ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
- Biomedical Research (2015) Volume 26, Issue 2
Method for qualitative and quantitative determination of gallic acid in Herba Gei.
Xinwu Huang1, Mingli Sun2, Guochun Li3*
1Department of Pharmacology, Luzhou Medical College, Luzhou Sichuan, 646000 China;
2College of pharmacy, Shandong University of Traditional Chinese Medicine, Jinan Shandong, 250000 China;
3Affiliated Traditional Chinese Medicine Hospital of LuZhou Medical College, Luzhou Sichuan, 646000 China.
- Corresponding Author:
- Guochun Li
Affiliated Traditional Chinese Medicine Hospital of LuZhou Medical College, Luzhou Sichuan, 646000 China.
Accepted date: March 03, 2015
To establish the method for qualitative and quantitative determination of gallic acid in Herba Gei. Gallic acid content is determined by HPLC with Diamonsil C18 column as the stationary phase, under the conditions of methanol-0.1% phosphoric acid in water (10:90) mobile phase, 271 nm detection wavelength, and 40? column temperature. Peak area of gallic acid shows a good linearity with the injection volume within a 0.202~1.212 μg range, r=0.9997; average recovery is 99.26%, RSD 2.20%. Through quantitative determination of Herba Gei of different origins, the method established herein is confirmed to be simple, fast, sensitive, accurate, reliable and reproducible.
Keywords
Herba Gei; Gallic Acid; HPLC; Hypertension
Introduction
Herba Gei is the dried whole plant of Geum japonicum Thunb. var. chinense F. Bolle in the genus Geum of the family Rosaceae, which is distributed throughout China. It is grown in the hillside meadows, ditchsides, river shoals, open space in forests and forest edges of 200-3500 meters above sea level [1]. The plant has analgesic, hypotensive, menstruation-regulatory, wind-dispelling and dampness-eliminating effects, and is used for hypertension, dizziness, headache, irregular menstruation, lower abdominal pain, leucorrhea, infantile convulsions and rheumatic lumbocrural pain; as well as external treatment of carbuncles, boils, pyogenic infections and traumatic injuries [2]. At present, a number of scholars have already studied the chemical composition of Herba Gei using modern analytical means and bioassay-guided fractionation, who found that Herba Gei mainly contains tannins, phenolic acids, triterpenoids, lignins, etc. [4-7] There has been only a few modern pharmacological research on Herba Gei, which primarily involves the effects on acyclovir treatment of HSV-1 infection [8], and on NO metabolism of RAW264.7 macrophages [9]. Research on quantitative determination of active constituents in Herba Gei is necessary from the perspectives of both basic and applied research, in order to better develop and utilize Herba Gei resources.
Instruments and Reagents
Instruments: HP-1100 series HPLC system (G1311A quaternary pump, G1313A autosampler, G1316A column compartment, G1314A VWD, G1315B DAD), Sartorius BP211D electronic balance. Reference substance: gallic acid reference substance (provided by the National Institute for the Control of Pharmaceutical and Biological Products, batch No. 0831-201432). Crude Herba Gei drug was purchased from the market, which was identified by Professor Hu Zhiqiang of the South University of Science and Technology of China as the dried whole plant of Geum japonicum Thunb. var. chinense F. Bolle in the genus Geum of the family Rosaceae. Methanol and phosphoric acid were of HPLC grade, water used was ultrapure water, and other reagents were all of analytical grade.
Methods and Results
Chromatographic conditions
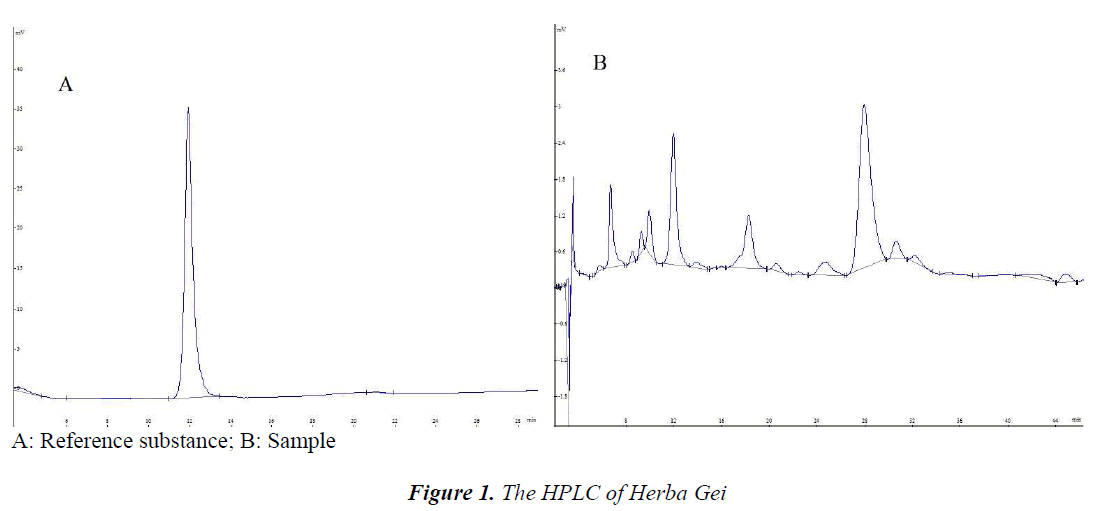
Column: Kromasil C18 (4.6 mm×250 mm, 5 μm); mobile phase: methanol-0.1% aqueous phosphoric acid (10:90); flow rate: 1 mL/min; detection wavelength: 273 nm; column temperature: 30℃. Number of theoretical plates should not be less than 4,000 calculated based on the gallic acid. Chromatogram is shown in Figure 1.
Plotting of standard curve
5.05 mg of gallic acid reference substance was accurately weighed, placed in a 25 mL volumetric flask, dissolved in methanol and diluted to the mark, and shaken well. 2.5 mL of 0.2020 mg/mL gallic acid reference solution was precisely drawn into a 5 mL volumetric flask, diluted to the mark with methanol, and shaken well to give a 0.1010mg/mL gallic acid reference solution. 2, 4, 6, 8, 10 and 12 μL of the reference solution were injected into the HPLC system, respectively, and peak areas were determined according to the above chromatographic conditions. Standard curve was plotted with the integral of peak area as ordinate, and injection volume of reference substance as abscissa, and regression equation was obtained as Y=3029.89 X+2.3241, r=0.9997, and linear range was 0.202-1.212 μg.
Preparation of test solution
About 1.0 g of crude Herba Gei drug powder (60 mesh) was accurately weighed, placed in a 100 mL conical flask, added precisely with of 25 mL 30% ethanol, weighed, and heated under reflux in a water bath for 90 min, let cool, then weighed again. After the lost weight was replenished with 30% ethanol, the solution was shaken well, clarified, and filtered, the subsequent filtrate was then passed through a 0.45 μm microporous membrane to give the test solution.
Accuracy test
10 μL of test solution was aspirated, injected repeatedly six times, and the RSD of gallic acid peak area was determined to be 0.29%.
Stability test
Herba Gei test solution was injected for HPLC determination at 0, 2, 4, 8, 12 and 24 h, respectively, and the RSD of gallic acid peak area was determined to be 1.216%.
Reproducibility test
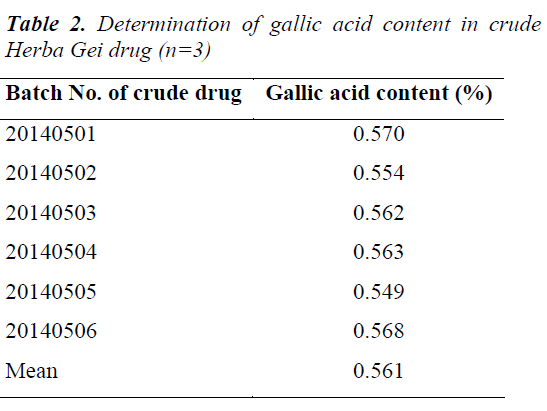
6 aliquots of samples with the same batch number were taken to separately prepare test solutions for determination; the results revealed that the average content of gallic acid in crude Herba Gei drug was 0.561 mg/g, with a RSD of 2.23%. The test results showed good reproducibility.
Recovery test
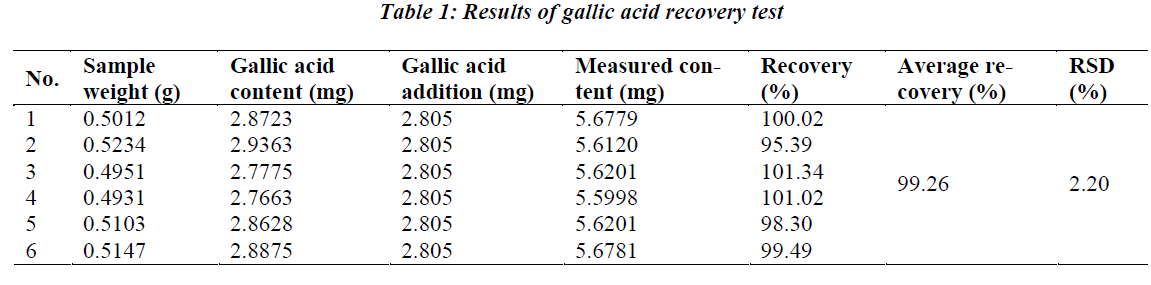
0.5 g of crude Herba Gei drug of known content was taken, added precisely with an appropriate amount of gallic acid reference solution, and prepared as per the above test solution preparation method, then determined according to the above chromatographic conditions, followed by calculation of recovery rate. The results are shown in Table 1.
Sample determination
Samples were prepared as per the test solution preparation method, and gallic acid contents in six batches of crude Herba Gei drugs were determined under the above chromatographic conditions. Each sample was tested in parallel three times. The results are shown in Table 2.
Discussion
Selection of detection wavelength: gallic acid was scanned at 200~400 nm with diode array detector, and maximum absorption was present at 273 nm, so detection wavelength was selected as 273 nm.
Selection of mobile phase: different proportions of methanol-aqueous phosphoric acid and methanol-aqueous formic acid were investigated during experiment; when the mobile phase was methanol-0.1% phosphoric acid (10:90), the resolution was high, retention time was adequate, and no tailing occurred.
Selection of extraction solvent: different extraction solvents were comparatively studied during experiment; 30% ethanol, 40% ethanol, 50% ethanol, 30% methanol, 40% methanol, 50% methanol and ethyl acetate were used as the extracting solvents. Experimental results showed that extraction efficiency was better when 30% ethanol was used, but the 30% ethanol extract was filtered slowly. Then, gallic acid content was investigated using 40% ethanol and 30% ethanol as the extraction solvents, which revealed that 40% ethanol differed little from 30% ethanol in terms of extraction efficiency, but it solved the filtration problem, so 40% ethanol was selected as the extraction solvent. The method for quantitative determination of gallic acid in Herba Gei established in this paper is simple fast, and has less interference, which can be used for quality control of crude Herba Gei drug.
Herba Gei has significant effects in treatment of hypertension, dizziness, headache and menstrual disorders; gallic acid has broad activity, according to some reports, gallic acid has a certain role in the above diseases [10-14]. Therefore, it is not difficult to see that gallic acid is the active constituent of Herba Gei, and determination of its content is of undeniable significance. This study establishes the method for quantitative determination of active constituent in Herba Gei, not only laying a firm foundation for the formulation of standards for quality control of its crude drug and preparations, but also providing a scientific evidence for the existing TCM pharmacology.
References
- Editorial Board of Flora of China, Chinese Academy of Sciences. Flora of China. Science Press 1996; 37: 221-223.
- Li JK, Liu HW, Wang NL, Li M, Yao XS. Chemical constituents from Geum japonicum Thunb. var. chinense F. Bolle. Journal of Shenyang Pharmaceutical University 2006: 23: 694-697.
- Yoshida T, Okuda T, Memon MU, Shingu T. Structureof gemin A, a new dimeric ellagitannin hayingand-glucose cores. J Chem Soc Chem Commun 1982; 6: 351-353.
- Cheng XR. Taraxasterane-Type Triterpene and Neolignans from Geum japonicum Thunb. var. chinense F. Bolle. Planta Medica 2011; 77: 2061-2065.
- Liu H, Li J, Zhao W, Bao L, Song X, Xia Y, Wang X, Zhang C, Wang X, Yao X, Li M. Fatty acid synthase inhibitors from Geum japonicum Thunb. var. chinense. Chem Biodivers 2009; 6: 402-410.
- Yoshida T, Okuda T, Memon MU, Shingu T. Tannins of rosaceous medicinal plants. Part 2. Gemins A, B, and C, new dimeric ellagitannins from Geum japonicum. J. Chem. Soc. Perkin Trans 1985: 315-321.
- Yoshida T, Okuda T, Memon MU, Shingu T. Structure of gemin A, a new dimeric ellagitannin having α- and β-glucose cores. J. Chem. Soc., Chem. Commun 1982; 6: 351-353.
- Kurokawa M, Nagasaka K, Hirabayashi T, Uyama S, Sato H, Kageyama T, Kadota S, Ohyama H, Hozumi T, Namba T. Efficacy of traditional herbal medicines in combination with acyclovir against herpes simplex virus type 1 infection in vitro and in vivo. Antiviral Research 1995; 27: 19-37.
- Zhang F. Studies on the Chemical Constituents and Quality Control of Geum Japonicum Thunb. Var. Chinense F. Bolle. Shanghai Jiaotong University, Master's Dissertation 2012.
- González-Muñoz A, Quesille-Villalobos AM, Fuentealba C, Shetty K, Gálvez Ranilla L. Potential of Chilean Native Corn (Zea mays L.) Accessions as Natural Sources of Phenolic Antioxidants and in Vitro Bioactivity for Hyperglycemia and Hypertension Man-agement. J Agric Food Chem 2013; 61: 10995-11007.
- Chinnadurai V, Khalid SA, Govindasamy C, Mohammed AA, Adel AA, Kodukkur VP. Antihypertensive effect of Melothria maderaspatana leaf fractions on DOCA-salt-induced hypertensive rats and identification of compounds by GC-MS analysis. Journal of Natural Medicines 2012; 66: 302-310.
- Vazirian M, Khanavi M, Amanzadeh Y, Hajimehdipoor H. Quantification of gallic acidin fruits of three medicinal plants. Iran J Pharm Res 2011; 10: 233- 236.
- Arumugam A, Gunasekaran N, Perumal S. In vitro antioxidant, anti-diabetic, cholinesterase and tyrosinase inhibitory potential of fresh juice from Citrus hystrix and C. maxima fruits. Food Science and Human Wellness 2014; 3: 16-25.
- Mabel P, Francis GS. In vitro antimicrobial activity and HPTLC analysis of hydroalcoholic seed extract of Nymphaea nouchali Burm. f. BMC Complementary and Alternative Medicine 2014; 14: 361-365.