ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 11
Investigation of autophagic effects of melatonin on breast cancer stem cells
Huseyin Donmez1, Nadir Kocak1* and Ibrahim Halil Yildirim2
1Department of Medical Genetics, Faculty of Medicine, Selcuk University, Konya, Turkey
2Department of Genetics, Faculty of Veterinary Sciences, Dicle University, Diyarbakir, Turkey
- *Corresponding Author:
- Nadir Kocak
Department of Medical Genetics
Faculty of Medicine
Selcuk University, Turkey
Accepted on April 1, 2017
Autophagy plays important roles in physiologic cellular events and also in cancers. It has been reported that cells escaped from death via autophagy and if autophagy inhibited, cells conducted to apoptosis. In some studies, protective effects of the melatonin on induced autophagy in cancer cells reported. In this study, we aimed to evaluate the autophagic effect of melatonin in cancer stem cells. For this purpose, CD44+/CD24- phenotype cells sorted from melatonin-treated and untreated MCF-7 and HEK293 cells. Effect of melatonin on autophagy was analysed by immunofluorescence and western blot analysis. Results showed that melatonin-induced LC3 (Microtubule-associated protein 1A/1B-light chain 3) aggregation and formation of autophagic vacuoles in MCF-7 derived cancer stem cells and also induced LC3-I to LC3-II conversion in these stem cells when compared to untreated control group. In conclusion, melatonin showed a pro-autophagic effect in CD44+/CD24- stem cells derived from MCF-7 cells and anti-autophagic effect in CD44+/CD24- stem cells obtained from HEK293 cells.
Keywords
Autophagy, MCF-7, HEK293, Melatonin, Cancer stem cells
Introduction
Cancer continues to be one of the major health problems as the most common cause of death after cardiovascular diseases. Lung cancer comes first in the incidence of death followed by stomach, liver, colorectal and breast cancers [1]. In the last 30 years, effective treatment methods are tried to be against cancer in addition to studies trying to understand formation and development mechanisms of cancer.
Cancer studies conducted have shown that cancer is created by a group of cells with stem cell properties [2,3]. It has been determined that these cells named Cancer Stem Cells (CSCs) and found in the total tumor mass have an intrinsic or acquired resistance to cytotoxic agents and radiation. The mechanisms underlying this resistance are reported as an increase in DNA damage recognition and repair capabilities, lack of activity in the apoptotic pathway, lack of chemotherapeutic agents into cells or increase in the excretion of these agents out of the cell [4,5]. However, in some tissues, cancer stem cells are observed to be in silent mode by slowing down the cell cycles and exhibiting a dormant character [6,7].
Since cancer stem cells show resistance to conventional chemotherapeutic methods and they become dormant, alternative compounds that do not harm normal cells are being investigated. In a study that we have conducted, we have observed that melatonin, which is a natural compound, induces apoptosis and cell differentiation in cancer stem cells. In studies conducted by other groups, it was reported that melatonin protected cancer cells from induced autophagy [8]. In a study conducted by Koh, melatonin has been shown to prevent ischemic brain damage with activation of the mTOR signaling pathway [9]. The mTOR protein is known to have a central regulatory role in the cell growth and cell cycle progression, and the mTOR pathway is thought to have an inhibitory effect on the autophagy [9].
In many studies investigated the relationship between cancer and autophagy, it was observed that there was an increase in the autophagy in tumor cells with no growth factors, cells escaped death with autophagy, cells died by apoptosis when autophagy in cells was inhibited [10,11]. In this study, we tried to understand whether melatonin can be used in the elimination of cancer stem cells and cancer treatment as an alternative treatment method by presenting the effect of melatonin, which induces autophagy in normal cancer cells, on cancer stem cells.
Material and Methods
Cell culture
MCF-7 human breast cancer cell line and HEK293 human embryonic kidney cell line were obtained from ATCC (American Type Culture Collection). Both cell types cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum, 1% L-glutamine and 1% streptomycin-penicillin at 37°C in a 5% CO2 humidified atmosphere.
Assessment of cell viability
Cell viability analysis carried out by MTT (Thiazolyl Blue Tetrazolium Bromide) assay. For this purpose, approximately 104 cells added into 100 μl culture medium and incubated in 96 well-plate at 37°C in 5% CO2. Cells washed twice with PBS after the removing the culture medium from the plate and incubated for 24 h in serum-free medium for synchronizing the mitotic cycle of each cell in wells. After the synchronizing procedure, cells treated with 100 μl of the medium that contains various concentrations of melatonin for 24, 48 and 72 h. Then, 10 μl 12 mM Thiazolyl Blue Tetrazolium Bromide (MTT; Sigma Aldrich®M2128) solution added to each well and incubated for 4 h. Absorbance were measured at 490 nm wavelength in the microplate reader (Biotek, USA).
Stem cell isolation
Stem cell isolation was carried out by using CD44/CD24 surface markers. CD44+/CD24- stem cells isolated by flow cytometry from the cells that treated with melatonin and C2Cer. Counted cells transferred to FACS test tubes as desired amounts. Cells in the FACS tubes centrifuged in 250 Xg for 5 minutes and then pellets suspended in 100 μl %2 FBS/HBSS. Twenty microliters of each conjugated antibodies (CD44-APC, Sigma-Aldrich Corporation, Germany; CD24-FITC, Sigma- Aldrich Corporation, Germany) were added to test tubes in the dark for 1 × 106 cells. Cells remained in the dark and rinsed for every 10 minutes for an hour at 4°C. Unconjugated antibodies washed with 3 ml 2% FBS/HBSS. Cell pellets diluted with 400 μl DAPI (0.4 ng/ml) (Sigma-Aldrich Corporation, Germany) and separated by FACS machine (FACS AriaIII-BD Pharmingen, ABD).
Sphere formation
After the CD44+/CD24- phenotype cells sorted from MCF-7 and HEK293, cells seeded to the media that not contain serum (1% PC+RPMI-1640) and transferred to 5% CO2, 37°C incubator. Cells cultured 45-60 days for tumor phase formation. At the end, tumor phase formations analysed and monitored.
Autophagy analysis
Immunofluorescence and western blot analysis performed to analyse the autophagic effect of melatonin on cancer stem cells. LC3-II aggregation determined by immunofluorescence analysis and LC3-I and II proteins analysed by western blot. MCF-7 and HEK293 cells treated for 48 h with 35 μM melatonin and 25 μM C2Cer which is autophagy marker. Then, CD44+/CD24- stem cells isolated and treated with labeled antibodies. Autophagy vacuoles analysed under the fluorescence microscopy.
Western blot analysis
Total protein extraction performed by a commercial kit (Millipore, cat. no: 2910) according to the manufacturer instructions. Protein samples were incubated at 95°C for 5 minutes after the addition of the same volume of 4X lammeli buffer. An amount of 20 μg of total protein was loaded onto each well of the 4-12% gradient gel and run at 40 mA electric current for 45 min. After the electrophoresis, proteins transferred onto PVDF membrane by using Trans- BlotRTurboTM Transfer system (Bio-Rad Laboratories, Inc., #170-4155) in 1.3 A electrical current. The immune blotting was accomplished by the incubation of the membrane with 1 μg/ml of primary antibody solution overnight and then 0.1 μg/ml of secondary antibody for 2 h at room temperature. At the end of the blotting, the membrane was incubated in Immune-star Western Chemiluminescent (Bio-Rad Laboratories, Berkeley, USA) substrate solution for 10 min and then exposed to X-ray film (Fujifilm, Tokyo, Japan) for one minute and imaged by using an X-ray film processor (Ecomax, Germany).
Statistical analysis
Statistical analyses were performed using GraphPad Prism 6.0 software (GraphPad Software Inc., La Jolla, CA, USA). Intra-group comparisons investigated by using dependent t test and difference between groups investigated by ANOVA test. Tests considered a basic significance level of p<0.05.
Results
Proliferation and viability test optimizations
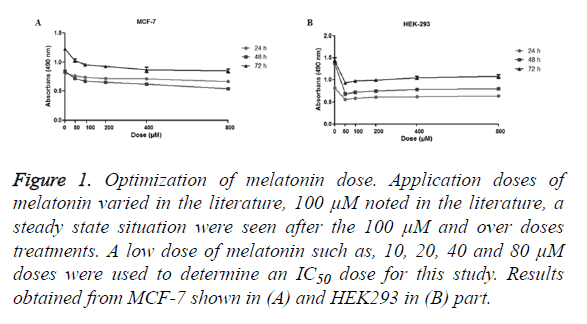
The MTT assay used to assess the cell viability. The dosage of the melatonin determined consistent with the literature, 100 μM and diluted concentrations were used (Figure 1). Results showed cells were stable over the 100 μM, so diluted dosages of melatonin (10, 20, 40, 80 μM) were used.
Figure 1. Optimization of melatonin dose. Application doses of melatonin varied in the literature, 100 μM noted in the literature, a steady state situation were seen after the 100 μM and over doses treatments. A low dose of melatonin such as, 10, 20, 40 and 80 μM doses were used to determine an IC50 dose for this study. Results obtained from MCF-7 shown in (A) and HEK293 in (B) part.
Melatonin reduces viability and cell proliferation in cancer cells
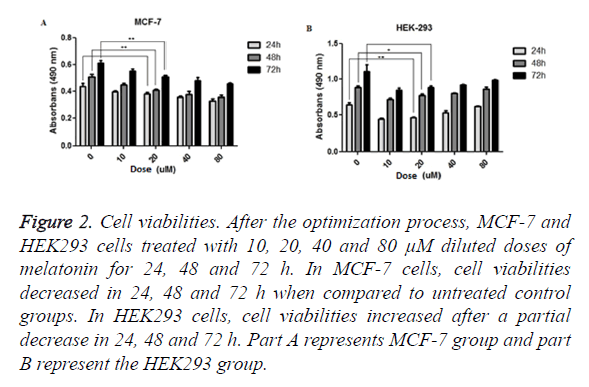
Viability analysis carried out by MTT assay. MCF-7 and HEK293 cells treated with various concentrations of melatonin (10, 20, 40, 80 μM) for periods of 24, 48 and 72 h. Results showed that melatonin reduces the viability of tumorigenic MCF-7 cells when compared to the untreated group of cell in the period of 24 (p=0.06549), 48 (p=0.0037) and 72 (p=0.0088) h. In melatonin-treated HEK293 cells, when compared with untreated cells, cell viability was increased through the time; 24 (p=0.0039), 48 (p=0.0341) and 72 (p=0.0599) h (Figure 2).
Figure 2. Cell viabilities. After the optimization process, MCF-7 and HEK293 cells treated with 10, 20, 40 and 80 μM diluted doses of melatonin for 24, 48 and 72 h. In MCF-7 cells, cell viabilities decreased in 24, 48 and 72 h when compared to untreated control groups. In HEK293 cells, cell viabilities increased after a partial decrease in 24, 48 and 72 h. Part A represents MCF-7 group and part B represent the HEK293 group.
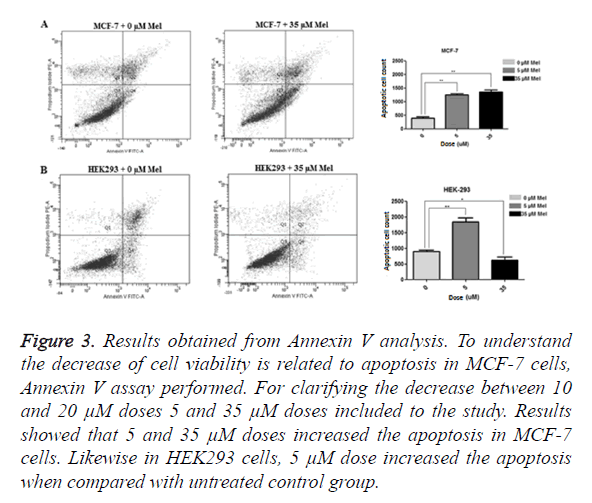
Annexin V analysis performed to determine whether the decrease in MCF-7 cell is related to apoptosis or not. To confirm the decrease in 10 and 20 μM, 5 and 35 μM dosages added to the study and MCF-7 and HEK293 cells treated for 48 h. Results showed 5 μM (p=0,0040) and 35 μM (p=0,0060) increased the apoptosis in MCF-7 cells. Likewise, increased apoptosis rate observed in HEK293 cells when compared to control group in 5 μM (p=0.0025) but, in 35 μM (p=0.0324) treated group, apoptosis was decreased (Figure 3). Efficient outcomes were taken in 35 μM concentration for both cell types and further studies done with this dosage.
Figure 3. Results obtained from Annexin V analysis. To understand the decrease of cell viability is related to apoptosis in MCF-7 cells, Annexin V assay performed. For clarifying the decrease between 10 and 20 μM doses 5 and 35 μM doses included to the study. Results showed that 5 and 35 μM doses increased the apoptosis in MCF-7 cells. Likewise in HEK293 cells, 5 μM dose increased the apoptosis when compared with untreated control group.
Determining the number of stem cells and sphere formation
The amount of CSCs determined against to the CD44/CD24 surface marker in flow cytometry. Also, the existence of stem cells verified by tumor phase (sphere) formation technique. In MCF-7 cell line CD44+/CD24- combination was 1, 2% and in HEK293 was 1, 1% found.
Melatonin reduces the amount of cancer stem cells
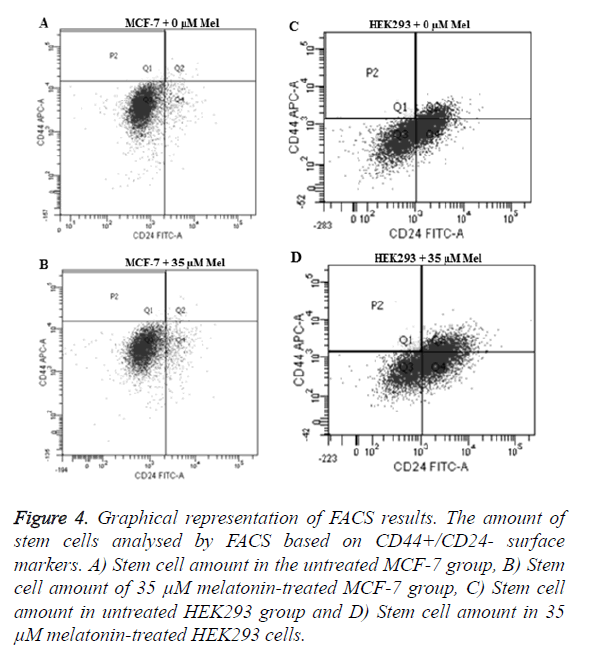
CD44+/CD24- cells isolated and assessed after the 35 μM melatonin treatment for 48 h. Results showed a decrease of CSCs in MCF-7 cells (p=0.0106) and an increase in HEK293 cells (p=0.0493) when compared to the control groups (Figure 4).
Figure 4. Graphical representation of FACS results. The amount of stem cells analysed by FACS based on CD44+/CD24- surface markers. A) Stem cell amount in the untreated MCF-7 group, B) Stem cell amount of 35 μM melatonin-treated MCF-7 group, C) Stem cell amount in untreated HEK293 group and D) Stem cell amount in 35 μM melatonin-treated HEK293 cells.
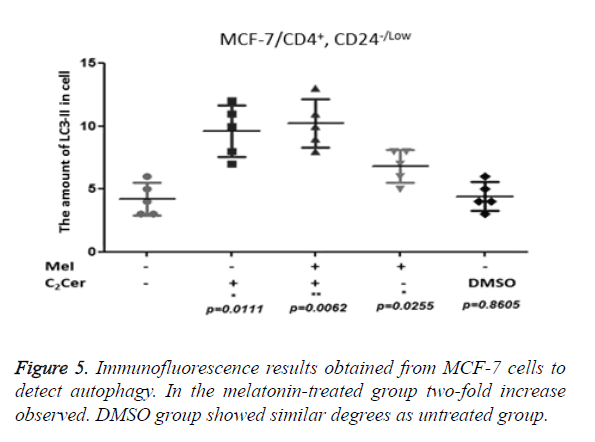
Melatonin induces LC3 aggregation and formation of autophagic vacuoles in Breast cancer stem cell line
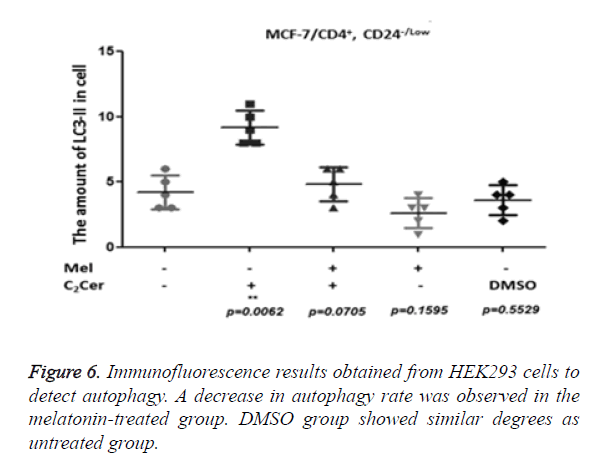
LC3-II aggregation determined by immunofluorescence in CD44+/CD24- cells after the melatonin, C2Cer, and DMSO treatment. An untreated group used as control group. Immunofluorescence results of MCF-7 cells showed a two-fold increase in the melatonin-treated group (p=0.0255) but the increase of autophagy in the melatonin-treated cells was not higher than the C2Cer-treated group. In the DMSO-treated group, an increase of autophagy detected as similar to the untreated group (p=0.8605) (Figure 5). In melatonin-treated CD44+/CD24- HEK293 cells, a decrease observed in autophagy when compared to untreated control group (p=0.1595). DMSO treated group showed similar degrees with the untreated control group (p=0.5529) (Figure 6).
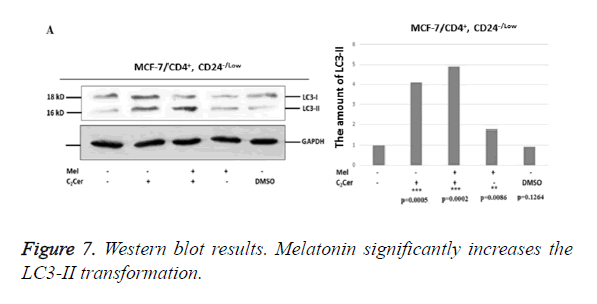
Melatonin induces LC3-I to LC3-II conversion in breast cancer stem cells
Western blot results showed an increase in conversion of LC3-I to LC3-II in melatonin-treated CD44+/CD24- stem cells derived from MCF-7 cells when compared to untreated group (p=0,0086). The most determined formation was the LC3-II formation in the combined treatment of C2Cer and melatonin (p=0.0002). In DMSO group, like immunofluorescence results, similar band densities observed in DMSO-exposed and untreated control group (p=0.1264). When results considered altogether, melatonin showed an autophagic effect on the CD44+/CD24- cancer stem cells that derived from MCF-7 cells (Figure 7).
In HEK293 cells, conversion of LC3-I to LC3-II seen decreased by half when melatonin-treated and untreated groups compared (p=0.0089). In C2Cer and melatonin combined group, LC3-II formation was similar to the untreated control group (p=0.1370) and this decrease was considered is a result of melatonin. DMSO group showed similar band density to the untreated control group. Results obtained from HEK293 cells demonstrate more efficiently that melatonin prevents autophagy in CD44+/CD24- stem cells.
Discussion
The synthesis of melatonin from the pineal gland exhibit a circadian rhythm. It is blood level is highest at night and decrease in daytime. This circadian feature is seen in almost all animals in a similar way regardless of time of daily activity [12]. It is reported that decreased function of the pineal leads to hyperestrogenism by reducing the secretion of melatonin and this may lead to breast cancer [12]. Melatonin is reported to have an oncostatic and antiproliferative effect on breast cancer development [13]. The positive effect of melatonin on the reduction of proliferation of cancer cells was shown by several studies [14]. However, its effect on stem cells is still unknown. In this study, the autophagic effect of melatonin on stem cells with CD44+/CD24- obtained from a breast cancer cell line MCF-7 was investigated. The same study was conducted on embryonic kidney cell line HEK293 cells in order to understand its effect on non-cancerous stem cells.
In the studies conducted on MCF-7 human breast cancer cells, it has been shown that the transition of the cell cycle from G0- G1 phase to S phase is blocked at physiological melatonin concentrations and this inhibits cell proliferation induced by estradiol [15]. In the literature, the doses administered are observed to be varying. Therefore, in our study, the viability analysis was performed in order to determine the common IC50 dose required for MCF-7 and HEK293 cells. As a result of analysis of viability, when groups with and without melatonin in MCF-7 cells were compared, a significant decrease was observed in viability as a function of time in the melatonintreated group. On the other hand, in HEK293 cells, there was an increase in viability depending on time in the melatonintreated group. These results are consistent with findings of Cos et al. [15]. In this study, a different dose of melatonin compared to doses used in the literature was shown to be appropriate to our study. In the vitality analysis, IC50 dose of melatonin forMCF-7 and HEK293 cells was found to be 35 μM and implementation period was set at 48 h.
Before investigating the effect of melatonin on the stem cells, a number of CD44+/CD24- marked stem cells in MCF-7 and HEK293 cells that are not treated with melatonin was determined. Considering the analysis results, a number of CD44+/CD24- isolated from MCF-7 were found as 1.2% while a number of CD44+/CD24- isolated from HEK293 was found as 1.1%, respectively. These results are found to be consistent with results of the earlier studies in the related literature [16,17]. Under serum-free suspension conditions, it is reported that stem cells and progenitor cells survive by creating tumor phase [18]. In our study, the development of the tumor phase by CD44+/CD24- marked cells isolated from MCF-7 and HEK293 was investigated in a serum-free medium. In this way, we have confirmed that the isolated cells are stem cells. In the earlier studies, it is reported that CD44+/CD24- marked cells develop the tumor phase [19,20].
The number of studies about the effect of melatonin on stem cells in the literature is very limited. Wang et al. determined that melatonin supports the ability of mesenchymal stem cells to survive in a hypoxia and serum-free medium and shown that this effect is performed through MAPK signaling pathway [21]. In another study, the effect of melatonin on the differentiation of embryonic stem cells into neural cells was investigated. As a result, the effect of melatonin on the regulation of neuronal differentiation was shown and it was suggested that it plays an important role in neurodegenerative diseases [22]. In another study conducted on brain tumor stem cells, it was shown that melatonin can overcome the multiple drug resistance with a different mechanism (its effect on ABCG2/BCRP promoter region methylations) [23]. In the present study, a decrease by 35-40% was detected in a number of CD44+/CD24- stem cells obtained from MCF-7 after administration of melatonin (p=0.0106). On the other hand, there was an increase by 20-25% in a number of CD44+/ CD24- stem cells obtained from HEK293 cells (p=0.0493). Our results show that melatonin reduces the number of cancer stem cells and increases normal stem cells. There is no information in the literature about reducing the effect of melatonin on the amount of MCF-7 CD44+/CD24- marked cancer stem cells. Its protective effect on HEK293 CD44+/ CD24- stem cells was found to be consistent with the protective effect of melatonin on mesenchymal stem cells in the literature [21].
Although autophagy physiology has been studied by many researchers, the number of studies about its effect on stem cells in the literature is very limited. Oliver et al. have shown the positive effect of melatonin on the survival of mesenchymal stem cells in the cellular stress conditions [24]. In another study conducted to investigate the relationship between cancer stem cells and their microenvironment, it was reported that cancer stem cells benefit from the autophagy protein catabolism functions under starvation conditions [25]. In the present study, we have investigated LC3-II aggregation in CD44+/CD24- stem cells obtained from MCF-7 and HEK293 cells with immunofluorescence analysis after administration of melatonin. Our results indicate that melatonin leads to an increase in LC3-II aggregation in MCF-7 CD44+/CD24- stem cells. This shows that melatonin has an effect on autophagy pathway in cancer stem cells. Administration of melatonin on HEK293 CD44+/CD24- stem cells reduces the LC3-II aggregation. In order to support our immunofluorescence results, we have investigated the conversion of LC3-I into LC3-II by western blot analysis. Melatonin increases conversion of LC3-I into LC3-II in MCF-7 CD44+/CD24- stem cells, whereas it inhibits the conversion of LC3-I into LC3-II in HEK293 CD44+/CD24- stem cells. Our western blot analysis results were found to be consistent with our immunofluorescence results. As a result, melatonin triggers the increase of autophagy, which is a mechanism of death, in MCF-7 CD44+/CD24-stem cells in cancer. Furthermore, melatonin suppresses autophagy pathway in HEK293 CD44+/ CD24- stem cells. Autophagy may have either positive or negative effects on cancer with its pro- or anti- properties. As a result of our findings which were found to be consistent with the literature [26], we have concluded that melatonin has a pro-autophagic effect on MCF-7 CD44+/CD24- stem cells and anti-autophagic effect on CD44+/CD24- stem cells obtained from HEK293.
References
- World Health Organization cancer report 2011.
- Dick JE. Looking ahead in cancer stem cell research. Nat Biotechnol 2009; 27: 44-46.
- Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature 2001; 414: 105-111.
- Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer 2002; 2: 48-58.
- Guzman ML, Neering SJ, Upchurch D, Grimes B, Howard DS, Rizzieri DA, Luger SM., Jordan CT. Nuclear factor kappaB is constitutively activated in primitive human acute myelogenous leukemia cells. Blood 2001; 98: 2301-2307.
- Guan Y, Gerhard B, Hogge DE. Detection, isolation, and stimulation of quiescent primitive leukemic progenitor cells from patients with acute myeloid leukemia (AML). Blood 2003; 101: 3142-3149.
- Kusumbe AP, Bapat SA. Cancer stem cells and aneuploidy populations within developing tumors are the major determinants of tumor dormancy. Cancer Res 2009; 69: 9245-9253.
- Alibek K, Mektepbayeva D, Irving S, Atinbayeva N, Zhaisanbayeva B, Mussurova S, Mussakhan S. Anticancer effects and uses of melatonin: a review. Austin J Cancer Clin Res 2015; 2: 1052.
- Koh PO. Melatonin prevents hepatic injury-induced decrease in Akt downstream targets phosphorylations. J Pineal Res 2011; 51: 214-219.
- Boya P, Gonzalez-Polo RA, Casares N, Perfettini JL, Dessen P. Inhibition of macroautophagy triggers apoptosis. Mol Cell Biol 2005; 25: 1025-1040.
- Lum JJ, Bauer DE, Kong M, Harris MH, Li C. Growth factor regulation of autophagy and cell survival in the absence of apoptosis. Cell 2005; 120: 237-248.
- Claustrat B, Brun J, Chazot G. The basic physiology and pathophysiology of melatonin. Sleep Med Rev 2005; 9: 11-24.
- Blask DE, Hill SM. Effects of melatonin on cancer: studies on MCF-7 human breast cancer cells in culture. J Neural Transm Suppl 1986; 21: 433-449.
- Margheri M, Pacini N, Tani A, Nosi D, Squecco R, Dama A, Masala E, Francini F, Zecchi-Orlandini S, Formigli L. Combined effects of melatonin and all-trans retinoic acid and somatostatin on breast cancer cell proliferation and death: Molecular basis for the anticancer effect of these molecules. Eur J Pharmacol 2012; 681: 34-43.
- Cos S, Blask DE, Lemus-Wilson A, Hill AB. Effects of melatonin on the cell cycle kinetics and estrogen-rescue of MCF-7 human breast cancer cells in culture. J Pineal Res 1991; 10: 36-42.
- Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA 2003; 100: 3983-3988.
- Yenigun VB, Ozpolat B, Kose GT. Response of CD44+/CD24-/low breast cancer stem/progenitor cells to tamoxifen and doxorubicin induced autophagy. Int J Mol Med 2013; 31: 1477-1483.
- Wolman SR, Heppner GH, Wolman E. New directions in breast cancer research. FASEB J 1997; 11: 535-543.
- Engelmann K, Shen H, Finn OJ. MCF7 side population cells with characteristics of cancer stem/progenitor cells express the tumor antigen MUC1. Cancer Res 2008; 68: 2419-2426.
- Hwang-Verslues WW, Kuo WH, Chang PH, Pan CC, Wang HH, Tsai ST, Jeng YM, Shew JY, Kung JT, Chen CH, Lee EY, Chang KJ, Lee WH. Multiple lineages of human breast cancer stem/progenitor cells identified by profiling with stem cell markers. PLoS One 2009; 4: e8377.
- Wang F, Zhou H, Du Z, Chen X, Zhu F, Wang Z, Zhang Y, Lin L, Qian M, Zhang X, Li X, Hao A. Cytoprotective effect of melatonin against hypoxia/serum deprivation-induced cell death of bone marrow mesenchymal stem cells in vitro. Eur J Pharmacol 2015; 748: 157-165.
- Sotthibundhu A, Phansuwan-Pujito P, Govitrapong P. Melatonin increases proliferation of cultured neural stem cells obtained from adult mouse subventricular zone. J Pineal Res 2010; 49: 291-300.
- Martin V, Sanchez-Sanchez M, Herrera F, Gomez-Manzano C, Fueyo J, Alvarez-Vega MA, Antolin I, Rodriguez C. Melatonin-induced methylation of the ABCG2/BCRP promoter as a novel mechanism to overcome multidrug resistance in brain tumour stem cells. Br J Cancer 2013; 108: 2005-2012.
- Oliver L, Hue E, Priault M, Vallette FM. Basal autophagy decreased during the differentiation of human adult mesenchymal stem cells. Stem Cells Dev 2012; 21: 2779-2788.
- Espina V, Mariani BD, Gallagher RI, Tran K, Banks S, Wiedemann J, Huryk H, Mueller C, Adamo L, Deng J, Petricoin EF, Pastore L, Zaman S, Menezes G, Mize J, Johal J, Edmiston K, Liotta LA. Malignant precursor cells pre-exist in human breast DCIS and require autophagy for survival. PLoS One 2010; 5: e10240.
- Klongpanichapaka S, Phansuwan-Pujitob P, Ebadic M, Govitrapong P. Melatonin inhibits amphetamine-induced increase in alpha-synuclein and decrease in phosphorylated tyrosine hydroxylase in SK-N-SH cells. Neurosci Lett 2008; 436: 309-313.