Keywords |
| Lipid peroxidation; Vitamin C; Vitamin E; TBARS-MDA; Hypertension; Diabetes. |
Introduction |
| A global study in the year 2000 showed that 972 million
people had hypertension with a prevalence rate of 26.4%. It
was projected that this figure will increase to 1.54 billion and a
prevalent rate of 29.4% in 2025 [1]. A recent community based
study of rural and semi-urban population in Enugu, Nigeria put
the prevalence of hypertension in Nigeria at 32.8% [2]. It has
been reported that Diabetes and Hypertension coexists in
40-60% of individuals with type 2 diabetes [3,4]. Arauz-
Pacheco et al. [4,5] reported that diabetic patients have a 1.5-3
times increased prevalence of hypertension compared with
non-diabetic patients, with 50% of adults with diabetes
reported to have hypertension at time of diagnosis [6]. Recent
reports have suggested that hypertension and diabetic
complications have a common aetiology involving oxidative
stress [7]. A group of authors, Friedman et al. [8] showed that
the combination of hypertension and diabetes mellitus is
accompanied by higher oxidative stress than that observed in
the individual disorder alone. |
| Oxidative stress occurs when there is an imbalance between
the generation of reactive oxygen species (ROS) and the
antioxidant defence systems so that the latter become overwhelmed [9]. Hypertension is associated with increased
oxidative stress; however, there is still a debate whether
oxidative stress is a cause or a result of hypertension. Animal
studies have generally supported the hypothesis that, increased
blood pressure is associated with increased oxidative stress;
however, human studies have been inconsistent [10]. Oxidative
stress promotes vascular smooth muscle cell proliferation and
hypertrophy and collagen deposition, leading to thickening of
the vascular media and narrowing of the vascular lumen. In
addition, increased oxidative stress may damage the
endothelium-dependent vascular relaxation and increases
vascular contractile activity [10]. |
| There is evidence that both free radical production and
antioxidant defences are disturbed in diabetes [11]. It has been
suggested over the last few years that oxidative stress in
diabetes may be partly responsible for the development of
diabetic complications [12] and the role of oxidative stress in
the pathogenesis of type 1 diabetes mellitus has been
implicated in several studies [13-15]. Increased lipid
peroxidation products and altered antioxidant enzyme activities
were also reported in type 2 diabetes mellitus [16]. Series of
clinical and experimental studies have shown that oxidative
stress, through free radical generation, plays a role in the onset of both diabetes [17] and hypertension [18]. Nevertheless, the
availability of data is not conclusive and the relationship
between hypertension and oxidative stress in diabetic patients
remains to be elucidated. This study is aimed to determine the
extent of attenuation of oxidative stress in hypertensive and
diabetic hypertensive patients receiving vitamin supplements
and lipid lowering drugs. |
Patients and Methods |
Study design and patients |
| Forty five (45) patients of both sexes and within the age
bracket of 49-72 years were included in this study. Previous
studies showed that the prevalence of hypertension and
diabetes is highest in age groups of 50-69 years [25-27]. The
study was conducted on the patients after informed consent
was obtained from each of the patients while approval for the
study was given by the ethical committee of Federal Medical
Centre Owerri, Imo State, Nigeria in accordance with the
Helsinki Declaration of 1975 (Edinburgh revision, 2008). Data
about the patients for example, age, and so forth was obtained
from administered questionnaire and information obtained
from their hospital folders and no complaints were encountered
during the study. They were subdivided into three main groups
as follows: |
| Group A (Normotensive patients): These were apparently
healthy normotensive patients recruited from some of the
employees of the Federal Medical Centre Owerri, Imo State.
They were 10 in number and served as control patients. They
were age and sex matched with the test patients (B and C). |
| Group B (Hypertensive patients): They were made up of 15
hypertensive patients, reporting to the Clinical Chemistry
Department of the Federal Medical Centre Owerri, Imo State
for laboratory examination. |
| Group C (Diabetic Hypertensive Patients): They were made up
of 20 hypertensive patients who are diabetics and were
reporting to the Clinical Chemistry Department of the Federal
Medical Centre Owerri, Imo State, Nigeria for laboratory
examination. |
Criteria for exclusion |
| Exclusion criteria were smoking, alcoholics, obesity (body
mass index [BMI]>30 kg/m2), hypercholesterolemia and
chronic diseases such as kidney failure and cardiovascular
disease. It is well known that oxidative stress is elevated in
patients with chronic disease such as cardiovascular damage,
renal damage. We did not exclude those receiving lipid
lowering drugs, insulin and antioxidant vitamins supplements
such as mineral ascorbates, probucol, allopurinol quinidine,
disopyramide, or other drugs known for affecting serum lipid
peroxidation and antioxidant values. |
| To confirm the hypertensive status of selected patients, blood
pressure (BP) was measured using OMRON type 711
Automatic IS Sphygmomanometer. Hypertension was defined as mean daytime blood pressure values = 135mmHg systolic
and = 85 mmHg diastolic, by ambulatory blood pressure
monitoring [19]. The diagnosis of diabetes was established
based on the World Health Organisation diagnostic criteria of
the fasting plasma glucose above 126 mg/dl and/or 2-hours
post-prandial plasma glucose above 200 mg/dl [20]. The
laboratory analysis on collected blood samples was conducted
at the Department of Biochemistry Laboratory of Imo State
University Owerri, Imo State, Nigeria. |
Sample collection |
| Blood samples were aseptically drawn from an antecubital vein
of patients by trained personnel and distributed into each of
plain centrifuge tubes for estimation of Serum Glucose,
TBARS-MDA, vitamin E and vitamin C. The samples in the
plain tubes were allowed to clot. All samples were spun at
1000 rpm for 10 minutes in a Jenlab bench centrifuge model
80-2, and the sera pipetted into serum bottles and analyzed.
The serum for serum glucose, TBARS-MDA, vitamin E and
vitamin C was separated from packed cells after the samples
were spun. |
Biochemical assay |
| Serum Glucose was determined using the modified glucose
oxidase method described by Trinder [21]. Vitamin C was
determined by the method of Nino and Shah [22], Vitamin E
estimation was according to the method described by Fabianek
et al. [23] while serum levels of lipid peroxidation were
estimated as TBARS and calculated as Malondialdehyde
(MDA) according to the method of Okhawa et al. [24]. |
Data Analysis |
| Statistical Package for Social Sciences (SPSS), Version 17.0,
was used for data analysis. Results were expressed as Mean ±
Standard deviation and tests of statistical significance were
carried out using one way analysis of variance (ANOVA).
Statistical significance was defined as P<0.05. Correlation
coefficient between analytes was calculated using Pearson
Correlation coefficient at 95% confidence interval. |
Results |
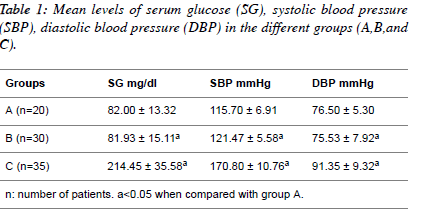
| Table 1 shows the mean values of serum glucose, systolic
blood pressure and diastolic blood pressure in normotensive
patients; hypertensive patients; diabetic hypertensive patients.
The serum glucose level (mg/dl) in the diabetic hypertensive
group (214.45 ± 35.58) and the hypertensive group (81.93 ±
15.11) compared to the normotensive patients (82.00 ± 13.32).
The systolic blood pressure (mmHg) in the diabetic
hypertensive group (170.80 ± 10.76) and the hypertensive
group (121.47 ± 5.58) significantly increased (P<0.05)
compared to the normotensive patients (115.70 ± 6.91). The
diastolic blood pressure (mmHg) in the diabetic hypertensive
group (91.35 ± 9.32) and the hypertensive group (75.53 ± 7.92) showed a significant increase (P<0.05) compared with the
normotensive patients. |
 |
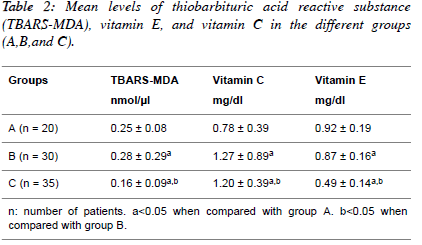
| Table 2 shows the mean values of Thiobarbituric acid reactive
substance measured as Malondialdehyde (TBARS-MDA),
vitamin C and vitamin E in normotensive patients;
hypertensive patients; diabetic hypertensive patients. The
diabetic hypertensive group showed significant increase
(P<0.05) in the levels of TBARS-MDA (0.16 ± 0.09) and
vitamin E (0.49 ± 0.14) compared to the Normotensive groups.
The hypertensive group showed significant increase (P<0.05)
in the levels of TBARS-MDA (0.28 ± 0.09) and vitamin E
(0.87 ± 0.17). However, the levels of TBARS-MDA and
vitamin C and vitamin E were significantly decreased (P<0.05)
in diabetic hypertensive group compared to the hypertensive
group. |
 |
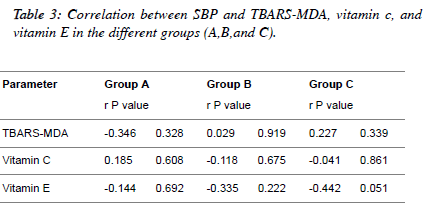
| Table 3 and Figures 1, 2, 3 depict the relationship between
TBARS-MDA, vitamin C and vitamin E and SBP in
normotensive patients, hypertensive patients; diabetic
hypertensive patients. There is a weak positive correlation
(r=0.227) between TBARS-MDA and SBP in diabetic
hypertensive patients (Figure 1), but there is a weak negative
correlation between vitamin C, vitamin E and SBP in diabetic
hypertensive patients (Figure 2 and Figure 3). With regards to
hypertensive patients, there is a weak positive correlation
(r=0.029) between TBARS-MDA and SBP and a weak
negative correlation (r=-0.118, r=-0.335) between vitamin C
SBP, vitamin E and SBP. |
 |
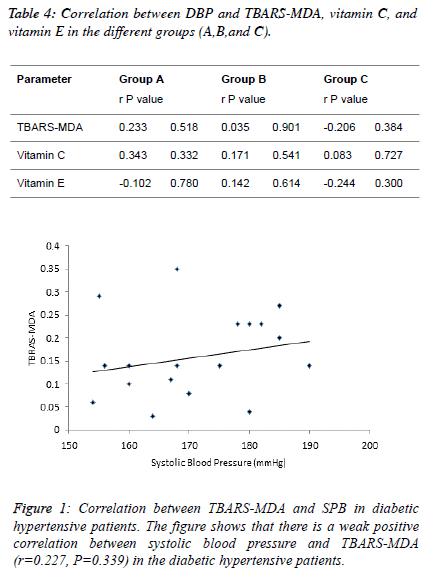
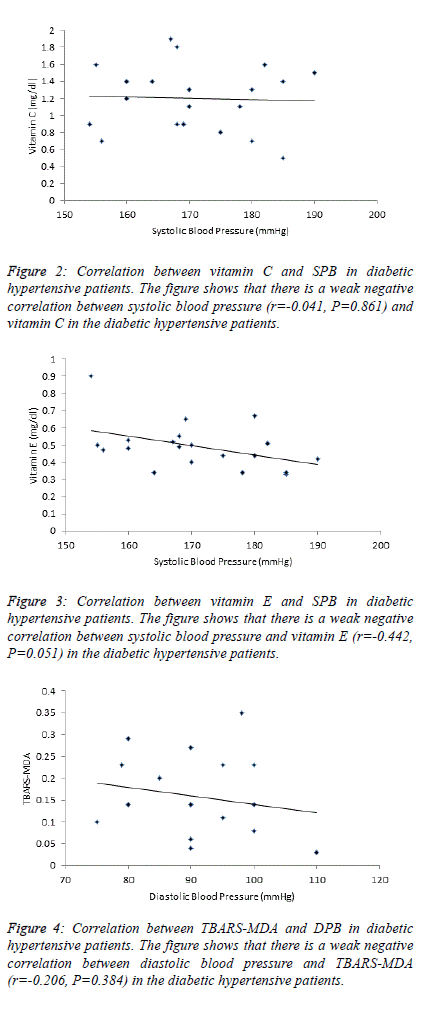
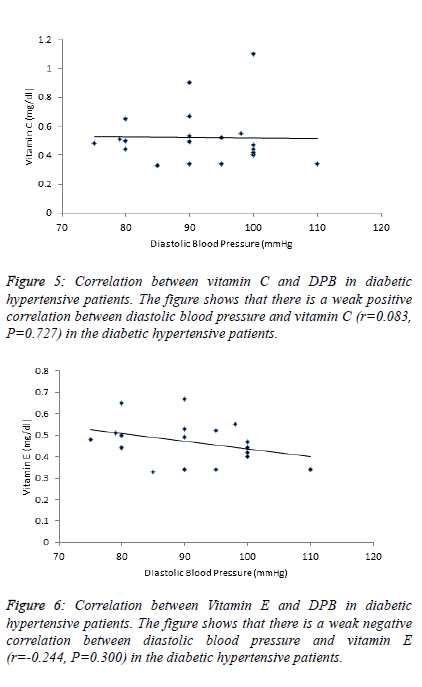
| Table 4 and figure 4, 5 and 6 depict the relationship between
TBARS-MDA, vitamin C and vitamin E and SBP in
normotensive patients, hypertensive patients; diabetic
hypertensive patients. There is a weak negative correlation
(r=-0.206) between TBARS-MDA and DBP in diabetic
hypertensive patients (Figure 4). There is also a weak negative
correlation (r=-0.244) between vitamin E and DBP in diabetic
hypertensive patients (Figure 6). A weak positive correlation
(r=0.083) was observed between vitamin C and DBP in
diabetic hypertensive patients (Figure 5). With regards to the
hypertensive patients, there is a weak positive correlation
between TBATS-MDA (r=0.035), vitamin C (r=0.171),
vitamin E (r=0.142) and DBP. |
 |
 |
 |
Discussion and Conclusion |
| Previous studies showed that the prevalence of hypertension
and diabetes is highest in age groups of 50-69 years [25-27].
This formed our decision to limit the study to patients of this
particular age bracket. The present findings demonstrate the
existence of a relationship between blood pressure and some
oxidative stress related parameters. The increased oxidative
stress levels observed in hypertensive patients are consistent
with the findings of several other studies [28,29] (Simic et al.,
2006, Kedziora-kornatowska et al., 2004). Previous findings
showed that diabetes and hypertension are associated with
increased oxidative stress, which results in higher serum
concentration of lipid peroxidation products like TBARSMDA
[25,30,31]. Malondialdehyde is a reliable marker of lipid
peroxidation and perioxidative tissue injury [32]. It has been
shown to be elevated in animal models of experimentally
induced hypertension, suggesting that it is a consequence
rather than a cause of hypertension |
| In this study, a significant decrease in TBARS-MDA levels
was observed in the diabetic hypertensive patients compared
with the hypertensive patients and normotensive patients. This
is due to the fact that the patients were receiving statin medication and antidiabetic drugs at the point of reporting to
the hospital [33], a confounding factor of oxidative stress [34].
Seghrouchi et al. [35] reported that in patients with type 2
diabetes mellitus, insulin treatment only partially improved
oxidative stress parameters. Fava et al. [36] also reported that
in type 2 diabetic patients, treatment with gliclazide for 12
weeks ameliorated oxidative stress better than did
glibenclamide. However significant increase in TBARS-MDA
level was noticed in the hypertensive patients compared with
the normotensive patients, suggesting that active lipid
peroxidation is occurring in essential hypertension. This is in
agreement with various findings by Dhananjay et al. [37];
Nwanjo et al. [38] and Ahmad et al. [39]. Malondialdehyde is a
highly toxic by-product, produced in part by oxidation; derived
from free radicals. MDA reacts both irreversibly and reversibly
with proteins and phospholipids with profound effects and
studies have shown significantly raised concentrations in
diabetes [40] and hypertension [37,38,39]. |
| The correlation of blood pressure levels with oxidative stress
related parameters in normotensive, hypertensive and diabetic
hypertensive patients suggests that these parameters have an
additional blood pressure modulating effect distinct from those
previously observed. Hypertensive and diabetic hypertensive
patients showed impairment of the antioxidant defence system
as assessed by a diminution of serum antioxidant status, in
agreement with previous data [34,41]. Furthermore, the
negative correlation between the SBP and both the antioxidant
vitamins level points out to the importance of blood
antioxidant status in blood pressure modulation. |
| Non enzymatic antioxidants such as vitamin C and vitamin E
play an excellent role in protecting the cells from oxidative
damage [42]. Previous findings showed that vitamin C level is
lower in hypertensive and diabetic patients compared to
general population [25,43,44]. Mayer Davis et al. [45] reported
that vitamin C level was unrelated to cardiovascular risk
factors in long term while Khaw et al. [46] reported a
significant association between plasma vitamin C levels and
long term sequels of hypertension. The result of the present
finding showed that vitamin C level significantly increased in
hypertensive and diabetic hypertensive patients. It has been
demonstrated that vitamin C supplementation showed a
significant decline in both systolic and diastolic blood pressure
which may persist for prolonged period. In addition, vitamin C
has been suggested to act more than an antioxidant and its
effects on neurotransmitters lead to its antihypertensive activity
[47]. |
| Vitamin E status in hypertensive and diabetic patients were
significantly lower than that of normotensive patients. Vitamin
E is a component of the total peroxylradical-trapping
antioxidant system, reacts directly with peroxyl and superoxide
radicals and singlet oxygen and protects membranes from lipid
peroxidation [48]. A study by Bernado Rodriguez-Iturbe et al.
[49] demonstrated that an antioxidant-enriched diet that
included vitamin E, vitamin C, selenium and zinc reduces the
renal interstitial inflammation, decreases renal tissue content of
Malondialdehyde and improves hypertension. Furthermore, the cardio-protective potential of vitamin E has been attributed to
its potent antioxidant action. This contention is supported by
the fact that a-tocopherol shows antioxidant potential by
donating hydrogen radical to remove the free radicals reacting
with it to form non-radical products or trapping of lipid
radicals [50]. |
| In conclusion, from the present study, antioxidant status and
lipid peroxidation are altered in hypertension and diabetic
hypertension. Although lipid peroxidation decreased in
diabetic hypertensive patients receiving vitamin supplements,
lipid lowering drugs and insulin, same cannot be said for
hypertensive patients. Use of lipid lowering drugs may
contribute to the lowering of lipid peroxidation in
hypertension. Vitamin E supplementation will produce a
beneficial effect in hypertensive and diabetic hypertensive
patients. The major limitation of our study was the small
sample size. Further studies employing larger population are
warranted to further confirm the results of present
investigation. |
Acknowledgement |
| Dr. Obinna Chijioke of Federal Medical Centre Owerri is
acknowledged for his help in collection of samples. The
research study was funded and done by the Undergraduate
Research Students of the Department of Biochemistry (2015),
Imo State University Owerri who are under the supervision of
Dr. Ekeanyanwu, R.C. |
Conflict of Interest |
| The authors declare that there is no conflict of interests
regarding the publication of this paper. |
|
References |
- Kearney PM, Whelton M, Reynolds K, Muntner P, WheltonPK,et al. Global burden of hypertension: analysis of world wide data. Lancet. 2005; 365: 217 -223.
- Ulasi II, Ijoma CK, Onodugo OD. A community based study of hypertension and cardiometabolic syndrome in semi- urban and rural communities in Nigeria. BMC Health Services Research 2010; 10: 71.
- Sowers JR, Epstein M, Frohlich ED. Diabetes, hypertension, and cardiovascular disease: an update. Hypertension 2001; 37: 1053-1059.
- Arauz-Pacheco C, Parrott MA, Raskin P. The treatment of hypertension in adult patients with diabetes. Diabetes Care 2002; 25: 134-147.
- Arauz-Pacheco C, Parrott MA, Raskin P. Hypertension management in adults with diabetes. Diabetes Care 2004; 27: S65-S67.
- Klein R, Klein BEK, Lee KE, Cruickshanks KJ, Moss SE. The incidence of hypertension in insulin-dependent diabetes. Archive of Internal Medicine 1996; 156: 622-627.
- Bayraktutan U. Free radicals, diabetes and endothelial dysfunction. Diabetes, Obesity and Metabolism 2002; 4: 224-238.
- Friedman J, Peleg E, Kagan T, Shnizer S, Rosenthal T. Oxidative stress in hypertensive, diabetic and diabetic hypertensive rats. American Journal of Hypertension 2003; 16: 1049-1052.
- Becker LB. New Concepts in reactive oxygen species and cardiovascular reperfusion physiology. Cardiovascular Research 2004; 61: 461-470.Â
- Bhale DV, Hivre MD, Mahat RH, Saudagar A, Mishra D, et al. Study of Oxidative stress in Patients with Hypertension. International Journal of Recent Trends in Science and Technology 2013;9: 157-158.
- Lyons TJ. Oxidized low density lipoproteins: a role in the pathogenesis of atherosclerosis in diabetes? Diabetic Medicine 1991;8: 411-419.
- Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes 1991;40: 405-412.
- MarjaniA. Lipid peroxidation alterations in type 2 diabetic patients. Pakistan Journal of Biological Sciences 2010; 13: 723-730.
- Orhan H, Sahin G. Erythrocyte glutathione S-transferase activity in diabetes mellitus: the effect of the treatment. Fabad Journal of Pharmaceutical Sciences 1999;24: 127-131.
- Sato Y, Hotta N, Sakamoto N. Lipid peroxide level in plasma of diabetic patients. Biochemical Medicine 1979;21: 104-107.
- Mansour MA, Al-shebly MM. Evaluation of oxidative stress and antioxidant status in diabetic and hypertensive women during labour. Oxidative Medicine and Cellular Longetivity 2012; 2012: 329743.
- Maritim AC, Sanders RA, Watkins III JB. Diabetes, oxidative stress and antioxidants: a review. Journal of Biochemical and Molecular Toxicology 2003;17: 24-38.
- Zhou XJ, Vaziri ND, Wang XQ, Silva FG, Laszik Z. Nitric oxide synthase expression in hypertension induced by inhibition of glutathione synthase. Journal of Pharmacology and Experimental Therapy 2002;300: 762-767.
- Myers MG, Tobe SW, McKay DW, Bolli P. New algorithm for the diagnosis of hypertension. American Journal of Hypertension 2005;18: 1369-1374.
- Okoduwa SIR, Umar AI, Ibrahim S, Bello F. Relationship of oxidative stress with type 2 diabetes and hypertension. Journal of Diabetology2013; 1:2.
- Trinder P. Determination of Glucose in Blood Using Glucose Oxidase with an Alternative Oxygen Acceptor. Annals of Clinical Biochemistry 1969; 6:24.
- Nino HV, Shah W. “Vitamins,” in Fundamentals of Clinical Chemistry, N.W. Tietz, Ed., pp. 547-550,WB Saunders, Philadelphia, Pa, USA, 2nd ed, 1986.
- Fabianek J, DeFilippi J, Richards T, Herp A. Micromethod for tocopherol determination in blood serum. Clinical Chemistry 1968; 14: 456-462.
- Stambouli-Gueviche AB, Mokhtari-Soulimane N, Merzouk H, Merzouk S, Bendedouche AS. Elevation of oxidative stress markers in type 1 diabetic children. Journal of Diabetes and Endocrinology 2015; 6: 5-11.
- Akinkugbe OO. Hypertensive disease in Ibadan, Nigeria. East Africa Medical Journal 1969;46: 313-320.
- Aksu H, Pala K, Aksu H. Prevalence and associated risk factors of type 2 diabetes mellitus in Nilufer district, Bursa, Turkey. International Journal of Diabetes and Metabolism 2006;14: 98-102.
- Idemudia J, Ugwuja E. Plasma lipid profiles in Hypertensive Nigerians. The Internet Journal of Cardiovascular Research 2009;6: 2-6.
- Simic DV, Mimic-Oka J, Pljesa-Ereegovac M. Byproducts of oxidative protein damage and antioxidant enzyme activities in plasma with different degrees of essential hypertension. Journal of Human Hypertension 2006;20: 149-155.
- Kedziora-kornatowska K, Czuezejko J, pawluk H. The markers of oxidative stress and activity of the antioxidant system in the blood of elderly patients with essential arterial hypertension. Cell and Molecular Biology Letters 2004;9: 635-641.
- Griesmacher A, Kindhauser M, Andert SE, Schreiner W, Toma C, et al. Enhanced serum levels of Thiobarbituric acid reactive substances in diabetes mellitus. American Journal of Medicine 1995;98: 469-475.
- Khenna HD, Sinha MK, Khenna S, Tandon R. Oxidative stress in Hypertension: Association with antihypertensive treatment. Indian Journal of Physiology and Pharmacology 2008;52: 283-287.
- Janero DR. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radical Biology and Medicine 1990; 9: 515-40.
- Ward NC, Hodgson JM, Puddey IB, Mori TA, Berlin LJ, et al. Oxidative stress in human hypertension association with antihypertensive treatment, gender, nutrition and lifestyle. Free Radical Biology and Medicine 2004;36: 226-232.
- Moreno MU, Jose GS, Fortuno A, Beloqui O, Diez J, et al. The C242T CYBA polymorphism of NADPH oxidase is associated with essential hypertension. Journal of Hypertension 2006;24: 1299-1306.
- Seghrouchni I, Drai J, Bannier E, Riviere J, CalmardiP, et al. Oxidative stress parameters in type 1, type 11 and insulin-treated type 2 diabetes mellitus; insulin treatment efficiency. ClinicaChimicaActa 2002; 321: 89-96.
- Fava D, Cassone-Faldetta M, Laurenti O, De luca O, Ghiselli A, et al. Glibenclamide improves antioxidants status and nitric oxide-mediated vasodilation in type 2 diabetes. Diabetic Medicine 2002; 19: 752-757.
- Dhananjay VB, Manjusha DH, Roshan KM, Aasiya S, Devendra M, et al. Study of oxidative stress in patients with hypertension. International Journal of Recent Trends in Science and Technology 2013; 9: 157-158.
- Nwanjo HU, Oze G, Okafor MC, Nwosu DI, Nwankpa P. Oxidative Stress and non-enzymic antioxidant status in hypertensive patients in Nigeria. African Journal of Biotechnology, 2007; 6: 1681-1684.
- Ahmad A, Hossain MM, Singhal U, Islam N. Comparative study of marker of oxidative stress among normotensive, pre-hypertensive and hypertensive patients. Biomedical Research 2013; 24: 491-495.Â
- Mahreeen R, Mohsin M, Nasreen Z, Siraj M, Ishaq M. Significantly increased levels of serum Malondialdehyde in type 2 diabetics with myocardial infarction. International Journal of Diabetes in Developing Countries 2010; 30: 49-51.
- Muda P, Kampus P, Zilmer M. Effect of antihypertensive treatment with candesartan or amlodipine on glutathione and its redox status, homocysteine and vitamin concentrations in patients with essential hypertension. Journal of Hypertension 2005; 23: 105-112.
- Farombi EO, Olowg BI, Emerole GO. Effect of three structurally related antimalarial drugs on liver microsomal components and lipid peroxidation in rats comp Biochemical Physiology 2000; 126: 217-224.
- Ness AR, Chee D, Elliot P. Vitamin C and blood pressure-an overview. Journal of Human Hypertension 1997;11: 343-350.
- Bates CJ, Wamsley CM, Prentice A. Does vitamin C reduce blood pressure? Results of a large study of people aged 65 or older. Journal of Hypertension, 1998; 16: 925-932.
- Mayer-Davis EJ, Monaco JH, Marshall JA. Vitamin C intake and cardiovascular disease risk factors in persons with non-insulin dependent diabetes mellitus. From the Insulin Resistance Atherosclerosis Study and the San Luis Valley Diabetes Study. Preventive Medicine 1997; 26: 277-283.
- Khaw KT, Bingham S, Welch A. Relation between plasma ascorbic acid and mortality in men and women in EPIC-Norfolk prospective study: a prospective population. European prospective population study. European Prospective Investigation into Cancer and Nutrition. Lancet 2001; 357: 657-663.
- Hernandez-Guerra M. Ascorbic acid improves the intrahepatic endothelial dysfunctioning of patients with cirrhosis and portal hypertension. Hepatology, 2006; 43: 485-491.
- Bisht S, Sosodia SS. Diabetes, Dyslipidaemia, Antioxidant and status of oxidative stress. International Journal of Research in Ayurveda 2010; 1: 33-42
- Bernardo Rodriguez-Iturbe, Chang-De Zhan, Yasmir Quiroz. Antioxidant-Rich Diet Relieves Hypertension and Reduces Renal Immune Infiltration in Spontaneously Hypertensive Rats. Hypertension 2003; 41: 341.
- Choi H. Mechanism of angiotensin induced superoxide production in cells reconstituted with angiotensin type 1 receptor and the components of NADPH oxidase. Journal of Biological Chemistry 2008;283: 255-267.
|





