ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 8
Lesser yam induces pgc-1α protein expression on brown adipose and skeletal muscle tissues in diabetic rats
Tri Setyawati1, Prasetyastuti2 and Sunarti2*
1Faculty of Medicine and Health Science, Universitas Tadulako, Central Sulawesi, Indonesia
2Department of Biochemistry, Faculty of Medicine, Universitas Gadjah Mada, Yogyakarta, Indonesia
- *Corresponding Author:
- Sunarti
Department of Biochemistry
Faculty of Medicine, Universitas Gadjah Mada, Indonesia
Accepted date: January 9, 2017
Background: Low expression of PGC-1α in Diabetes Mellitus (DM) is closely related to impaired function of mitochondria. Secondary metabolites from fermented lesser yam can increase the expression of PGC-1α protein.
Aim: This study evaluated the effect of lesser yam flour on Pgc-1α expression of brown adipose and skeletal muscle in diabetic rats.
Method: Twenty-five male Wistar rats, divided into 5 groups: 1) normal rats, 2) diabetic rats, 3) diabetic rats with 1.25 g, 4) diabetic rats with 2.5 g, and 5) diabetic rats with lesser yam flour 5 g per day. Diabetic rats were conducted by inducing of nicotinamide and streptozotocin via intraperitoneal injection. Blood glucose levels were measured before and after administration of the lesser yam flour. After 4 weeks of treatment, the brown adipose and the skeletal muscle tissues were taken under anesthesia, and the expression of Pgc-1α protein was analysed by immunohistochemistry method.
Results: Blood glucose levels were significantly reduced in the group of lesser yam compared to diabetic control group (P=0.001). The expression of Pgc-1α protein is highly expressed in both brown adipose and skeletal muscle tissues compared to diabetic control group (P=0.162; P=0.004), respectively.
Conclusion: Lesser yam may improve mitochondrial function through increased the expression of Pgc-1α protein.
Keywords
Lesser yam, Diabetes, Pgc-1α expression, Brown adipose, Skeletal muscle.
Introduction
Hyperglycemia in type 2 Diabetes Mellitus (T2DM) leads to increased Reactive Oxygen Species (ROS) generation which lowers mitochondrial biogenesis and causes mitochondrial dysfunction [1]. In the condition of mitochondrial dysfunction, the expression of Peroxisome Proliferator Activated Receptor-γ (PPAR-γ) coactivator 1α (Pgc-1α), especially in skeletal muscle and adipose tissues decreased [2]. Pgc-1α has an important role as regulator of mitochondrial biogenesis and energy metabolism in several tissues [3,4]. PGC-1α activation in cells increases the oxidative capacity of cells and mitochondrial biogenesis [4].
Pgc-1α is highly expressed in tissues where oxidative metabolism and mitochondria are mostly found, such as Brown Adipose Tissue (BAT), skeletal muscle, heart, brain and kidney [5]. The expression of Pgc-1α in induction depends on the physiological and environmental signals [6], when Pgc-1α is exposed by cold; its expression in Brown Adipose Tissue (BAT) dramatically increased [5]. Similarly, the expression of Pgc-1α in the liver increased during the fasting condition in response to glucagon [7]. Otherwise, Attie et al. [8] reported that individuals with T2DM experienced a 20% decrease in the expression of Pgc-1α in skeletal muscle. On the other hand, the expression of Pgc-1α was found decreased in the condition of insulin resistance and obese [4]. The decreased of Pgc-1α expression lead to reduced fatty acid oxidation, resulting in the lipid accumulation and decreased response to insulin, thus worsening the condition of diabetes [9]. Therefore, targeting Pgc-1α pathway may have benefits in the prevention and treatment of metabolic disease, including T2DM [4].
Nutrition has a role as regulator for the expression of Pgc-1α [10]. In 2005 Sparks et al. [11] reported that a high-fat diet may downregulated the expression of Pgc-1α in skeletal muscle either in human or murine. However, dietary strategies increasing Pgc-1α expression remains the question [10]. Lagouge et al. [12] reported that resveratrol, a natural polyphenol mainly found in grape skin, can increase the activation of Pgc-1α. In addition, Short Chain Fatty Acids (SCFAs) especially butyrate has been reported to increase the expression of Pgc-1α both at the mRNA and protein. The ability of butyrate enhances Pgc-1α expression mediated by inhibition of histone deacetylase activity of butyrate [13].
The SCFAs, especially butyrate, can be produced by colonic bacteria through fermentation of resistant starch. Resistant starch is a type of starch that is resistant to digestive enzymes in the gut [14]. Previous study showed that the beneficial effects of resistant starch in improving metabolic syndrome [15]. Lesser yam (Dioscorea esculenta) is one of resistant starch sources, Marsono [16] reported that lesser yam containing resistant starch 10.4 mg/g dry basis. Therefore, the aim of this study was to evaluate the impact of lesser yam flour in Pgc-1α expression in both brown adipose and skeletal muscle in diabetic rats.
Materials and Methods
Preparation of lesser yam flour
Lesser yam flour was prepared according to Richana et al. [17]. In brief, lesser yams were purchased from the local market, then peeled, washed and cut into small pieces. The lesser yams then dried in an oven (50°C for 24 h) and mashed. The lesser yam flour was stored in vacuum bags before use.
Animals and diets
A total of twenty-five male Wistar rats (8 weeks old, ± 180-200 g) were obtained from the Unit of Animal Development for Research, Institut Pertanian Bogor (IPB). Rats were individually housed in a temperature and humidity chamber controlled with good lighting (12 h light/12 h dark). During the acclimatization for 7 days, the rats were fed a standard diet (Table 1).
| Composition | Standard diet (%) | Modification diet lesser yam flour (%) | ||
|---|---|---|---|---|
| 1.25 g | 2.5 g | 5 g | ||
| Casein | 24 | 24 | 24 | 24 |
| DL-Metionine | 0.3 | 0.3 | 0.3 | 0.3 |
| Corn starch | 61 | 52.67 | 44.33 | 27.67 |
| Vitamin Mix AIN 93 | 1 | 1 | 1 | 1 |
| Mineral Mix AIN 93 | 3.5 | 3.5 | 3.5 | 3.5 |
| Choline Chloride | 0.2 | 0.2 | 0.2 | 0.2 |
| Agar-agar | 5 | 5 | 5 | 5 |
| Corn oil | 5 | 5 | 5 | 5 |
| Lesser yam | 8.33 | 16.67 | 33.33 | |
Table 1: Composition of animal diet.
Rats were divided into 5 groups: normal rats (K1), diabetic rats (K2), diabetic rats+lesser yam flour 1.25 g (P1: 1.25 g), diabetic rats+lesser yam flour 2.50 g (P2: 2.5 g), and diabetic rats+lesser yam flour 5.0 g (P3: 5 g). Diabetes was conducted in rats according to Broca et al. [18]. In brief, rats were injected with 65 mg/kg BW streptozotocin (Nacalai Tesque, Japan), 15 minute after being injected with 120 mg/kg BW nicotinamide (Sigma Aldrich, USA). Diabetic was confirmed by plasma glucose after 5 days induction. In this study, rats were considered in diabetic condition if their fasting blood glucose>140 mg/dL. Animal diet was derived from the standard diet by substituting corn flour with lesser yam flour (Table 1). All animals were treated for 4 weeks. Blood was drawn from orbital sinus of the animals before and after treatment with anesthesia. At the end of the treatment, the animals were sacrificed to collect the BAT from interscapular, and skeletal muscle from gastrocnemius. The tissues were fixed in 10% of formalin and embedded in paraffin before analysis. This study was approved by the Medical and Health Research Ethics Committee (MHREC), Faculty of Medicine, Universitas Gadjah Mada.
Biochemical analysis
Plasma glucose was analysed using a commercial kit (Diasys, Holzheim, Germany), according to the manufacturers’ protocol.
Pgc-1α expression in both Brown adipose tissue and skeletal muscle
Around 3 μm paraformaldehyde-fixed tissues with deparafined were committed by xylol I, II, III and then rehydrated with absolute alcohol, 95% and 70% for 3 minute. Streptavidin-peroxydase was inactivated by 1% hydrogen peroxide in methanol for 15 min at 4°C. The tissues were added a specific antibody or primary antibody (bs-1832R; 1: 200 dilution) and incubated overnight at 4°C. The secondary antibody was added and incubated for 30 minutes at room temperature. This study used diaminobenzydine as the chromogen, and hematoxylin meyer as a counterstain and mounting using E-Z mount. The expression of Pgc-1α protein was signed with the brown color in the nucleus of tissues (skeletal and brown adipose). The expression of Pgc-1α was calculated as follows: expression (%)=brown nucleus cell (Pgc-1α)/total cell × 100%.
Statistical analysis
All values are presented as mean ± Standard Deviation (SD). One-way ANOVA was used to analyse differences in levels of plasma glucose and the expression of Pgc-1α protein in both BAT and skeletal muscle between groups. Paired t-test was used to analyse differences in levels of plasma glucose before and after administration of lesser yam flour. Correlation between the plasma glucose levels and the expression of Pgc-1α protein in BAT and skeletal muscle examined by Pearson correlation. Differences were considered statistically significant at p<0.05.
Results
Plasma glucose levels
After 4 weeks of administration of the lesser yam flour, the fasting plasma glucose of the diabetic rats was reduced, and the largest decreased of it were found in the diabetic rats with lesser yam flour 2.5 g. The decreased of the fasting plasma glucose also occur in the diabetic control rats, but it was smallest than it in those with the lesser yam flour. The decreased percentage of the fasting plasma glucose is shown in Table 2.
| Before administration (mg/dL) | After administration (mg/dL) | Reduction (%) | |
|---|---|---|---|
| K1 | 81.32 ± 12.78a | 94.90 ± 13.76a | 16.70% |
| K2 | 146.22 ± 30.90b | 125.67 ± 9.15a | -14.05% |
| P1 | 152.33 ± 20.69b | 79.77 ± 9.07a,b | -47.63% |
| P2 | 152.11 ± 24.30b | 67.14 ± 4.98a,b | -55.86% |
| P3 | 148.81 ± 4.90b | 90.16 ± 35.63a,b | -39.41% |
| p | <0.001 | 0.001 |
Table 2: Plasma glucose levels before and after administration lesser yam flour.
Expression of Pgc-1α in Brown adipose tissue and skeletal muscle
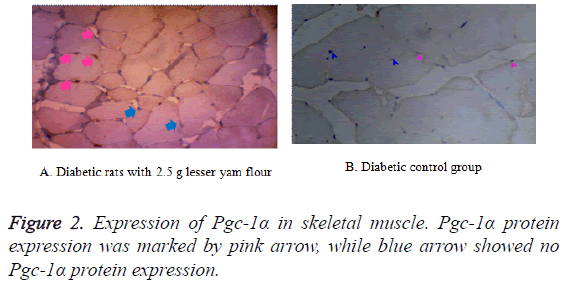
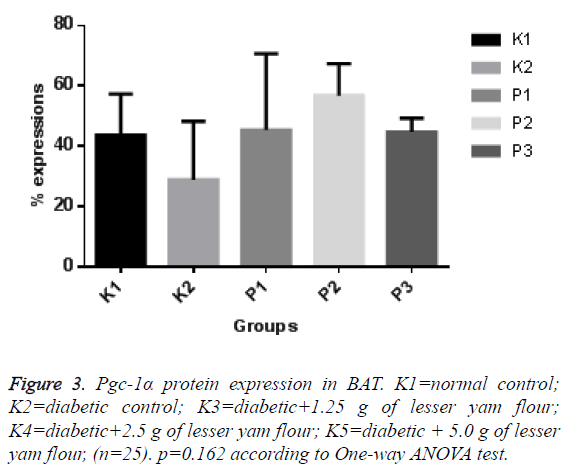
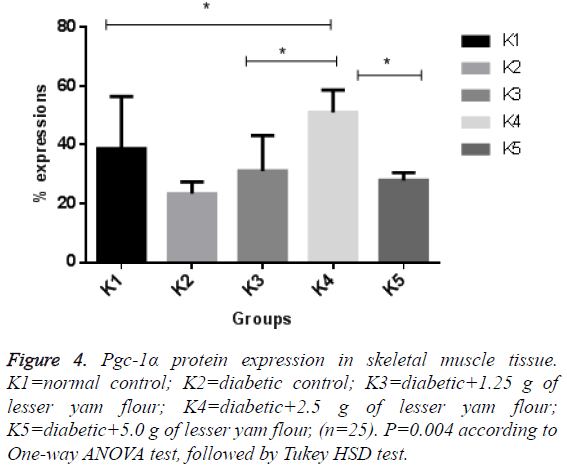
The expression of Pgc-1α in both BAT and skeletal muscle of the diabetic control rats and those with lesser yam flour be shown in Figures 1 and 2. The expression of Pgc-1α in the nucleus cell is marked by brown color. The lowest expression of Pgc-1α protein in BAT was found in the diabetic rats control (K2), while the highest expression was found in the diabetic rats with lesser yam flour 2.5 g (P2), even though they did not differ significantly (p=0.162) (Figure 3). Similar to the expression of Pgc-1α protein in BAT, the lowest expression of Pgc-1α protein in skeletal muscle was found in the diabetic rats control, whereas the highest expression was found in the diabetic rats with lesser yam 2.5 g (P2) (p=0.004) (Figure 4). The correlation between the plasma glucose levels and the expression of Pgc-1α protein in both BAT and skeletal muscle is shown in Table 3. In present study, insignificantly negative correlation between plasma glucose levels seen after 4 weeks of treatment (r=-0.356; p=0.070) for BAT and (r=-0.418; p=0.037) for skeletal muscle.
| BAT | Skeletal muscle | ||
|---|---|---|---|
| Plasma glucose level | Pearson correlation (r) | -0.356 | -0.418 |
| p | 0.07 | 0.037a | |
| N | 25 | 25 | |
Table 3: Correlation between plasma glucose level and Pgc-1α expression in BAT and skeletal muscle.
Discussion
The decreased of the fasting plasma glucose in the diabetic rats after administration of the lesser yam flour for 4 weeks showed that the lesser yam flour has antihyperglycemic. These results are consistent with previous studies; Harijono et al. [19] reported that the administration of water-soluble polysaccharides of lesser yam can lower fasting plasma glucose levels of hyperglycaemic rats. Lesser yam is a good source of dietary fiber and resistant starch [16]. Treatment of dietary fiber and resistant starch in the diabetic subjects were able to reduce plasma glucose levels [20,21]. It is also known that dietary fiber is able to control blood glucose levels through several mechanisms such as slowing of gastric emptying and absorption of nutrients, including glucose [22]. However, in this study, the diabetic rats that received 5 g of lesser yam flour had glucose levels higher than those receiving 1.25 g and 2.5 g of lesser yam flour. This might be due to a high intake of dietary fiber or resistant starch may supply energy through fermentation by colonic bacteria [23]. Resistant starch is one type of starch that resistant to enzymatically degradation in the gut [14], while inulin can be slowly digested [22]. Both carbohydrates can be fermented by colonic bacteria to produce SCFAs, especially butyrate [24]. A previous study showed that administration of sodium butyrate could increase expression of Pgc-1α in BAT and skeletal muscle. Butyrate is able to induce the expression of Pgc-1α due to inhibition of Histone Deacetylation (HDAC) activity. Inhibition of HDAC activates Histone Acetylation (HAT), and opens chromatin DNA for transcription initiation and elongation of Pgc-1α mRNA [13].
In this study, the expression of Pgc-1α protein in both BAT and skeletal muscle in the diabetic rats with the lesser yam flour was higher than in those without the lesser yam flour. It shows that the reduced of the fasting plasma glucose may through increased of the expression of Pgc-1α protein. The expression of Pgc-1α in several organs, mainly BAT and skeletal muscle, represents mitochondrial function in both animal and human model [25]. Recent study suggested that mitochondrial function was closely related to homeostasis of glucose and dysfunction of mitochondrial that found in T2DM and insulin resistance condition [1]. Impaired of mitochondrial function lead to decreased oxidative capacity [26]. Previous report showed in T2DM rats was found decreased of expression of cytochrome C-oxidase (COX), an enzyme that play a role in production Adenosine Triphosphate (ATP), thus result in decreased ATP production [27]. Under a condition of chronic low energy due to impairment mitochondrial respiration, the insulin-independent glucose transport system may possibly upregulated; result in an accumulation of glucose. Increased glucose load is accompanied by a reduction in aerobic glucose oxidation, can lead to excess glucose. Excess glucose can be streamed to gluosamine pathway that leads to insulin resistance. Under hyperinsulinemic condition, glucosamine can cause downregulation of genes that involve in Oxidative Phosphorylation (OXPHOS), including Pgc-1α [8]. Previous research has shown that expression of Pgc-1α decreased in the diabetic subject [28].
Our study also shows that increased the expression of Pgc-1α in both BAT and skeletal muscle are consistent with a decrease in plasma glucose levels, especially in the diabetic rats with lesser yam flour 2.5 g. This may be due to an increase in mitochondrial function in both BAT and skeletal muscle. This is supported by previous study showing an increase in mitochondrial function accompanied by up regulation of Pgc-1α expression [13,29]. Improved of mitochondrial function, especially in the skeletal muscles, can improve insulin sensitivity which mediated by increasing of insulin sensitivity in the skeletal muscle [30,31]. It is showed that lesser yam has hypoglycaemic effect through increased the expression of Pgc-1α, especially in skeletal muscle. The limitations of this study are no measuring SCFAs profile. In conclusion, the lesser yam improves mitochondrial function in the diabetic rats, which characterized by increased expression of Pgc-1α, thus reducing the fasting glucose levels.
Acknowledgement
This research was supported by funding from the Community Fund, Faculty of Medicine, Universitas Gadjah Mada. We also admit to Dianandha Septiana Rubi.
References
- Kim JA, Wei Y, Sowers JR. Role of mitochondrial dysfunction in insulin resistance. Circ Res 2008; 102: 401-414.
- Puigserver P, Spiegelman BM. Peroxisome proliferator-activated receptor-1α coactivator 1a (PGC-1a): transcriptional coactivator and metabolic regulator. Endocrin Rev 2003; 24: 79-90.
- Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E, Ridderstrale M, Laurila E, Houstis N, Daly M, Patterson N, Mesirov JP, Golub TR, Tamayo P, Spiegelman B, Lander ES, Hirschhorn JN, Altshuler D. PGC-1alpha-responsive genes involved in oxidative phosphorylation are co-ordinately downregulated in human diabetes. Nat Genet 2003; 34: 267-273.
- Wu Z, Boss O. Targeting PGC-1 alpha to control energy homeostasis. Expert Opin Ther Targets 2007; 11: 1329-1338.
- Liang H, Ward WF. PGC-1alpha: a key regulator of energy metabolism. Adv Physiol Educ 2006; 30: 145-151.
- Villena JA. New insights into PGC-1 coactivators: redefining their role in the regulation of mitochondrial function and beyond. FEBS J 2015; 282: 647-672.
- Fernandez-Marcos PJ, Auwerx J. Regulation of PGC-1α, a nodal regulator of mitochondrial biogenesis. Am J Clin Nutr 2011; 93: 884-890.
- Attie AD, Kendziorski CM. PGC-1alpha at the crossroads of type 2 diabetes. Nat Genet 2003; 34: 244-245.
- Lukaszuk B, Miklosz A, Chabowski A, Gorski J. Modest decrease in PGC-1α results in TAG accumulation but not in insulin resistance in L6 myotubes. Cell Physiol Biochem 2015; 35: 1609-1622.
- Benton CR, Wright DC, Bonen A. PGC-1alpha-mediated regulation of gene expression and metabolism: implications for nutrition and exercise prescriptions. Appl Physiol Nutr Metab 2008; 33: 843-862.
- Sparks LM, Xie H, Koza RA, Mynatt R, Hulver MW, Bray GA, Smith SR. A high-fat diet co-ordinately downregulates genes required for mitochondrial oxidative phosphorylation in skeletal muscle. Diabetes 2005; 54: 1926-1933.
- Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 2006; 127: 1109-1122.
- Gao Z, Yin J, Zhang J, Ward RE, Martin RJ. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 2009; 58: 1509-1517.
- Higgins JA. Resistant starch: metabolic effects and potential health benefits. J AOAC Int 2004; 87: 761-768.
- Johnston KL, Thomas EL, Bell JD, Frost GS, Robertson MD. Resistant starch improves insulin sensitivity in metabolic syndrome. Diab Med 2010; 27: 391-397.
- Marsono Y. Perubahan Kadar Resisten Starch (RS) dan Komposisi Kimia Beberapa Bahan Pangan Kaya Karbohidrat Dalam Pengolahan. Agritech 2004; 3: 124-127.
- Richana N, Sunarti TC. Karakterisasi sifat fisiokimia tepung umbi dan tepung pati dari umbi ganyong, suweg, ubi kelapa, dan gembili. J Pascapanen 2004; 1: 29-33.
- Broca C, Gross R, Petit P, Sauvaire Y, Manteghetti M, Tournier M, Masiello P, Gomis R, Ribes G. 4-Hydroxyisoleucine: experimental evidence of its insulinotropic and antidiabetic properties. Am J Physiol 1999; 277: 617-623.
- Harijono ET, Sunarharum WB, Suwita IK. Efek Hipoglikemik Polisakarida Larut Air Gembili (Dioscorea esculenta) yang Diekstrak dengan Berbagai Metode. J Teknol dan Industri Pangan 2012; 23: 1-8.
- Chandalia M, Garg A, Lutjohann D, von Bergmann K, Grundy SM. Beneficial effects of high dietary fiber intake in patients with type 2 diabetes mellitus. N Engl J Med 2000; 342: 1392-1398.
- Gargari BP, Namazi N, Khalili M, Sarmadi B, Jafarabadi MA, Dehghan P. Is there any place for resistant starch, as alimentary prebiotic, for patients with type 2 diabetes? Complement Ther Med 2015; 23: 810-815.
- Slavin J. Fiber and prebiotics: mechanisms and health benefits. Nutrients 2013; 5: 1417-1435.
- Nugent AP. Health properties of resistant starch. Nutr Bull 2005; 30: 27-54.
- Cummings JH, Macfarlane GT, Englyst HN. Prebiotic digestion and fermentation. Am J Clin Nutr 2001; 73: 415-420.
- Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev 2004; 84: 277-359.
- Montgomery MK, Turner N. Mitochondrial dysfunction and insulin resistance: an update. Endocr Connect 2015; 4: 1-115.
- Sunarti, Kusuma RJ, Luglio HF. Dioscorea esculenta increase cytochrome c oxidase 1 expression and adenosine triphosphate in diabetic rats. Mediterranean J Nutr Metabol 2015; 8: 217-224.
- Patti ME, Butte AJ, Crunkhorn S, Cusi K, Berria R, Kashyap S, Miyazaki Y, Kohane, I, Costello M, Saccone R, Landaker EJ, Goldfine AB, Mun E, DeFronzo R, Finlayson J, Kahn CR, Mandarino LJ. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: Potential role of PGC1 and NRF1. Proc Natl Acad Sci USA 2003; 100: 8466-8471.
- Henagan TM, Lenard NR, Gettys TW, Stewart LK. Dietary quercetin supplementation in mice increases skeletal muscle PGC1a expression improves mitochondrial function and attenuates insulin resistance in a time-specific manner. PLoS One 2014; 9: e89365.
- Liang H, Balas B, Tantiwong P, Dube J, Goodpaster BH, ODoherty RM, DeFronzo RA, Richardson A, Musi N, Ward WF. Whole body overexpression of PGC-1alpha has opposite effects on hepatic and muscle insulin sensitivity. Am J Physiol Endocrinol Metab 2009; 296: 945-954.
- Winarti S, Harmayani E, Nurismanto R. Karakteristik dan profil inulin beberapa jenis uwi (Dioscorea spp.). Agritech 2011; 31: 2-6.