ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Health Science and Bio Convergence Technology: Edition-II
Joint effects of galaxolide and cadmium on soil microbial community function
Ze Lv1,2, Xiaomin Hu1*, Jing An2 and Wei Wei3
1School of Resources and Civil Engineering, Northeastern University, Shenyang, PR China
2Key Laboratory of Pollution Ecology and Environmental Engineering, Institute of Applied Ecology, Chinese Academy of Sciences, Shenyang, PR China
3School of Municipal and Environmental Engineering, Shenyang Jianzhu University, Shenyang, PR China
- *Corresponding Author:
- Xiaomin Hu
School of Resources and Civil Engineering
Northeastern University, PR China
Accepted date: March 28, 2017
Background: Synthetic musk has been considered as emerging organic pollutant in pharmaceutical and personal care product pollution. Toxicology experiments show that it has potential developmental toxicity and endocrine disrupting effects. However, there is little concern regarding galaxolide and heavy metals as emerging pollutants in medicine and cleaning industry pollution of soil. In this paper, the joint effect of galaxolide and cadmium on soil microbial community function was studied.
Methods: Soil culture experiments and Biolog analyses.
Results: Results showed that the carbon sources utilizing ability of soil microorganisms have been improved under galaxolide and its combination with cadmium. The utilization efficiency of esters was the highest in the six kinds of carbon sources, while it was lower for sugars and acids. Under the joint stress of 500 and 1000 mg•kg-1 galaxolide and cadmium, the Shannon index was significantly increased by 14.04% and 11.51% (p<0.05), the Simpson index was 54.64% and 41.49% (p<0.05), the McIntosh index was 224.12% and 179.51% (p<0.05), respectively.
Conclusions: The utilization of carbohydrate, esters, alcohols and carboxylic acids, as well as species richness and dominant population, were significantly promoted under the joint stress of 500 mg•kg-1 galaxolide and cadmium (p<0.05). The homogeneity of the population was broken after galaxolide addition.
Keywords
Galaxolide (HHCB), Cadmium, Combined pollution, Soil microbial community function
Introduction
Synthetic musk is widely used in pharmaceutical, cosmetics, and personal care products as an additive [1]. Toxicology experiments show that it has potential developmental effects on toxicity and endocrine disrupting [2]. Synthetic musk is able to be accumulated in the body tissue, appear in breast milk, and through the placental barrier to enter the embryo or fetus body [3]. It found that Synthetic musk can also increase the sensitivity of carcinogens and lead to testicular atrophy in animal experiments [4]. Galaxolide (HHCB) is one of the typical synthetic musk. As a new type of pollutants, it has become an important part of the drug and Personal Care Category of Pollutants (PPCPs). Its existence, distribution, migration, transformation and potential toxic effects in the environment have been received more and more concerns by researchers [5,6].
Soil is not only an important part of the ecological environment, but also the main receptor of the complex pollution of many environmental pollutants. The research on soil compound pollution has become one of the hot spots in the field of environment [7,8]. The Galaxolide (HHCB) and Cadmium (Cd) in soil were derived from sewage irrigation and sludge utilization, thus causing the combined pollution. The Combination of HHCB and Cd can enhance the toxicity of single pollution to animals and plants [6,9]. Therefore, it has become a new potential hazard in the environment. Microorganism is an important component of soil ecosystem, which plays a key role in the process of organic matter decomposition, nutrient cycling and plant nutrient utilization [10]. The differences in the utilization of various carbon sources in Biolog micro plate by soil microbes were responded to the differences of microbial community metabolism in soil. The method has been widely used to evaluate the diversity of soil microbial metabolism caused by pollutants [11,12]. At present, there is no report about the research on the microbial functional diversity of HHCB and Cd contaminated soil using Biolog method. In this work, Joint effects of HHCB and Cd on soil microbial community function were studied using Biolog at community level. The paper provides basic data and theoretical basis for establishing the ecological toxicity diagnosis index, risk assessment and restoration criteria.
Materials and Methods
Experimental soil
Brown soil was collected from the surface layer (0-20 cm) of farmland (42º08.606’ N, 123º20.712’ E) in Shenyang City, Liaoning Province in the Northeast of China, which was permitted by the Shenyang Environmental Protection Bureau of China. After transferring samples to our lab, the fresh soil was sieved through a 2 mm mesh, stored at 4°C refrigerator. The general physicochemical properties of the soil and its background concentrations of Cd and HHCB are listed in Table 1.
| pH | CCE/cmol•kg-1 | Organic matter/% | Cd/mg•kg-1 | HHCB/μg•kg-1 | N/mg•kg-1 | P/mg•kg-1 | K/mg•kg-1 | Soil texture/% | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Clay<0.001 mm | Silt (0.001-0.01 mm) | Sand>0.01 mm | ||||||||
| 5.77 | 21.1 | 2.46 | 0.75 | 19.53 | 34 | 18 | 164 | 66.51 | 27.61 | 5.88 |
Table 1. Physical and chemical properties of the soil before experiment.
Experimental method
Soil culture experiments: Each soil microcosm was carried out by the cultivation of two 200 g of the collected soil (spiked with different concentrations of Cd and HHCB). The HHCB stock solution was diluted with acetone to a concentration of 20 g•L-1 to facilitate solubilization in the aqueous system. A given amount of the stock solution of HHCB or CdCl2 was transferred into the soil, stirred, and mixed thoroughly. The mixed soil was put into a fume cupboard until the acetone completely volatilized. The soil samples were then covered with aluminium foil and incubated in a glasshouse for 4 weeks at 25 ± 2°C. During the incubation period, the soil moisture contents of the samples were periodically monitored by weighing and were adjusted to 60% of the WHC by adding sterile water. Samples from each soil microcosm were collected at 4 weeks for the subsequent testing. The final nominal concentrations of Cd and HHCB were 0 or 10 and 0, 100, 500 or 1000 mg•kg-1 dry weight soil, respectively. A control microcosm was also prepared without the addition of HHCB or Cd. Each treatment contained three replicates to minimize experimental errors.
Biolog analyses: Briefly, 5 g of fresh soil was suspended in 45 ml sterile saline solution (0.145 mol•L-1) on a rotary shaker at 180 r•min-1 for 30 min at 25°C. Suspensions were left to settle for 10 min before diluting. Ten-fold serial dilutions were prepared with 0.145 mol•L-1 of sterile saline solution. The 10-3 dilution was used to inoculate the Biolog Eco microplates with 150 μL of the diluted solution added to each well of a Biolog Eco plate. The plates were then sealed and incubated at 28°C in the dark for 10 days, and the absorbance at 590 nm was measured every 24 h. The absorbance of each well was read using an automated Biolog Microplate Reader (Biolog Inc.) at a wavelength of 590 nm (A590). And the data were collected using Microlog 4.01 software (Biolog Inc.). The readings at 72 h of incubation were used for subsequent the analysis of index and carbon source.
Statistical analysis
Average Well Color Development (AWCD) of substrate utilization at A590 was calculated as the average OD across all wells per plate using AWCD.
AWCD=(Σ(ni))/31
Three types of index computation formula are as follows [13]:
Shannon index (H’)=-ΣPi × ln (Pi);
Simpson index (D)=1/(Σ(Pi)2);
McIntosh index (U)=(Σni2).
Where ni represents the relative optical density of the ith well which was corrected by color development in the control well (water only).
Where pi is the ratio of individual carbon source utilization against the sum utilization.
The data from the interaction between HHCB and Cd was analysed by a two-way ANOVA with SPSS 20.0 software for Windows. All values were mean ± SD of three replicates. Data in the same column followed by different letters were significantly different (P<0.05). The experimental figures were plotted by Origin 8.
Results
The effect of soil microbial population activity under the stress of HHCB and Cd
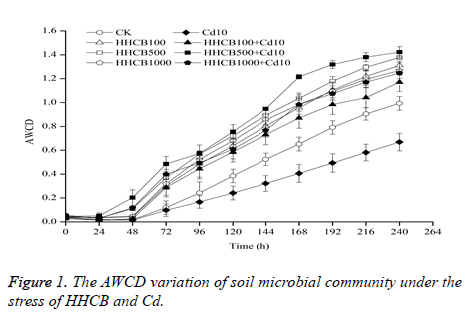
Average Well Color Development (AWCD) is an important indicator to reflect the metabolic activity of soil microbes. The carbon source utilization of microbial biomass can be characterized by the AWCD value [14]. The AWCD variation of soil microbial community under the stress of HHCB and Cd are shown in Figure 1.
CK: 0 mg•kg-1 HHCB+0 mg•kg-1 Cd; HHCB100: 100 mg•kg-1 HHCB; HHCB: 500 mg•kg-1 HHCB; HHCB1000: 1000 mg•kg-1 HHCB; Cd10: 10 mg•kg-1 Cd; HHCB100+Cd10: 100 mg•kg-1 HHCB+10 mg•kg-1 Cd; HHCB500+Cd10: 500 mg•kg-1: HHCB+10 mg•kg-1 Cd; HHCB1000+Cd10: 1000 mg•kg-1 HHCB+10 mg•kg-1 Cd. The same below.
As shown in Figure 1, under the stress of HHCB, the overall change trend of the AWCD value is HHCB500>HHCB1000>HHCB100>CK. The AWCD values of HHCB100, HHCB500 and HHCB1000 were higher than those of blank control for about 31.97%~309.62%, 17.19%~315.75% and 24.73%~287.56%. And under the stress of HHCB and Cd, the overall change trend of the AWCD value is HHCB500 + Cd10 > HHCB1000 + Cd10 > HHCB100 + Cd10 > CK > Cd10. The AWCD values of HHCB100 + Cd10, HHCB500 + Cd10 and HHCB1000 + Cd10 were higher than those of blank control for about 15.10%~141.12%, 23.11%~967.37% and 5.46%~516.84%. The change trend is consistent with the single HHCB; it showed that the promoting effect of HHCB on AWCD was not affected by Cd. However, the AWCD value was increased. This demonstrated that the soil microorganisms kept higher activity, HHCB and Cd pollution which stimulated the reproduction of soil microbes, enhanced the ability of carbon source utilization, and kept the soil microorganisms activity higher.
The effect of soil microbial population diversity indexes under the stress of HHCB and Cd
The effect on Shannon richness index: For Biolog Eco microplate, the higher Shannon index, the more carbon source species available for soil microbial and higher the species richness. As shown in Table 2, the Shannon index was significantly higher than the blank control 12.25% and 11.02% under the single stress of 500 and 1000 mg•kg-1 HHCB (p<0.05). The Shannon index was significantly higher than the blank control 14.04% and 11.51% under the joint stress of 500 and 1000 mg•kg-1 HHCB and Cd (p<0.05). The soil microbial metabolic function was improved and the species richness was increased after HHCB addition.
| Treatment | Shannon index (H’) | Simpson index (D)•10-1 | McIntosh index (U)•10-1 |
|---|---|---|---|
| CK | 2.69 ± 0.18c | 11.62 ± 1.81c | 1.09 ± 0.46bc |
| HHCB100 | 2.89 ± 0.04abc | 13.99 ± 1.64abc | 2.43 ± 0.67abc |
| HHCB500 | 3.01 ± 0.02ab | 16.70 ± 0.53ab | 2.80 ± 0.09ab |
| HHCB1000 | 2.98 ± 0.06ab | 16.01 ± 1.46ab | 2.47 ± 0.30abc |
| Cd10 | 2.78 ± 0.05bc | 11.23 ± 1.05c | 0.89 ± 0.45c |
| HHCB100+Cd10 | 2.80 ± 0.06bc | 12.81 ± 1.37bc | 2.39 ± 0.89abc |
| HHCB500+Cd10 | 3.06 ± 0.03a | 17.98 ± 0.56a | 3.52 ± 0.77a |
| HHCB1000+Cd10 | 2.99 ± 0.05ab | 16.45 ± 0.85ab | 3.04 ± 0.27a |
Table 2. Diversity indices of soil microbial community.
The effect on Simpson dominance index: The Simpson dominance index reflects the changes in the number of species population, the greater the index, the more uneven distribution of species within the community, the dominant species of the more prominent position. As shown in Table 2, the Simpson index was significantly higher than the blank control 43.67% and 37.73% under the single stress of 500 and 1000 mg•kg-1 HHCB (p<0.05). The Simpson index was significantly higher than the blank control 54.64% and 41.49% under the joint stress of 500 and 1000 mg•kg-1 HHCB and Cd (p<0.05). The number of species in the community was not uniform and the dominant population was increased in the soil after HHCB addition.
The effect on McIntosh homogeneity index: The McIntosh diversity index is based on the diversity index of Euclidian distance in the soil microbial community. As shown in Table 2, the McIntosh index was significantly higher than the blank control 224.12% and 179.51% under the joint stress of 500 and 1000 mg•kg-1 HHCB and Cd (p<0.05). The McIntosh index was significantly increased and the homogeneity of the population was broken after HHCB addition.
The effects on carbon source utilization potential of the soil microbial community under the stress of HHCB and Cd
Biolog Eco microplate containing 31 carbon sources, the carbon source will be divided into 6 categories according to the different functional groups, carbohydrates, amino acids, esters, alcohols, amines and carboxylic acids. Utilization of carbon substrates by soil microbial community under the stress of HHCB and Cd are shown in Table 3. As shown in Table 3, polymers were the most strongly utilized carbon source by the soil microbial community, and carbohydrates and carboxylic acids were the least utilized. Compared with the blank control, carbohydrate utilization was significantly promoted under the single stress of 500 mg•kg-1 HHCB and the joint stress of 500 mg•kg-1 HHCB and Cd (p<0.05). Esters utilization was significantly promoted under the joint stress of 500 mg•kg-1 HHCB and Cd (p<0.05). Alcohols utilization was significantly promoted under the joint stress of 500 and 1000 mg•kg-1 HHCB and Cd (p<0.05). Carboxylic acids utilization was significantly promoted under the single stress of 500 and 1000 mg•kg-1 HHCB, and it was significantly promoted under the joint stress of 500 and 1000 mg•kg-1 HHCB and Cd (p<0.05). The utilization of carbohydrate, esters, alcohols and carboxylic acids were significantly promoted under the joint stress of 500 mg•kg-1 HHCB and Cd (p<0.05). There were no significant effects on amino acids and amines utilization under the single and joint stress of HHCB and Cd (p>0.05).
| Treatment | Carbohydrate•10-1 | Amino acids•10-1 | Alcohols•10-1 | Esters•10-1 | Amines•10-1 | Carboxylic acid•10-1 |
|---|---|---|---|---|---|---|
| CK | 0.25 ± 0.09c | 1.49 ± 0.81a | 3.04 ± 1.34b | 1.69 ± 1.02c | 1.40 ± 0.83a | 0.61 ± 0.2d |
| HHCB100 | 0.71 ± 0.14c | 4.39 ± 0.90a | 6.63 ± 1.52ab | 2.83 ± 0.75abc | 4.59 ± 1.51a | 1.66 ± 0.45cd |
| HHCB500 | 3.82 ± 0.44a | 2.84 ± 0.10a | 7.22 ± 0.21ab | 3.76 ± 0.59abc | 3.59 ± 0.76a | 2.46 ± 0.13bc |
| HHCB1000 | 1.06 ± 0.35bc | 3.90 ± 0.10a | 7.48 ± 0.56ab | 3.34 ± 0.61abc | 3.61 ± 0.97a | 2.18 ± 0.28c |
| Cd10 | 0.20 ± 0.03c | 1.14 ± 0.54a | 3.15 ± 1.85b | 0.85 ± 0.50c | 0.97 ± 0.80a | 0.47 ± 0.24d |
| HHCB100+Cd10 | 0.66 ± 0.32c | 4.79 ± 0.33a | 6.29 ± 2.43ab | 2.58 ± 1.12bc | 4.25 ± 1.93a | 1.28 ± 0.59cd |
| HHCB500+Cd10 | 3.58 ± 0.21ab | 4.70 ± 0.80a | 7.84 ± 0.79a | 5.91 ± 1.62a | 4.47 ± 1.09a | 4.32 ± 0.68a |
| HHCB1000+Cd10 | 1.76 ± 0.21abc | 3.98 ± 0.55a | 7.09 ± 0.17ab | 5.35 ± 1.03ab | 4.71 ± 1.31a | 3.60 ± 0.16ab |
Table 3. Utilization of carbon substrates by soil microbial community under the stress of HHCB and Cd.
Discussion
The AWCD value of soil microbial community was higher than that of the blank control under the stress of HHCB alone and in combination with Cd. The results showed that the addition of HHCB improved the ability of soil microorganisms to utilize carbon sources. HHCB is a typical polycyclic musk and has the similar structure and effect with hormones. The research found that hormones can stimulate biological growth [6], so, we think that the HHCB also can stimulate the growth of soil microorganisms, and improve the utilization of carbon sources. Some organic pollutants can enhance the metabolic activity of microorganisms, and improve the utilization of carbon sources. It was found that the uterine cavity of female rats treated with HHCB had been expanded in chronic experiments [15]. Our results are consistent with previous study, which found that AWCD values were higher than that of the blank control and improved the ability of soil microorganisms to utilize carbon sources under the bensulfuron methyl pollution [16] and the high concentration fomesafen pollution [17].
The Shannon, Simpson and McIntosh indices were significantly promoted under the joint stress of 500 and 1000 mg•kg-1 HHCB and Cd. It indicated that the species richness and the dominant population were significantly promoted in the compound contaminated soil, and the homogeneity of the population was broken after HHCB addition. This phenomenon may be due to the improvement of the microbial metabolic function of compound contaminated soil and the species of carbon source utilization by HHCB. Also, some bacteria can promote its own growth and reproduction rate using a single carbon source. The characterization bacteria, which can make full use of a single carbon source, may become the dominant population and destroy the population uniformity of the soil. The other possibility is that the degradation of HHCB in soil, the biodegradation of HHCB has been supported and confirmed by many studies [18,19]. Sikkema [20] and his team worker has put forward the toxic effect of anesthesia hypothesis. They thought that the fat-soluble compounds would combine with fat-soluble molecules on the bacteria cell membrane, thus affecting the membrane structure and penetrating. In the same way, HHCB is a fat-soluble compound. It may also change the permeability of cell membrane, which makes it easier for Cd to get into the microbial cells, and improve the microbial toxicity of Cd. There were some other people who thought that Cd has inhibitory effect on soil microbial activity, it may decline the number and activity of microorganisms in soil, leading to the inhibition of microbial biodegradation, resulting in longer retention time of HHCB in soil [21]. Chen [22] has reported that HHCB can improve other toxic effects by disrupting cellular defense system and produce inhibitory effect of xenobiotic system for a long time, leading to the reducing of the cell's ability to resist exogenous chemicals and resulting in amplification of the toxic effects of exogenous chemicals. The existence of HHCB can promote the activity of these microorganisms and it leads to the increase of the carbon sources utilization and the three indexes.
The utilization efficiency of esters was the highest, while sugars and acids have lower utilization efficiency compared with others 6 kinds of carbon sources. A previous study reported that carbohydrates were the carbon source most strongly utilized by soil microbes under the stress of the veterinary antibiotic oxytetracycline [23]. This discrepancy may be attributed to the different chemical characteristics and compositions of HHCB and oxytetracycline. On the other hand, the mechanism of combined toxicity of compound pollution is obviously different from that of single pollution.
Conclusions
The ability of utilizing carbon sources in soil microorganisms has improved under HHCB and its combination with Cd. The utilization efficiency of esters was the highest, while sugars and acids have lower utilization efficiency compared with other 6 kinds of carbon sources. The utilization of carbohydrate, esters, alcohols and carboxylic acids were significantly promoted under the joint stress of 500 mg•kg-1 HHCB and Cd (p<0.05).
The Shannon, Simpson and McIntosh indices were significantly promoted under the joint stress of 500 and 1000 mg•kg-1 HHCB and Cd (p<0.05). The species richness and the dominant population were significantly promoted in the compound contaminated soil and the homogeneity of the population was broken after HHCB addition.
Acknowledgements
We gratefully acknowledged financial supports provided by the National Natural Science Foundation of China (Grantor. 21277150 and 31370523).
References
- Wang M, Peng C, Chen W, Markert B. Ecological risks of polycyclic musk in soils irrigated with reclaimed municipal wastewater. Ecotoxicol Environ Saf 2013; 97: 242-247.
- Van der Burg B, Schreurs R, van der Linden S. Endocrine effects of polycyclic musks: do we smell a rat. Int J Androl 2008; 31: 188-193.
- Lee S, Kim S, Park J, Kim HJ, Lee JJ. Synthetic musk compounds and benzotriazole ultraviolet stabilizers in breast milk: Occurrence, time-course variation and infant health risk. Environ Res 2015; 140: 466-473.
- Melmed S. Fertility and fragrance: another cause of Kallmann syndrome. J Clin Invest 2015; 125: 2275-2278.
- Liu S, Zhou Q, Wang Y. Ecotoxicological responses of the earthworm Eisenia fetida exposed to soil contaminated with HHCB. Chemosphere 2011; 83: 1080-1086.
- Chen C H, Zhou Q X, Cai Z. Effects of soil polycyclic musk and cadmium on pollutant uptake and biochemical responses of wheat (Triticum aestivum). Arch Environ Contam Toxicol 2010; 59: 564-573.
- Long XX, Zhang YG, Jun D. Zinc, cadmium and lead accumulation and characteristics of rhizosphere microbial population associated with hyperaccumulator Sedum alfredii hance under natural conditions. Bull Env Contam Toxicol 2009; 82: 460-467.
- Lu M, Zhang ZZ, Wang JX, Zhang M, Xu YX. Interaction of heavy metals and pyrene on their fates in soil and tall fescue (Festuca arundinacea). Environ Sci Technol 2014; 48: 1158-1165.
- Chen F, Gao J, Zhou QX. Toxicity assessment of simulated urban runoff containing polycyclic musks and cadmium in Carassius auratus using oxidative stress biomarkers. Env Pollut 2012; 162: 91-97.
- Kirk JL, Beaudette LA, Hart M, Moutoglis P, Klironomos JN. Methods of studying soil microbial diversity. J Microbiol Methods 2004; 58: 169-188.
- Cebron A, Arscne-Ploetze F, Bauda P, Bertin PN, Billard P. Rapid impact of phenanthrene and arsenic on bacterial community structure and activities in sand batches. Microb Ecol 2014; 67: 129-144.
- Liu B, Li YX, Zhang XL. Effects of chlortetracycline on soil microbial communities: Comparisons of enzyme activities to the functional diversity via Biolog EcoPlates (TM). Eur J Soil Biol 2015; 68: 69-76.
- Hu JL, Lin XG, Wang JH. Microbial functional diversity, metabolic quotient, and invertase activity of a sandy loam soil as affected by long-term application of organic amendment and mineral fertilizer. J Soils Sediments 2011; 11: 271-280.
- Haack SK, Garchow H, Klug MJ. Analysis of factors affecting the accuracy, reproducibility, and interpretation of microbial community carbon source utilization patterns. Appl Env Microbiol 1995; 61: 1458-1468.
- Api AM, Ford RA. Evaluation of the oral subchronic toxicity of HHCB (1,3,4,6,7,8-hexahydro-4,6,6,7,8,8-hexamethylcyclopenta-gamma-2 -benzopyran) in the rat. Toxicol Lett 1999; 111: 143-149.
- Zhang WW, Xu J, Dong FS. Responses of microbial community functional diversity to bensulfuron-methyl in paddy soil. J Agro Env Sci 2014; 33: 1749-1754.
- Wu XH. Effects of fomesafen on the soil microbial diversity. Beijing Inst Plant Protect Chin Acad Agr Sci 2014; 53-55.
- Kuhlich P, Gostl R, Teichert P. Transformations of polycyclic musks AHTN and HHCB upon disinfection with hypochlorite: two new chlorinated disinfection by-products (CDBP) of AHTN and a possible source for HHCB-lactone. Analyt Bioanalyt Chem 2011; 399: 3579-3588.
- Bester K. Retention characteristics and balance assessment for two polycyclic musk fragrances (HHCB and AHTN) in a typical German sewage treatment plant. Chemosphere 2004; 57: 863-870.
- Sikkema J, de Bont JA, Poolman B. Interactions of cyclic hydrocarbons with biological membranes. J Biol Chem 1994; 269: 8022-8028.
- Maliszewska-Kordybach B, Smreczak B. Habitat function of agricultural soils as affected by heavy metals and polycyclic aromatic hydrocarbons contamination. Env Int 2003; 28: 719-728.
- Chen F, Gao J, Zhou Q. Toxicity assessment of simulated urban runoff containing polycyclic musks and cadmium in Carassiusauratus using oxidative stress biomarkers. Environ Pollut 2012; 162: 91-97.
- Kong WD, Zhu YG, Fu BJ. The veterinary antibiotic oxytetracycline and Cu influence functional diversity of the soil microbial community. Env Pollut 2006; 143: 129-137.