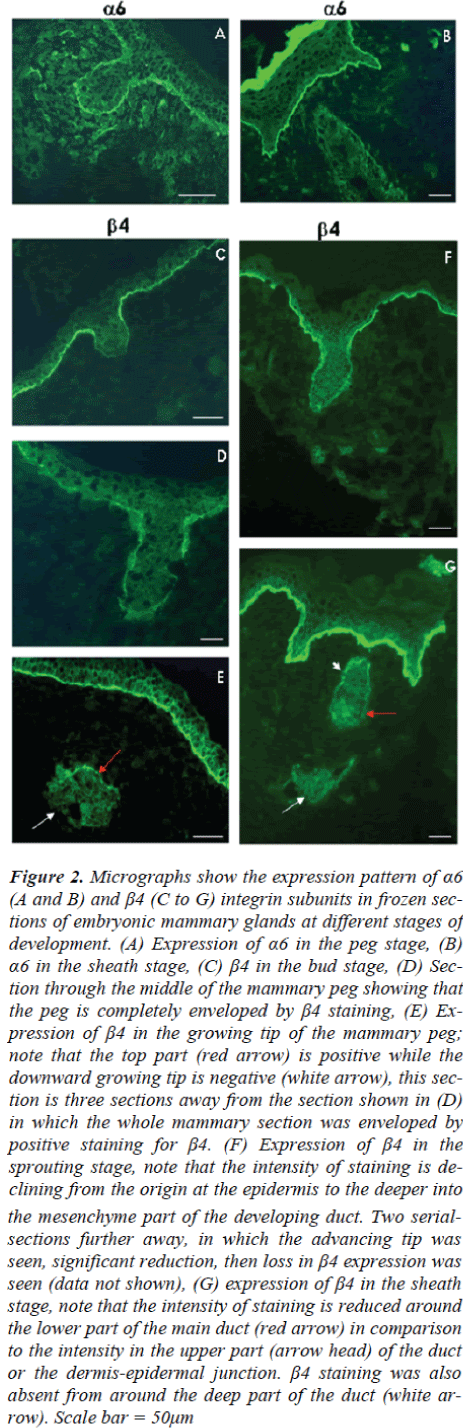
Using antibody GoH3, α6 integrin was detected duringthe early stages (the bud and peg stages) of prenatalmammary gland development, α6 integrin was expressedby basal cells at the dermal-epidermal junction (Fig 2A).At the sheath stage of mammary development, however,α6 integrin was not detected in basal cells of the mammarymain ducts, although it was retained by the basalcells of the embryonic epidermis and those of the nipplesheath (Fig 2B).
The expression pattern of β4 integrin was also examinedusing antibody 346-11A in frozen sections of mammarygland of mice at different embryonic days of development(E12.5, E14.5, E16.5 and E18.5). It was observed thatfrom early stages of mammary gland development, β4integrin is absent from the growing tip of the mammarybud. However, it was present at the dermal-epidermal junctionof the basal cells in the adjacent ectoderm and the distal part of the mammary bud close to the ectoderm(Fig. 2C). The intensity of β4 expression declined from the ectoderm downwards and completely disappeared inthe tip of the mammary bud. This suggests an altered attachmentof the epithelial cells to the ECM at the downwardgrowing tip of the mammary bud, while attachmentmechanism at the connection of the mammary bud to theectoderm appeared to be unchanged.
At the peg stage, a similar pattern to that seen during thebud stage was also observed. β4 integrin-positive stainingwas seen at the basal aspect of the developing epidermisand around the mammary neck and body (Fig 2D), whilstβ4 expression was absent from a small part of the downwardgrowing tip of the mammary peg (Fig 2E white arrow).This appears to indicate the advancing edge of themammary body, and was only seen in the rare sectionsthat pass through these very restricted and localizedpatches.
In the early sprouting stage, β4 expression maintained asimilar pattern to that seen during the peg and bud stages.Expression of β4 in the dermal-epidermal junction zoneextended across the newly forming nipple sheath (Fig 2F),but the growing end of the mammary duct was negative.
At the sheath stage of prenatal mammary gland development,β4 was absent from the dermal-epidermal junctionaround the mammary ducts. Weak staining could be detectedin the upper (distal) segment while the lower(deeper) segments were clearly negative. On other handpositive staining for β4 was maintained at a high level inthe embryonic basement membrane zone of the epidermisincluding that of the nipple sheath (Fig 2G).
Figure 2: Micrographs show the expression pattern of α6 (A and B) and β4 (C to G) integrin subunits in frozen sections of embryonic mammary glands at different stages of development. (A) Expression of α6 in the peg stage, (B) α6 in the sheath stage, (C) β4 in the bud stage, (D) Section through the middle of the mammary peg showing that the peg is completely enveloped by β4 staining, (E) Expression of β4 in the growing tip of the mammary peg; note that the top part (red arrow) is positive while the downward growing tip is negative (white arrow), this section is three sections away from the section shown in (D) in which the whole mammary section was enveloped by positive staining for β4. (F) Expression of β4 in the sprouting stage, note that the intensity of staining is declining from the origin at the epidermis to the deeper into the mesenchyme part of the developing duct. Two serialsections further away, in which the advancing tip was seen, significant reduction, then loss in β4 expression was seen (data not shown), (G) expression of β4 in the sheath stage, note that the intensity of staining is reduced around the lower part of the main duct (red arrow) in comparison to the intensity in the upper part (arrow head) of the duct or the dermis-epidermal junction. β4 staining was also absent from around the deep part of the duct (white arrow). Scale bar = 50μm
Expression pattern of Laminin 5
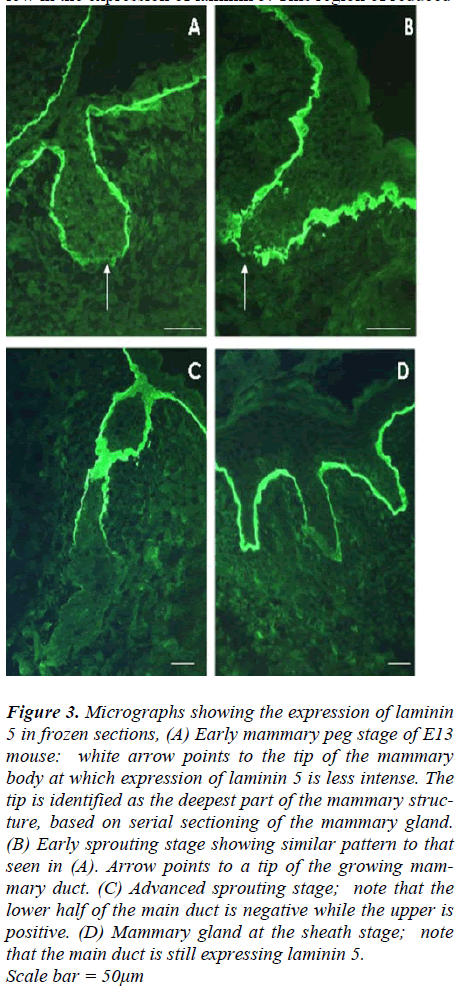
An antibody to laminin 5, 1110T, which recognizes the α3subunit, was used to examine the expression pattern oflaminin 5 during prenatal mammary gland development.Frozen sections of mammary glands at different stages ofdevelopment were obtained from mouse embryos at E13,E15, E16 and E18. Mammary glands at the late bud stageand early peg stage of E13 mice show very similar patternsconsisting of highly positive staining with laminin 5antibody around the newly developing mammary neckand most of the mammary body (Fig 3 A). The most proximal region of the body however was significantlylow in the expression of laminin 5. This region of reduced expression could be easily missed depending on the planeof the section.
Later on during the sheath stage at E18, laminin 5 positivereactivity was retained by the basement membrane of theembryonic epidermis, around the nipple sheath and mostof the upper (close to the epidermis) part of the mammarygland main duct (Fig 3D). However, none of the small ducts showed positive reactivity for laminin 5 at thisstage.
Figure 3: Micrographs showing the expression of laminin 5 in frozen sections, (A) Early mammary peg stage of E13 mouse: white arrow points to the tip of the mammary body at which expression of laminin 5 is less intense. The tip is identified as the deepest part of the mammary structure, based on serial sectioning of the mammary gland. (B) Early sprouting stage showing similar pattern to that seen in (A). Arrow points to a tip of the growing mammary duct. (C) Advanced sprouting stage; note that the lower half of the main duct is negative while the upper is positive. (D) Mammary gland at the sheath stage; note that the main duct is still expressing laminin 5. Scale bar = 50μm
Discussion
Prenatal mammogenesis does not disrupt collagen IV
Our detailed immunofluorescence studies using antibodyfor collagen IV showed that during prenatal developmentcollagen IV is present throughout the basement membraneof the mouse skin and around the developing mammaryglands at all of the different stages. Such a pattern is similarto that seen during human foetal hair development [6]and mouse hair development [32]. These results are notentirely surprising as collagen IV can stimulate motility innormal and tumour cells in vitro [33], and should nottherefore have an inhibitory effect in the downwardgrowth. Mammary glands of virgin mice also express collagenIV in all ducts including the terminal end bud [30].The only conflicting report is one saying that in prepubertalmammary glands of heifers, collagen IV is primarilyin the basement membrane of mature ducts andless in other parts of the gland [34]. The conclusion wouldbe that the tissue reorganization of prenatal mammarygland development does not require, or depend upon, disruptionor interruption of the basement membrane collagenIV.
Integrins and prenatal mammogenesis
Immunofluorescence examination of α6 and β4 integrinsshowed that α6 was present during the mammary bud andpeg stages and was lost from the main duct at the sheathstage. In contrast, β4 integrin was absent from advancingtip of the mammary gland at all of the prenatal mammogenesisstages, yet it was present around the rest of thedeveloping mammary gland.
The α6 integrin expression pattern suggests that α6 ismost likely required for prenatal mammary gland developmentat the early stages (bud and peg stages) when itcompletely envelops the advancing mammary bud andpeg.
However, this was not the case in later stages (at least thesheath stage), since it was seen in the basement membranezone of the epidermis while being absent from that of themammary duct. However, the fact that α6-null mice shownormal mammary glands at E17 (no distortion or delay),and also developed normally when transplanted into fatpads of syngeneic hosts [28], remains a mystery.
The expression pattern of β4 integrin, provides furtherunderstanding of the structural development of mammarygland. As a gap in the expression of β4 integrin was identifiedat the tip of the growing mammary gland bud andpeg stage. This gap indicates differences in the biochemistryof cell-substrate junction, which probably occurs toallow easier penetration of the basement membrane andpromotes mammary invasion at that specific site. The β4 integrin was missing from only a very restricted smallpart of the mammary gland; at its’ advancing tip. Thisobservation could be easily missed, and may explain thereport by Nanba and colleagues [29].
Laminin 5 shows a similar distribution to β4 integrin
Although laminin 5 was present at the junction of thebasal cells with the basement membrane and around thedeveloping mammary bud and peg, gaps in staining werealso seen in the proximal (deepest) part. Our serial sectionssuggests that this may be the growing tips of themammary bud and pegs, as these gaps are also in thedeepest parts. At later stages the gaps became much moreapparent when the mammary ducts had grown deep intothe mesenchyme during the advanced sprouting andsheath stages. This result again is contrary to Nanba andcolleagues [29], reporting that laminin 5 is in the basementmembrane completely enclosing the mammary budand peg. Yet it is in agreement with the recently reports oflaminin 5 expression during mouse hair follicle development[32,35], as laminin 5 was seen around the developinghair follicle except in the deep invading part of thehair follicle.
Our results show that the expression pattern of β4 integrinis similar to that of laminin 5, but different from that of α6integrin. The β4- and laminin 5- negative patterns suggestthat the tip of the growing mammary gland may have abasement membrane with a different biochemical compositionfrom that in the rest of the mammary tree. Thus β4with α6 integrin may bind to laminin 5 in the wholemammary gland except at the growing end where an alternativeintegrin isotype, most likely β1 as suggested bysome preliminary observations (not shown), joins α6 andmaybe binds to a different ligand, such as laminin 10 orlaminin 1 or collagen. Both laminin 10 and 1 have beenfound in the basement membrane surrounding the developinghair follicle [20,32], in a similar expression patternto that of α6 found in this study.
In conclusion, it appears that cells at the growing tip ofthe developing mammary gland and hair follicles behavedifferently from cells further back in the duct. A differentcomposition of the basal lamina at the growing tip may besignificant as it may facilitate invasive epithelial behaviourfor downward growth, either mechanically (if thiscomposition of basement membrane is more deformable)or indirectly via an alteration in signal transduction pathways.
At the beginning of this study we hypothesized thatlaminin 5 is involved in initiation of mammary gland developmentwhilst laminin 10 is needed for maintainingthe mammary bud down-growth into the dermis, and thatα6β4 is the major receptor for laminin 5 while α3β1 is themajor receptor for laminin 10. Although laminin 10 was not investigated due to the lack of specific anti-laminin 10antibodies that can be used on mouse tissue, the resultsdescribed here partially support this hypothesis. From theexpression pattern it appears that α6β4 is most likely thereceptor for laminin 5 around the developing mammarygland, however, further work is needed regarding the specifictype of laminin(s) and its/their receptor(s) at the advancingtip of the mammary structure.
References
- Clark RAF, Tonnesen MG. Integrins in skin biology and pathophysiology. In: Freinkel RK, Woodly DT, editors. The Biology Of The Skin: The Parthenon Publishing Group; 2001. p. 333-352.
- Uitto J, Pulkkinen L, Christiano AM. Molecular basis of the dystrophic and junctional forms of epidermolysis bullosa: mutations in the type VII collagen and kalinin (laminin 5) genes. J Invest Dermatol 1994; 103 (5 Suppl): 39S-46S.
- Mellerio JE, Ashton GH, Mohammedi R, Lyon CC, Kirby B, Harman KE, et al. Allelic heterogeneity of dominant and recessive COL7A1 mutations underlying epidermolysis bullosa pruriginosa. J Invest Dermatol 1999; 112(6): 984-987.
- McGrath JA, Schofield OM, Eady RA. Epidermolysis bullosa pruriginosa: dystrophic epidermolysis bullosa with distinctive clinicopathological features. Br J Dermatol 1994; 130(5): 617-625.
- Jiang W, Bu D, Yang Y, Zhu X. A novel splice site mutation in collagen type VII gene in a Chinese family with dominant dystrophic epidermolysis bullosa pruriginosa. Acta Derm Venereol 2002; 82 (3): 187-191.
- Karelina TV, Bannikov GA, Eisen AZ. Basement membrane zone remodeling during appendageal development in human fetal skin. The absence of type VII collagen is associated with gelatinase-A (MMP2) activity. J Invest Dermatol 2000; 114 (2): 371-375.
- Colognato H, Yurchenco PD. Form and function: the laminin family of heterotrimers. Dev Dyn 2000; 218 (2): 213-234.
- Aumailley M, Smyth N. The role of laminins in basement membrane function. J Anat 1998; 193 ( Pt 1): 1- 21.
- Nanba D, Hieda Y, Nakanishi Y. Remodeling of desmosomal and hemidesmosomal adhesion systems during early morphogenesis of mouse pelage hair follicles. J Invest Dermatol 2000; 114 (1): 171-177.
- Hayashi K, Mochizuki M, Nomizu M, Uchinuma E, Yamashina S, Kadoya Y. Inhibition of hair follicle growth by a laminin-1 G-domain peptide, RKRLQVQLSIRT, in an organ culture of isolated vibrissa rudiment. J Invest Dermatol 2002; 118 (4): 712-718.
- Schuler F, Sorokin LM. Expression of laminin isoforms in mouse myogenic cells in vitro and in vivo. J Cell Sci 1995; 108 ( Pt 12): 3795-3805.
- Aberdam D, Galliano MF, Vailly J, Pulkkinen L, Bonifas J, Christiano AM, et al. Herlitz's junctional epidermolysis bullosa is linked to mutations in the gene (LAMC2) for the gamma 2 subunit of nicein/kalinin (LAMININ-5). Nat Genet 1994; 6 (3): 299-304.
- Baudoin C, Miquel C, Blanchet-Bardon C, Gambini C, Meneguzzi G, Ortonne JP. Herlitz junctional epidermolysis bullosa keratinocytes display heterogeneous defects of nicein/kalinin gene expression. J Clin Invest 1994; 93(2): 862-869.
- Baudoin C, Miquel C, Gagnoux-Palacios L, Pulkkinen L, Christiano AM, Uitto J, et al. A novel homozygous nonsense mutation in the LAMC2 gene in patients with the Herlitz junctional epidermolysis bullosa. Hum Mol Genet 1994; 3(10): 1909-1910.
- Pulkkinen L, Christiano AM, Gerecke D, Wagman DW, Burgeson RE, Pittelkow MR, et al. A homozygous nonsense mutation in the beta 3 chain gene of laminin 5 (LAMB3) in Herlitz junctional epidermolysis bullosa. Genomics 1994; 24 (2): 357-360.
- Pulkkinen L, Christiano AM, Airenne T, Haakana H, Tryggvason K, Uitto J. Mutations in the gamma 2 chain gene (LAMC2) of kalinin/laminin 5 in the junctional forms of epidermolysis bullosa. Nat Genet 1994; 6 (3): 293-7.
- Kivirikko S, McGrath JA, Baudoin C, Aberdam D, Ciatti S, Dunnill MG, et al. A homozygous nonsense mutation in the alpha 3 chain gene of laminin 5 (LAMA3) in lethal (Herlitz) junctional epidermolysis bullosa. Hum Mol Genet 1995; 4(5): 959-962.
- Vailly J, Pulkkinen L, Miquel C, Christiano AM, Gerecke D, Burgeson RE, et al. Identification of a homozygous one-basepair deletion in exon 14 of the LAMB3 gene in a patient with Herlitz junctional epidermolysis bullosa and prenatal diagnosis in a family at risk for recurrence. J Invest Dermatol 1995; 104(4): 462-466.
- Vailly J, Pulkkinen L, Christiano AM, Tryggvason K, Uitto J, Ortonne JP, et al. Identification of a homozygous exon-skipping mutation in the LAMC2 gene in a patient with Herlitz's junctional epidermolysis bullosa. J Invest Dermatol 1995; 104(3): 434-437.
- Li J, Tzu J, Chen Y, Zhang YP, Nguyen NT, Gao J, et al. Laminin-10 is crucial for hair morphogenesis. Embo J 2003; 22(10): 2400-2410.
- Hynes RO. Integrins: a family of cell surface receptors. Cell 1987; 48(4): 549-554.
- Sonnenberg A, Calafat J, Janssen H, Daams H, van der Raaij-Helmer LM, Falcioni R, et al. Integrin alpha 6/beta 4 complex is located in hemidesmosomes, suggesting a major role in epidermal cell-basement membrane adhesion. J Cell Biol 1991; 113(4): 907-917.
- Watt FM, Hertle MD. Keratinocyte integrins. In: Leigh IM, Lane EB, Watt FM, editors. The Keratinocyte Handbook. Cambridge, Uk: Cambridge University Press; 1994. p. 153-164.
- Georges-Labouesse E, Messaddeq N, Yehia G, Cadalbert L, Dierich A, Le Meur M. Absence of integrin al pha 6 leads to epidermolysis bullosa and neonatal death in mice. Nat Genet 1996; 13(3): 370-373.
- Niessen CM, van der Raaij-Helmer MH, Hulsman EH, van der Neut R, Jonkman MF, Sonnenberg A. Deficiency of the integrin beta 4 subunit in junctional epidermolysis bullosa with pyloric atresia: consequences for hemidesmosome formation and adhesion properties. J Cell Sci 1996; 109 (Pt 7): 1695-1706.
- Prince JM, Klinowska TC, Marshman E, Lowe ET, Mayer U, Miner J, et al. Cell-matrix interactions during development and apoptosis of the mouse mammary gland in vivo. Dev Dyn 2002; 223 (4): 497-516.
- Monaghan P, Warburton MJ, Perusinghe N, Rudland PS. topographical arrangement of basement membrane proteins in lactating rat mammary gland: Comparison of the distribution of type IV collagen, laminin, fibronectin, and Thy-1 at the ultrastructural level. Proc Natl Acad Sci USA 1983; 80: 3344-3348.
- Klinowska TC, Alexander CM, Georges-Labouesse E, Van der Neut R, Kreidberg JA, Jones CJ, et al. Epithelial development and differentiation in the mammary gland is not dependent on alpha 3 or alpha 6 integrin subunits. Dev Biol 2001; 233(2): 449-467.
- Nanba D, Nakanishi Y, Hieda Y. Changes in adhesive properties of epithelial cells during early morphogenesis of the mammary gland. Dev Growth Differ 2001; 43 (5): 535-544.
- Klinowska TC, Soriano JV, Edwards GM, Oliver JM, Valentijn AJ, Montesano R, et al. Laminin and beta1 integrins are crucial for normal mammary gland development in the mouse. Dev Biol 1999; 215(1): 13-32.
- DiPersio CM, Hodivala-Dilke KM, Jaenisch R, Kreidberg JA, Hynes RO. alpha3beta1 Integrin is required for normal development of the epidermal basement membrane. J Cell Biol 1997; 137(3): 729-742.
- Chuang YH, Dean D, Allen J, Dawber R, Wojnarowska F. Comparison between the expression of basement membrane zone antigens of human interfollicular epidermis and anagen hair follicle using indirect immunofluorescence. British Journal of dermatology. 2003; 149: 274-281.
- Aznavoorian SA, Stracke ML, Krutzsch H, Schiffmann E, Liotta LA. J Cell Biol. 1990; 110: 1427-1438.
- Berry SDK, Howard RD, Akers RM. Mammary localization and abundance of laminin, fibronectin, and collagen IV proteins in prepubertal heifers. j. Dairy. Sci. 2003; 2003(86): 2864-2874.
- Joubeh S, Mori O, Owaribe K, Hashimoto T. Immunofluorescence analysis of the basement membrane zone component in human anagen hair follicles. Exp Dermatol 2003; 12: 365-370.