ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 7
Lung injury following inhaled chemotherapy
Ming Cai* and Yuan Weng
Department of Thoracic and Cardiovascular Surgery, the Fourth People’s Hospital of Wuxi, Affiliated Hospital of Jiangnan University, 200 Huihe Road, Wuxi, Jiangsu, China
- *Corresponding Author:
- Ming Cai
Department of Thoracic Surgery
The Fourth People’s Hospital of Wuxi
Affiliated Hospital of Jiangnan University, China
Accepted date: December 2, 2016
Objective: We evaluated lung injury following inhaled treatment with paclitaxel, and determined the potential mechanism underlying the lung injury and remodeling following inhaled treatment.
Methods: Sprague-Dawley rats were treated with paclitaxel (3 mg/kg), via tracheal intubation and mechanical aeration. Histopathological changes in lung tissue were determined by haematoxylin and eosin staining. In addition, activity of matrix MMP-2 and MMP-9 was measured by gelatin zymography.
Results: Significant lung injury was found in paclitaxel-treated rats of ≥ 3 mg/kg group, P<0.05. In contrast, lung injury was mild in<3 mg/kg group. Activity of MMP-9 and MMP-2 in lung tissue was significantly increased in all rats received inhaled treatment, P<0.05. However, the magnitude of increase was greater in ≥ 3 mg/kg paclitaxel-treated group compared to<3 mg/kg groups. In addition, the increase of MMP-9 activity appeared to be time-dependently suppressed, whereas the increase of MMP-2 activity was time-dependent exacerbated.
Conclusion: Inhaled chemotherapy with paclitaxel at dose 3 mg/kg and over may lead to significant lung injury. MMP-9 may play important role in lung injury, whereas MMP-2 may play important role in lung remodeling following inhaled treatment.
Keywords
Inhaled chemotherapy, Paclitaxel, Lung injury, Matrix metalloproteinase.
Introduction
Lung cancer is the leading cause of cancer-related death worldwide [1-5]. Inhaled chemotherapy, which was first reported in 1993, may help fighting lung cancer [6-11]. Inhaled chemotherapy directly delivers chemotherapy drugs to the primary and/or metastatic cancer tissues via nebulization inhalation [12,13]. The concentration of the drugs in the tumor tissue reaches higher than those delivered systemically. During inhaled chemotherapy, the chemotherapy drugs will be absorbed in places where bronchial alveolar capillary and lymphatic network abundantly exist and in active absorption [14]. These places may include tumor tissues. After absorbed by the lung and maybe lymph system nearby, the drugs will enter into general circulation and eventually degraded in the liver [15]. Thus, inhaled chemotherapy may help patients overcome some of the obstacles to chemotherapy, such as cancer cell resistance, low drug accumulation in the lungs, and adverse side effects [16-18]. Previous studies have shown the benefits of inhaled chemotherapy in animal experiment and human beings [19-25]. However, inhaled chemotherapy with drugs commonly used for systematic chemotherapy may produce pathological changes in normal lung tissues [26-30]. Thus, the first goal of the present study was to evaluate the lung injury following inhaled treatment with Paclitaxel (PTX) [31-35]. Matrix metalloproteinases (MMP)-9 and (MMP)-2 have been demonstrated to play important roles in lung injury and remodeling following lung injury [36-40]. Thus, the second goal was to determine the activity of MMP-9 and MMP-2 in lung tissue following inhaled treatment with PTX.
Materials and Methods
Materials
PTX was purchased from Jiangsu Haosen Pharmaceutical Co., Ltd, (Jiangsu, and China). Ventilator (DW-2000) was purchased from Shanghai Praiseworthy Peng Co., Ltd of Science and Technology (Shanghai, China). Inhalation therapy nebulizer (Pari 085) was purchased from PARI (Berlin, Germany).
Animals
All procedures were approved by the Institutional Animal Care and Use Committee. At 4-8 weeks of age (body weight: 150 g) SD rats (n=40) were randomly divided into three groups, control (n=10, male: female=1:1), PTX<3 mg/kg (n=10, male: female=1:1), PTX ≥ 3 mg/kg (n=10, male: female=1:1). All rats were housed in standard cages and freely reach chow food (Shanghai Laboratory Animal Center (SLAC)) and water. This study was approved by the ethic committee of The Fourth People’s Hospital of Wuxi, Affiliated Hospital of Jiangnan University.
Experimental protocols
On the experimental day, rats were anesthetized with phenobarbital sodium (i.p.) and atropine (i.m.). After endotracheal intubation was performed, rats were ventilated with room air. Chemotherapy drugs were imbibed by the ventilator via an aspirator pump. Rats in control group were inhaled with saline (30 min/time, once a day) for seven days. Rats in PTX group were inhaled with PTX (3 mg/kg, 0.2 g/L, 30 min/time) on the 1st day of the week for 3 weeks. Rats were sacrificed at 1,2 and 4 weeks after inhalation. The left lungs were immediately fixed with formaldehyde and the right lungs were quickly frozen by liquid nitrogen and then stored at -80°C before measuring MMPs activity.
Haematoxylin and eosin (H&E) staining
The histopathological changes in lung tissues were observed by three pathologists who were blinded to the studies.
Gelatin zymography
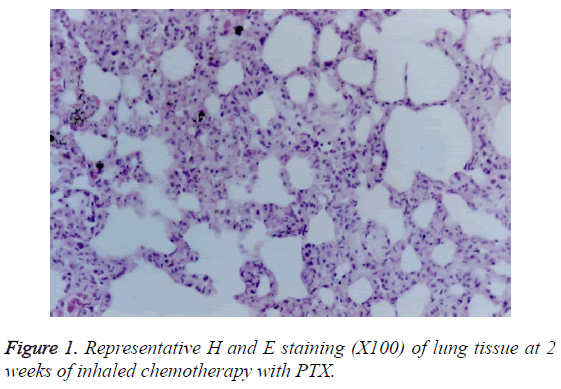
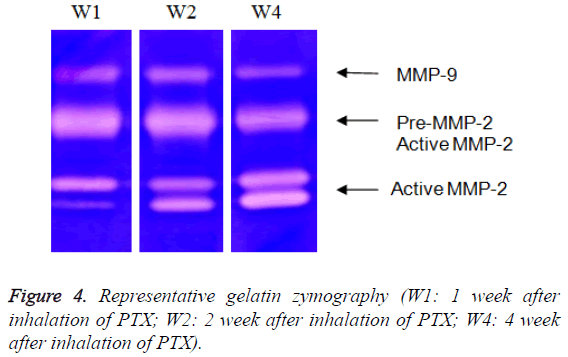
Gelatin zymography was used to measure the activity of MMPs 2 and 9 as described previously [36]. Briefly, tissue extracts were electrophoreses 10% sodium dodecyl sulphateacrylamide gel containing 0.1% gelatin, rinsed in dd-H2O followed by incubation with bulky volume of renaturation buffer (2.7% TX-100 in dd-H2O) at room temperature for one hour with gentle shaking. The enzyme activity was developed in 50 mM Tris pH 7.5, 0.2 M NaCl, 5 mM CaCl2 and 0.2% Brij 35 at 37°C for 18 hours and stained with Coomassie Blue. The activity of MMP is analysed with NIH image software (edition 1.62). Activities of pro-MMP-2 and MMP-2 were quantified at 66 and 62 kDa, respectively. Activity of MMP-9 was considered a lucid band at 92 KDa (Figure 1). Activities of MMPs were calculated according to the method of Davies et al. [6]. Briefly, the ratio of activated MMP-2 to total MMP-2 activities (62 kDa/66 kDa+62 kDa) was calculated from their gelatinolytic activities measured by computer-assisted image analysis.
Statistical analysis
Experiment data were analysed with a SAS software package. For comparison of MMP-9 and MMP-2 activities, results were compared using unpaired student t test. Values are means ± SE. A p value of 0.05 or less was considered to be significant.
Results
Histopathological changes
PTX-treated group: At 1 week of the treatment, only infiltration of small amount of lymphocytes was observed. At 2 weeks of the treatment, serious injury in large and medium bronchi was found (Figure 1). The histopathological changes included hyperplasia of bronchial epithelia and interstitial cells, epithelial exfoliation, papillary degeneration, oedema in peripheral tissue of walls, mucous degeneration, alveolar wall thickening in the central area, infiltration of large amount of lymphocytes and small amount of neutrophil cells, and duct thickening and alveoli fusion of small bronchus. At 4 weeks of the treatment, reduced oedema, increased bulla, but no fibrosis was observed.
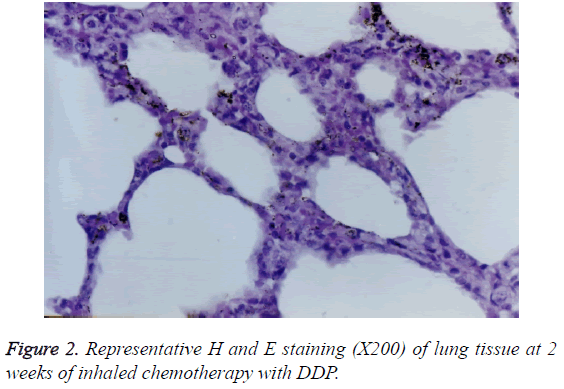
With 2 weeks of the treatment, histopathological changes including hyperplasia of columnar epithelium, erosion, cilia damage, mild congestion in interstitial spaces, and infiltration of lymphocytes were observed in large bronchi (Figure 2).
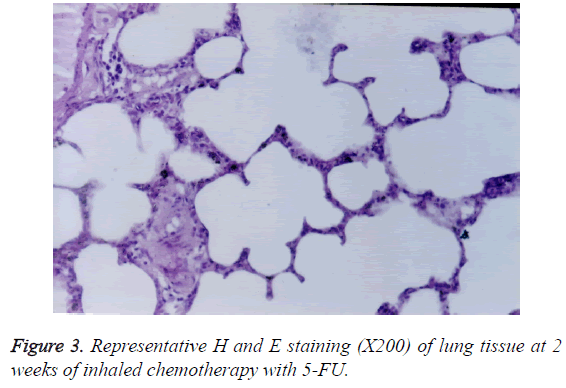
The spaces around the bronchial walls and interstitial tissues were infiltrated by lymphocytes (Figure 3). In addition, oedema and congestion appeared in interstitial spaces. At 2 weeks of the treatment, interalveolar interval and walls of small vessels were thickened. However, no fibrosis was observed.
MMP-9 and MMP-2 activity
Both MMP-9 activity (Table 1 and Figure 4) and MMP-2 activity (Table 2 and Figure 4) significantly increased at alltime points in PTX-treated groups. However, the magnitude of increase was greater in PTX-treated ≥ 3 mg/kg group compared to PTX<3 mg/kg group. Interestingly, MMP-9 activity appeared to positively correlate to the lung injury. The increase in MMP-9 activity was suppressed time-dependently in ≥ 3 mg/kg group. The increase of MMP-9 activity in PTXtreated group peaked at 2 weeks. On the other hand, MMP-2 activity appeared to negatively correlate to the lung injury. The increase in MMP-2 activity was exacerbated time-dependently in all PTX groups.
| 1 week After inhalation | 2 weeks after inhalation | 4 weeks after inhalation | |
|---|---|---|---|
| Control group | 34 862 ± 385 | 30 256 ± 295 | 31 064 ± 315 |
| PTX group | 78 758 ± 421** | 83 465 ± 412** | 67 841 ± 234** |
Table 1: MMP-9 activity following PTX- inhaled chemotherapy.
| 1 week after inhalation | 2 weeks after inhalation | 4 weeks after inhalation | |
|---|---|---|---|
| Control group | 13.8 ± 1.3 | 13.2 ± 1.2 | 12.4 ± 1.4 |
| PTX group | 45.6 ± 1.1** | 56.5 ± 1.6** | 62.3 ± 0.9** |
Table 2: MMP-2 activity following inhaled chemotherapy.
Discussion
In 1993, Tatsumura et al. first proposed the nebulized inhalation chemotherapy to treat lung cancer [1,41-45]. Since then, progress has been obtained by both experimental research and clinical trial. At normal conditions, the cilia on bronchial epithelium function to discharge secretion and foreign bodies of airway through their swinging activity. But in case of lung cancer, the cilia are damaged and their movements are impaired by excessive secretion of tumor tissue. A reduced capability to exclude foreign bodies may make the inhaled drug to be absorbed better by tumor tissue and remained longer in tumor tissue than normal lung tissue [46]. In addition, angiogenesis featured with a defective, leaky and fragile vascular construction, also occurs in lung cancer [47]. Therefore, the absorption and storage capacity for inhaled drugs may be higher in tumor tissue. This possibility is supported by the evidence that the concentration of the inhaled drugs in tumor tissue reached five times higher than those in normal lung tissues. A higher concentration of inhaled drugs in tumor tissue is beneficial by to get better therapeutic effects without systemic reactions.
As a new therapeutic approach, its safety and side effects should be extensively investigated. Surprisingly, there was no sufficient information regarding the histopathological changes in the lung tissue following inhaled chemotherapy. PTX are commonly used in systemic chemotherapy for lung cancer. In the present study, we evaluated their influences on lung tissue. The doses we used in the present study were determined based on the systematic chemotherapy. We found that inhaled treatment with PTX<3 mg/kg group. Only produced mild histopathological changes in lung tissue, whereas inhaled treatment with PTX>3 mg/kg produced a serious lung injury. In addition, PTX induced some general symptom, including slow movement during first two weeks, poor appetite, bodyweight loss and hair chaos. However, all these symptoms were gradually disappeared at 4 weeks of the treatment. Important future studies would be to identify the impact of low-dose PTX on normal lung tissue.
MMPs are major extracellular matrix-degrading enzymes playing important role in the tissue injury and remodeling. In the present study, MMP-9 and MMP-2 activity slightly increased in animals with mild lung injury, but dramatically increased in animals with serious lung injury. Thus, MMP-9 and MMP-2 may play roles in inhaled chemotherapy-induced lung injury. We noticed that the changing pattern of MMP-9 was different from the one of MMP-2. The increase of MMP-9 activity appeared to be time-dependently suppressed as well as lung injury, whereas the increase of MMP-2 activity was timedependent exacerbated as well as lung remodeling. Thus, increased MMP-9 activity may be related to the lung injury, whereas increased MMP-2 activity may be related to the lung remodeling. In the future, it will be important to determine whether inhibition of MMP-9 will reduce inhaled chemotherapy-induced lung injury.
In summary, we evaluated histopathological changes of lung tissues following inhaled chemotherapy. We found that inhalation of 3 mg/kg PTX resulted in a significant lung injury. In contrast, the doses of 3 mg/kg used in the present study appeared relative safe. In addition, MMP-9 may play important role in lung injury, whereas MMP-2 may play important role in lung remodeling following inhaled chemotherapy.
References
- Rami-Porta R, Bolejack V, Crowley J. The IASLC lung cancer staging project: proposals for the revisions of the t descriptors in the forthcoming eighth edition of the TNM classification for lung cancer. J Thorac Oncol 2015; 10: 990-1003.
- Shaw AT, Kim DW, Nakagawa K, Seto T, Crino L. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 2013; 368: 2385-2394.
- Brahmer J, Reckamp KL, Baas P. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015; 373: 1627-1639.
- Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015; 372: 2018-2028.
- Gilbert ES, Stovall M, Gospodarowicz M, Van Leeuwen FE, Andersson M. Lung cancer after treatment for Hodgkins disease: focus on radiation effects. Radiat Res 2003; 159: 161-173.
- Tatsumura T, Koyama S, Tsujimoto M. Further study of nebulization chemotherapy, a new chemotherapeutic method in the treatment of lung carcinomas: fundamental and clinical. Br J Cancer 1993; 68: 1146-1149.
- Hershey AE, Kurman ID, Forrest LJ. Inhalation chemotherapy for macroscopic primary or metastatic lung tumors: proof of principle using dogs with spontaneously occurring tumors as a model. Clin Cancer Res 1999; 5: 2653-2659.
- Zarogoulidis P, Chatzaki E, Porpodis K, Domvri K, Hohenforst-Schmidt W. Inhaled chemotherapy in lung cancer: future concept of nanomedicine. Int J Nanomedicine 2012; 7: 1551-1572.
- Velkov T, Rahim NA, Qi Z. Inhaled anti-infective chemotherapy for respiratory tract infections: Successes, challenges and the road ahead ? Adv Drug Del Rev 2015, 85: 5-7.
- Vento S, Cainelli F, Temesgen Z. Lung infections after cancer chemotherapy. Lancet Oncol 2008; 9: 982-992.
- Zarogoulidis P, Darwiche K, Hohenforst-Schmidt W, Huang H, Li Q. Inhaled gene therapy in lung cancer: proof-of-concept for nano-oncology and nanobiotechnology in the management of lung cancer. Future Oncol 2013; 9: 1171-1194.
- Zarogouldis P, Karamanos NK, Porpodis K, Domvri K, Huang H. Vectors for inhaled gene therapy in lung cancer. Application for nano oncology and safety of bio nanotechnology. Int J Mol Sci 2012; 13: 10828-10862.
- Pandey R, Khuller GK. Anti-tubercular inhaled therapy: opportunities, progress and challenges. J Antimicrob Chemother 2005; 55: 430-435.
- Zarogoulidis P, Darwiche K, Kalamaras G, Huang H, Hohenforst-Schmidt W. Targeted versus chrono-targeted chemotherapy for inhaled chemotherapy in non-small cell lung cancer. Transl Lung Cancer Res 2013; 2: 17-22.
- Weinberg OK, Marquez-Garban DC, Fishbein MC, Goodglick L, Garban HJ. Aromatase inhibitors in human lung cancer therapy. Cancer Res 2005; 65: 11287-11291.
- Rebucci M, Michiels C. Molecular aspects of cancer cell resistance to chemotherapy. Biochem Pharmacol 2013; 85: 1219-1226.
- Wang Y, Liu T, Zhang E, Luo S, Tan X. Preferential accumulation of the near infrared heptamethine dye IR-780 in the mitochondria of drug-resistant lung cancer cells. Biomaterials 2014; 35: 4116-4124.
- Beer TM, Armstrong AJ, Rathkopf DE. Word of wisdom. Re: enzalutamide in metastatic prostate cancer before chemotherapy. European Urology 2014; 66: 174.
- Bart PHW, Peter WAK, Kasper B, Atie WW, Walter P, Frank GP, Roman PS, Susan N, Godefridus JP, Pieter EP. Phase I study of aerosolized slit cisplatin in the treatment of patients with carcinoma of the lungs. Clin Cancer Res 2007; 13: 2414-2421.
- Nadezhda VK, Clifford W, Luz E, Eva G, Sara M, Vernon K. Paclitaxel liposome aerosol treatment induces inhibition of pulmonary metastases in murine renal carcinoma model. Clin Cancer Res 2001; 7: 3258-3262.
- Mauger DT, Sorkness CA. Step-up therapy for children with uncontrolled asthma receiving inhaled corticosteroids. N Eng J Med 2010; 362: 975-985.
- Barnes PJ. Scientific rationale for inhaled combination therapy with long-acting beta2-agonists and corticosteroids. Eur Respir J 2002; 19: 182-191.
- Ghofrani HA, Wiedemann R, Rose F, Olschewski H, Schermuly RT. Combination therapy with oral sildenafil and inhaled iloprost for severe pulmonary hypertension. Ann Intern Med 2002; 136: 515-522.
- Cochrane MG, Bala MV, Downs KE. Inhaled corticosteroids for asthma therapy : patient compliance, devices, and inhalation technique. Chest 2000; 117: 542-550.
- Mclaughlin VV, Benza RL, Rubin LJ. Addition of inhaled treprostinil to oral therapy for pulmonary arterial hypertension : a randomized controlled clinical trial. J Am Coll Cardiol 2010; 55: 1915-1922.
- Mortensen NP, Hickey AJ. Targeting inhaled therapy beyond the lungs. Respiration 2014; 88: 353-354.
- Pilcer G, Rosière R, Traina K. New co-spray-dried tobramycin nanoparticles–clarithromycin inhaled powder systems for lung infection therapy in cystic fibrosis patients. J Pharm Sci 2013; 102: 1836-1846.
- Wauthoz N. In vivo assessment of temozolomide delivery systems for inhalation therapy of lung cancer. Bioconjug Chem 2015; 26: 120-133.
- Plant BJ, Downey D, Eustace JA. WS74 inhaled aztreonam lysine (Cayston) therapy significantly improves lung function, weight, hospitalisations and excerbation rates prospectively-an Irish and UK real world experience. J Cystic Fibrosis 2014; 13: 7189-7206.
- Semykin SY, Polikarpova SV, Dubovik LG. Efficiency of the inhalational tobramycin therapy in complex antibacterial therapy of lung exacerbation in cystic fibrosis children with chronic Pseudomonas aeruginosa infection. J Cystic Fibrosis 2010; 9: S55.
- Liu WJ, Zhong ZJ, Cao LH, Li HT, Zhang TH. Paclitaxel-induced lung injury and its amelioration by parecoxib sodium. Sci Rep 2015; 5: 12977.
- Itoh Y, Sendo T, Hirakawa T. Similarity and difference in the acute lung injury induced by a radiographic contrast medium and an anticancer agent paclitaxel in rats. Toxicol Lett 2004; 152: 27-34.
- Wang B, Yang J, Luo D. Complete remission and fatal interstitial pneumonitis related to nab-paclitaxel in refractory small cell lung cancer: A case report and review of the literature. Thoracic Cancer 2012; 3: 84-87.
- Herscher LL, Hahn SM, Kroog G, Pass H, Temeck B. Phase I study of paclitaxel as a radiation sensitizer in the treatment of mesothelioma and non-small-cell lung cancer. J Clin Oncol 1998; 16: 635-641.
- Belknap SM, Kuzel TM, Yarnold PR, Slimack N, Lyons EA. Clinical features and correlates of gemcitabine-associated lung injury: findings from the RADAR project. Cancer 2006; 106: 2051-2057.
- Corbel M, Boichot E, Lagente V. Role of gelatinases MMP-2 and MMP-9 in tissue remodeling following acute lung injury. Braz J Med Biol Res 2000; 33: 749-754.
- Pirrone F, Pastore C, Mazzola S. In vivo study of the behaviour of matrix metalloproteinases (MMP-2, MMP-9) in mechanical, hypoxic and septic-induced acute lung injury. Veter Res Commun 2009; 33: 121-124.
- Nolan A, Kwon S, Cho SJ, Naveed B, Comfort AL. MMP-2 and TIMP-1 predict healing of WTC-lung injury in New York City firefighters. Respir Res 2014; 15: 5.
- Steinberg J, Halter J, Schiller HJ. Metalloproteinase inhibition reduces lung injury and improves survival after cecal ligation and puncture in rats. J Surg Res 2003; 111: 185-195.
- Wu Z, Ruan Y, Chang J. Angiotensin II is related to the acute aortic dissection complicated with lung injury through mediating the release of MMP9 from macrophages. Am J Transl Res 2016; 8: 1426-1436.
- Shen YF, Han ZQ, Jing juan DU. Clinical study of 5-fluorouracil nebulized inhalation combined with radio chemotherapy for non-small cell lung cancer. J Qilu Oncol 2004.
- Zhou FW, Fang N, Wu XR. Clinical study of TCM nebulized inhalation combined with radio chemotherapy for non-small cell lung cancer. Medical J West China 2012.
- Hunter CJ, Dejam A, Blood AB. Inhaled nebulized nitrite is a hypoxia-sensitive NO-dependent selective pulmonary vasodilator. Nature Med 2004; 10: 1122-1127.
- Lødrup-Carlsen KC, Carlsen KH. Inhaled nebulized adrenaline improves lung function in infants with acute bronchiolitis. Respir Med 2000; 94: 709-714.
- Leflein JG, Szefler SJ, Murphy KR. Nebulized budesonide inhalation suspension compared with cromolyn sodium nebulizer solution for asthma in young children: results of a randomized outcomes trial. Pediatrics 2002; 109: 866-872.
- Janowska-Wieczorek A, Wysoczynski M, Kijowski J. Microvesicles derived from activated platelets induce metastasis and angiogenesis in lung cancer. Int J Cancer 2005; 113: 752-760.
- Strieter RM, Polverini PJ, Arenberg DA, Walz A, Opdenakker G. Role of C-X-C chemokines as regulators of angiogenesis in lung cancer. J Leukoc Biol 1995; 57: 752-762.