ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2020) Volume 31, Issue 6
Intratracheal administration of diesel exhaust particles causes male reproductive toxicity and enhances liver cytochrome P450 1A1 activity.
Department of Nutrition, Faculty of Health Sciences, Aomori University of Health and Welfare, Aomori 030-8505, Japan
- Corresponding Author:
- Hiromi Izawa
Department of Nutrition, Faculty of Health Sciences Aomori University of Health and Welfare Aomori 030-8505 Japan
Accepted on October 15, 2020
Diesel Exhaust Particles (DEPs) contain Polycyclic Aromatic Hydrocarbons (PAHs) and the Aryl hydrocarbon Receptor (AhR) is closely involved in the endocrine disrupting action of PAHs. Activation of the AhR is thought to cause various effects such as reproductive dysfunction. In our previous studies, Subcutaneous (SC) administration of DEPs to male mice caused reproductive toxicity and increased hepatic Cytochrome P450 1A1 (CYP1A) enzyme activity, as an indirect index of AhR activity. Air pollutants in the environment enter the body through the airways. In this study, we examined the effect of Intratracheally (IT) administered DEP on spermatogenesis and hepatic CYP1A1 in male mice. The amount of DEP administered IT in this study was lower than that administered SC in previous studies. The result showed that similar to SC, IT administered DEP decreased daily sperm production and hepatic CYP1A1 activity significantly. Thus, DEP toxicity was suggested to be more pronounced with IT administration than with SC administration.
Keywords
Diesel Exhaust Particle, Sperm, Testosterone, CYP1A1.
Introduction
Diesel exhaust gas is known to trigger carcinogenesis and allergic diseases [1,2], and has been revealed to cause damage to the reproductive system through the so-called environmental hormone-like effect [3]. The causative substance is the coal powder in diesel exhaust gas, named diesel exhaust particles (DEPs), which are aggregate particles composed of elemental carbon that further adsorb organic compounds and sulfates. Organic compounds include a numerous chemicals such as polycyclic aromatic hydrocarbons (PAHs), nitro compounds of PAHs [4], and endocrine disrupting chemicals such as dioxin derivatives [5]. The aryl hydrocarbon receptor (AhR) is involved in mediating the endocrine disrupting activity of PAHs [6] and its activation is thought to cause various effects such as reproductive dysfunction.
When PAHs are incorporated into cells, they bind with AhR to form a PAH-AhR complex, which is transferred into the nucleus and combines with the AhR nuclear translocator. This complex further combines with the dioxin response element in DNA and enhances the expression of genes of the Cytochrome P450 (CYP) family, such as CYP1A1, which are involved in carcinogenesis, immunosuppression, hepatotoxicity, teratogenicity and reproductive toxicity [6- 8]. CYP1A1 enzyme activity is recognized as an indirect index of AhR activity.
In our previous studies, Subcutaneous (SC) administration of DEPs to male mice caused reproductive toxicity and increased plasma testosterone levels and hepatic CYP1A1 enzyme activity [9]. In addition, the expression of this reproductive toxicity was different based on AhR reactivity where strains with high AhR responsiveness showed strong reproductive toxicity [10]. Quercetin, which is one of the flavonoids contained in onions, strongly suppressed AhR activation in an ex vivo system using an Ah-immunoassay kit [11], which suggests that quercetin may also antagonize PAHs and suppress AhR activity in vivo. Indeed, SC administration of DEPs to mice treated quercetin reduced the male reproductive toxicity [12].
These results strongly suggested that quercetin reduced male reproductive toxicity by suppressing the DEP- induced AhR activation. However, these findings were from studies of mice exposed to DEPs by SC administration. Generally environmental air pollutants enter the body through the airways. Therefore, in this study, we examined the effect of Intratracheal (IT) administration of DEP on spermatogenesis and hepatic CYP1A1 in male mice.
Materials and Methods
Chemicals and DEPs
NADPH and fluorescamine were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Resorufin and ethoxyresorufin were obtained from Sigma-Aldrich Chemical Co. Ltd (St. Louis, MO, USA). DEPs were collected, and a suspension was prepared according to Sagai’s method [13]. In brief, DEPs were collected on a glass fiber filter after driving a 2,740 cc, 4-cylinder, direct injection diesel engine (Isuzu 4JB-1) installed at the animal experiment facility of the National Institute for Environmental Studies (Tsukuba, Ibaraki, Japan) at 1,500 rpm with a load of 10 kg/m. DEPs were stored at -30°C until use. A nine-fold volume of phosphate-buffered saline (PBS) containing 0.05% Tween 20 (PBST) was added to the DEPs, and the mixture was suspended by sonication for 5 min on ice. Subsequently, the suspension was diluted with PBST solution to 0.5 mg/mL.
Animals and DEP injection
Six-week-old Balb/c male mice were purchased from Clea Japan, Inc. (Tokyo, Japan). The mice were housed in an environmentally controlled room at 23 ± 2°C with 55 ± 7% humidity on a 12 h light/dark cycle and were allowed a 1-week acclimation period. AIN93G diet (Clea Japan, Inc.) and water were provided ad libitum. After acclimation, the mice were divided into vehicle (n=6) and DEP (n=5) groups that were administered (IT) 50 μL of DEP suspension and PBST, respectively using a canula under anesthesia with 4% halothane 12 times during 6 weeks. Then, 3 days after the last treatment, all mice were weighed and sacrificed, blood samples were collected into individual heparinized centrifuge tubes, and centrifuged to separate the plasma, which was stored at −80°C until assayed for testosterone. The testes and liver were harvested from each animal, weighed, and then they were stored at −80°C until assayed for daily sperm production (DSP) and ethoxyresorufin-O-deethylase (EROD) activity, respectively. This study was approved by the Animal Research Committee, Aomori University of Health and Welfare, and all experimental procedures were performed in accordance with the Guidelines for Animal Experimentation of Aomori University of Health and Welfare.
Sperm analysis
DSP was determined using the procedure of Joyce et al. [14] and Yoshida et al. [15]. The left testis was homogenized for 30 s in 5 mL 10 mM PBS containing 0.05% Triton X-100 using a Polytron homogenizer. Aliquots of the suspensions were placed in a hematocytometer chamber, and the number of step 14-16 spermatids per testis was counted. The DSP obtained by dividing the spermatid count by 4.84, which is the number of days developing mouse spermatids spend in steps 14-16 during spermatogenesis [14,15].
Measurement of plasma testosterone level
Plasma testosterone levels were measured using a testosterone enzyme immunosorbent assay (EIA) kit (Cayman Chemical Company, Ann Arbor, MI, USA).
Measurement of hepatic EROD activity
The harvested livers were homogenized in 0.15 M potassium chloride (KCl) using a Polytron homogenizer. Microsomes were isolated by ultracentrifugation (105,000 × g, 60 min), and EROD activity was measured using the procedure of Kennedy et al. [16].
Statistical analysis
All data are expressed as means ± standard error of the mean (SEM). Differences between the DEP and vehicle groups were evaluated using the Student’s t-test. A P-value<0.05 was considered significant and the statistical analyses were performed using the statistical package for the social sciences (SPSS) version 20.0.0 for Windows (IBM SPSS Statistics; IBM Corporation, Somers, NY, USA).
Results
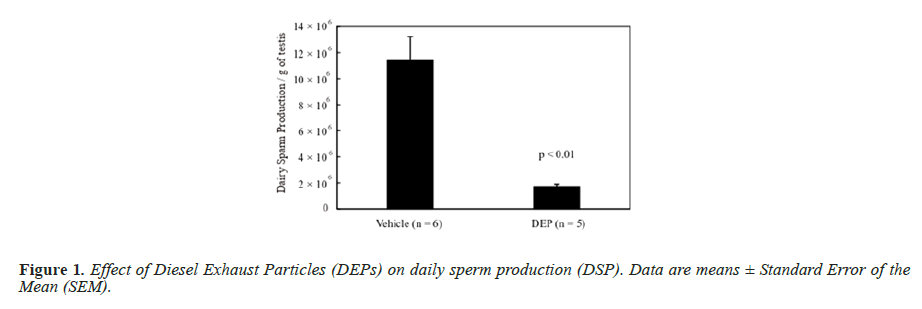
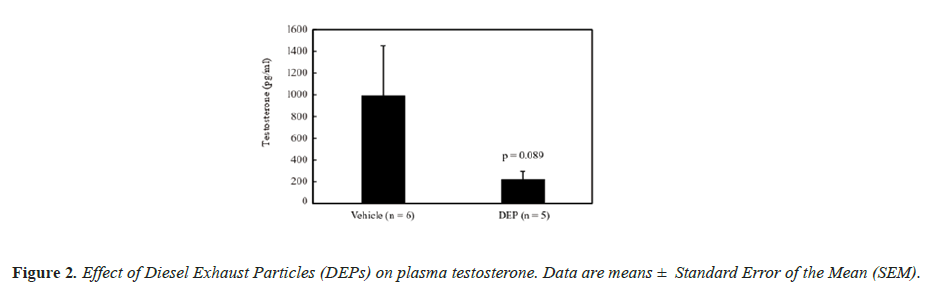
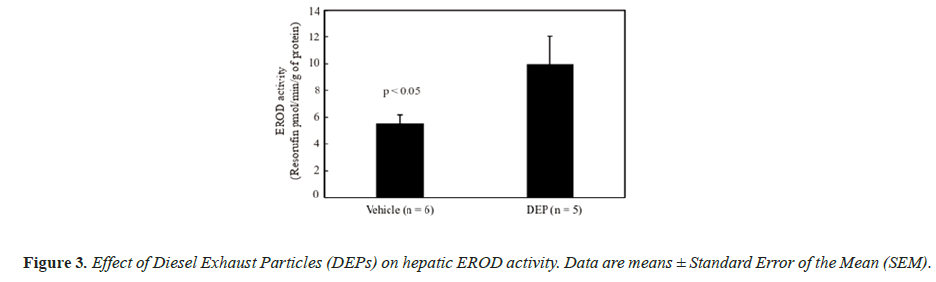
The body weight and testis and liver weights (both absolute and relative to body weight) did not differ significantly between the groups (data not shown). Figure 1 shows the DSP of the mice, and the DSP per gram of testis tissue (DSP/g) of the DEP-treated group was significantly decreased compared with that of the vehicle group. Figure 2 shows the plasma testosterone levels of the mice, which were not significantly deferent between the vehicle and DEP-treated groups. However, plasma testosterone levels of the DEP-treated group were lower than those of the vehicle group (P=0.0829). Figure 3 shows the hepatic EROD activity of the mice, which was significantly increased in the DEP-treated group compared with that of the vehicle group.
Discussion
In the present study, we examined whether IT administration of DEPs affects reproductive function and hepatic CYP1A1 activity of male mice. Balb/c male mice were used this study because they have highly responsive AhRs [7,17-19] and are easily affected by DEP toxicity [9,10]. The DEPs had no effect on body weight or absolute and relative (to body weight) testis and liver weights, which is not entirely in agreement with a previous report that inhalation of diesel exhaust alters body weight, but not testes weight in mice [15]. The negative effect of DEPs on body or organ weights may be dose-dependent.
DSP is a quantitative index of sperm producing ability [20] and the value per gram of testis tissue of the DEP-treated group was significantly decreased compared with that of the vehicle group. In our previous study, Balb/c male mice were administered DEP suspension at 24.7, 27.0, or 220 μg/mouse into the dorsal SC layer 10 times during a 5 weeks period [9], making a study total of 247, 270 or 2,200 μg/mouse, respectively. In the present study, each mouse received 25.0 μg during the experimental period, which is similar to the 24.7 μg/mouse in our previous study and the DSP per gram od testis tissue of the 24.7 μg/ mouse DEP group was not significantly lower than that of the vehicle group in the previous study [9]. However, IT administration of DEPs decreased spermatogenesis, suggesting a more potent effect than that induced by SC administration.
Testosterone is a crucial hormone for spermatogenesis and in the present study, the plasma levels of the DEP-treated group were decreased compared with that of the vehicle group, while the factor treatment was almost significant (P=0.0829). Both 3-methyl-4-nitrophenol (PNMC) [21,22] and 4-nitrophenol (PNP) [23], which are components of DEP, have reported to have antiandrogenic activity and they significantly decreased plasma testosterone levels in rats [21,22]. These findings suggested that PNMC and PNP have a direct effect on the testes that reduces testosterone secretion and our present study results suggest a similar trend.
In contrast, DE gas inhalation from birth to 3 months has been reported to have no effect on plasma testosterone levels in rats [24], whereas inhalation from birth to 8 months significantly increased levels of adult rats [25]. In the present study, DEPs were administered to adult mice and the effects of DEPs on spermatogenesis may differ between young and adult rodents. In our previous study, plasma testosterone levels increased significantly in mice administered (SC) approximately three times the amount of DEP suspension administered in this study [9]. However, testosterone levels of mice treated SC with the same amount of DEPs administered in this study were almost the same as those of the vehicle mice [9]. The effect of DEPs on plasma testosterone may be different between SC and IT administration, and the present results indicate that testosterone production was inhibited by IT administration of DEPs.
Our previous study suggested that the toxicity of SC administered DEPs to the male reproductive system may occur in an AhR-dependent manner [10]. AhR is a ligand-activated transcription factor [6] that upregulates CYP1A expression, which has EROD activity [26]. Thus, hepatic EROD activity is an indirect endpoint of AhR responsiveness and in this study, we demonstrated that the toxicity of IT administered DEP is also similarly mediated. The EROD activity of DEP-treated group was significantly increased compared with that of the vehicle group. In our previous study, hepatic EROD activity did not increase in mice treated with approximately three times the amount of DEPs administered SC in this study [9]. However, in this study, hepatic EROD activity significantly increased following IT administration of DEPs, indicating that this route induces higher toxicity than SC administration. It has been reported that CYP1A1 does not induce toxicity by metabolic activation of Benzo[a]Pyrene (BaP), which is a carcinogen contained in PAHs, a major component of the organic compounds in DEPs, but it is an essential enzyme for detoxification and excretion of BaP [27,28]. This suggests that the increase in EROD activity in this study was due to the increased expression of the drug metabolizing enzyme CYP1A1 due to detoxification of toxic substances contained in DEPs. However, we did not generate data to prove this, so additional detailed studies are required in the future.
Conclusion
In conclusion, our study showed that DSP and plasma testosterone were lowered and liver EROD activity was increased by IT administration of DEPs at amounts that showed no effects following SC administration in previous studies. Thus, these findings suggest DEP toxicity to be more pronounced with IT than with SC administration.
References
- Silverman DT. Diesel Exhaust and Lung Cancer-Aftermath of Becoming an IARC Group 1 Carcinogen. Am J Epidemiol 2018; 187: 1149-1152.
- Rider CF, Carlsten C. Air pollution and DNA methylation: effects of exposure in humans. Clinical Epigenetics 2019; 11: 131.
- Ema M, Naya M, Horimoto M, Kato H. Developmental toxicity of diesel exhaust: a review of studies in experimental animals. Reprod Toxicol 2013; 42: 1-17.
- Wichmann HE. Diesel exhaust particles. Inhal Toxicol 2007; 19 Suppl 1: 241-244.
- Clunies-Ross C, Stanmore BR, Millar GJ. Dioxins in diesel exhaust. Nature 1996; 381: 379.
- Hankinson O. The aryl hydrocarbon receptor complex. Annu Rev Pharmacol Toxicol 1995; 35: 307-340.
- Pohjanvirta R, Tuomisto J. Short-term toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin in laboratory animals: effects, mechanisms, and animal models. Pharmacol Rev 1994; 46: 483-549.
- Poland A, Knutson JC. 2,3,7,8-tetrachlorodibenzo-p-dioxin and related halogenated aromatic hydrocarbons: examination of the mechanism of toxicity. Annu Rev Pharmacol Toxicol 1982; 22: 517-554.
- Izawa H, Kohara M, Watanabe G, Taya K, Sagai M. Diesel exhaust particle toxicity on spermatogenesis in the mouse is arylhydrocarbon receptor dependent. J Reprod Dev 2007; 53: 1069-1078.
- Izawa H, Kohara M, Watanabe G, Taya K, Sagai M. Effects of diesel exhaust particles on the male reproductive system in strains of mice with different aryl hydrocarbon receptor responsiveness. J Reprod Dev 2007; 53: 1191-1197.
- Izawa H, Watanabe G, Taya K, Sagai M. Inhibitory effects of foods and polyphenols on the activation of the aryl hydrocarbon receptor induced by diesel exhaust particles. Environ Sci 2007; 14: 149-156.
- Izawa H, Kohara M, Aizawa K, Suganuma H, Inakuma T, Watanabe G, et al. Alleviative effects of quercetin and onion on male reproductive toxicity induced by diesel exhaust particles. Biosci Biotechnol Biochem 2008; 72: 1235-1241.
- Sagai M, Saito H, Ichinose T, Kodama M, Mori Y. Biological effects of diesel exhaust particles. I. In vitro production of superoxide and In vivo toxicity in mouse. Free Radic Biol Med 1993; 14: 37-47.
- Joyce KL, Porcelli J, Cooke PS. Neonatal goitrogen treatment increases adult testis size and sperm production in the mouse. J Androl 1993; 14: 448-455.
- Yoshida S, Sagai M, Oshio S, Umeda T, Ihara T, Sugamata M, et al. Exposure to diesel exhaust affects the male reproductive system of mice. Int J Androl 1999; 22: 307-315.
- Kennedy SW, Jones SP. Simultaneous measurement of cytochrome P4501A catalytic activity and total protein concentration with a fluorescence plate reader. Anal Biochem 1994; 222: 217-223.
- Poland A, Glover E. Characterization and strain distribution pattern of the murine Ah receptor specified by the Ahd and Ahb-3 alleles. Mol Pharmacol 1990; 38: 306-312.
- Zacharova LY, Gulyaeva LF, Lyakhovich VV, Mikhailova ON, Timofeeva OA, Filipenko ML, et al. Cytochrome P4501A1 and 1A2 gene expression in the liver of 3-methylcholanthrene- and o-aminoazotoluene-treated mice: a comparison between PAH-responsive and PAH-nonresponsive strains. Toxicol Sci 2003; 73:108-113.
- Thomas RS, Penn SG, Holden K, Bradfield CA, Rank DR. Sequence variation and phylogenetic history of the mouse Ahr gene. Pharmacogenetics 2002; 12: 151-163.
- Russsel LD, Ettlin RA, SinhaHikim AP, Clegg ED. Historogical and Histopathological Evaluation of the Testis. Int J Androl 1990; 16: 83.
- Li C, Takahashi S, Taneda S, Furuta C, Watanabe G, Suzuki AK, et al. Impairment of testicular function in adult male Japanese quail (Coturnix japonica) after a single administration of 3-methyl-4-nitrophenol in diesel exhaust particles. J Endocrinol 2006; 189: 555-564.
- Li C, Taneda S, Suzuki AK, Furuta C, Watanabe G, Taya K. Anti-androgenic activity of 3-methyl-4-nitrophenol in diesel exhaust particles. Eur J Pharmacol 2006; 543: 194-199.
- Li C, Taneda S, Suzuki AK, Furuta C, Watanabe G, Taya K. Estrogenic and anti-androgenic activities of 4-nitrophenol in diesel exhaust particles. Toxicol Appl Pharmacol 2006; 217: 1-6.
- Watanabe N. Decreased number of sperms and Sertoli cells in mature rats exposed to diesel exhaust as fetuses. Toxicol Lett 2005; 155: 51-58.
- Tsukue N, Toda N, Tsubone H, Sagai M, Jin WZ, Watanabe G, et al. Diesel exhaust (DE) affects the regulation of testicular function in male Fischer 344 rats. J Toxicol Environ Health A 2001; 63: 115-126.
- Nebert DW, Roe AL, Dieter MZ, Solis WA, Yang Y, Dalton TP. Role of the aromatic hydrocarbon receptor and [Ah] gene battery in the oxidative stress response, cell cycle control, and apoptosis. Biochem Pharmacol 2000; 59: 65-85.
- Uno S, Dalton TP, Derkenne S, Curran CP, Miller ML, Shertzer HG, et al. Oral exposure to benzo[a]pyrene in the mouse: detoxication by inducible cytochrome P450 is more important than metabolic activation. Mol Pharmacol 2004; 65: 1225-1237.
- Uno S, Dalton TP, Dragin N, Curran CP, Derkenne S, Miller ML, et al. Oral benzo[a]pyrene in Cyp1 knockout mouse lines: CYP1A1 important in detoxication, CYP1B1 metabolism required for immune damage independent of total-body burden and clearance rate. Mol Pharmacol 2006; 69: 1103-1114.