ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
- Biomedical Research (2013) Volume 24, Issue 4
Influence of different proton pump inhibitors on platelet function in acute myocardial infarction patients receiving clopidogrel treatment after percutaneous coronary intervention.
Heart Center, Chaoyang Hospital, Capital Medical University, Beijing 100020, China
- *Corresponding Author:
- Xinchun Yang
Heart Center, Chaoyang Hospital
Capital Medical University
Beijing 100020
China
Accepted date: July 18 2013
Citation: Wang Z , Yang X, Cai J, Chen M, Wan X. Influence of different proton pump inhibitors on platelet function in acute myocardial infarction patients receiving clopidogrel treatment after percutaneous coronary intervention. Biomedical Research 2013; 24 (4): 453-457.
The objective of the present study is to investigate the effect of clopidogrel and proton pump inhibitors (PPIs) on upper gastrointestinal bleeding and adverse cardiovascular events in acute myocardial infarction (MI) patients after percutaneous coronary intervention (PCI). A total of 240 patients receiving emergent PCI due to acute MI were recruited from January 2009 to November 2010. After admission, patients were treated with aspirin (300 mg) and clopidogrel (600 mg) on the first day and then with aspirin at 100 mg/d and clopidogrel at 75 mg/d for antiplatelet therapy. These patients were randomly assigned into Omeprazole (40 mg/d) group (n=83), Pantoprazole (40 mg/d) group (n=80) and Famotidine (40 mg/d) group (n=77). Treatment was done for 5-7 days and patients were followed up for 1 month. The gastrointestinal bleeding and in-stent restenosis were observed. There was no marked difference in the incidence of gastrointestinal bleeding between Omeprazole group and Pantoprazole group, but that in the former two groups was significantly higher than in the Famotidine group. No dramatic difference was observed in the incidence of in-stent restenosis among three groups. Treatment with PPI may not increase the risk for in-stent re-stenosis in acute MI patients receiving PCI, but PPI treatment can significantly reduce the incidence of gastrointestinal bleeding when compared with histamine H2-receptor antagonist.
Keywords
proton pump inhibitor; clopidogrel; percutaneous coronary intervention; bleeding; Stent thrombosis
Introduction
To date, aspirin in combination with clopidogrel, a platelet ADP receptor antagonist, has been used as a standardized anti-platelet therapy in patients receiving percutaneous coronary intervention (PCI), which aims to prevent the stent thrombosis. With the application of two antiplatelet drugs, the incidence of gastrointestinal bleeding is increasing concomitantly. In 2007, American College of Cardiology (ACC) and American Heart Association (AHA) recommended in their guidelines that proton pump inhibitor (PPI) can be applied in patients with a history of gastrointestinal bleeding when the aspirin and/or clopidogrel is used. In recent years, some studies revealed that PPI may not only effectively prevent the gastrointestinal bleeding but influence the anti-platelet therapy of clopidogrel to increase the risk for adverse cardiovascular events (ACE) [1,2]. In addition, a variety of studies have shown that combined use of clopidogrel and aspirin may not compromise the platelet function and has no influence on the risk for ACE [3,4]. Currently, there is still controversy on the influence of PPI on the anti-platelet effect of clopidogrel. In the present study, the short term influence of omeprazole and pantoprazole on the anti-platelet effect of clopidogrel was investigated aiming to explore the correlation between PPI and ACE after PCI.
Subjects and Methods
Subjects
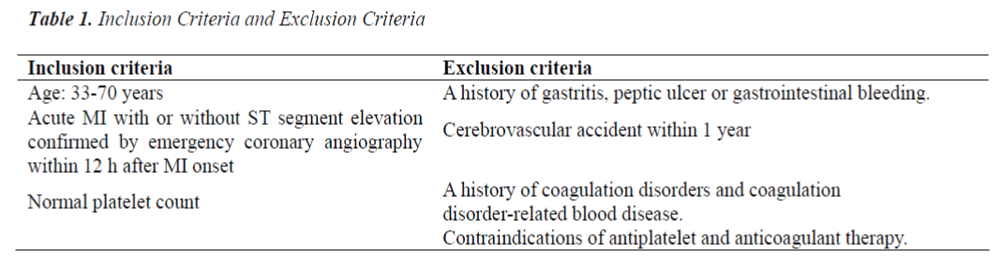
A total of 240 patients receiving emergent PCI due to acute myocardial infarction (MI) in ICU of Beijing Chaoyang Hospital were recruited from March 2010 to March 2011. The inclusion criteria were as follows (Table 1): 33- 77 years; MI with or without ST segment elevation which was confirmed by coronary angiography within 12 h after MI onset; normal platelet count. Exclusion criteria: a history of gastritis, gastrointestinal ulcer or gastrointestinal bleeding; cerebrovascular events within past 1 year; a history of coagulation disorders or coagulation related hematological diseases; presence of contradictions for anti-platelet therapy or anti-coagulation therapy. MI was defined as a creatine kinase MB level that was more than twice the upper limit of normal range and either symptoms consistent with acute MI or electrocardiographic changes in at least two contiguous leads (pathologic Q waves _ 0.04 sec in duration), persistent ST-segment elevation, or ST-segment depression > 0.1 mV) [5,6].
Methods
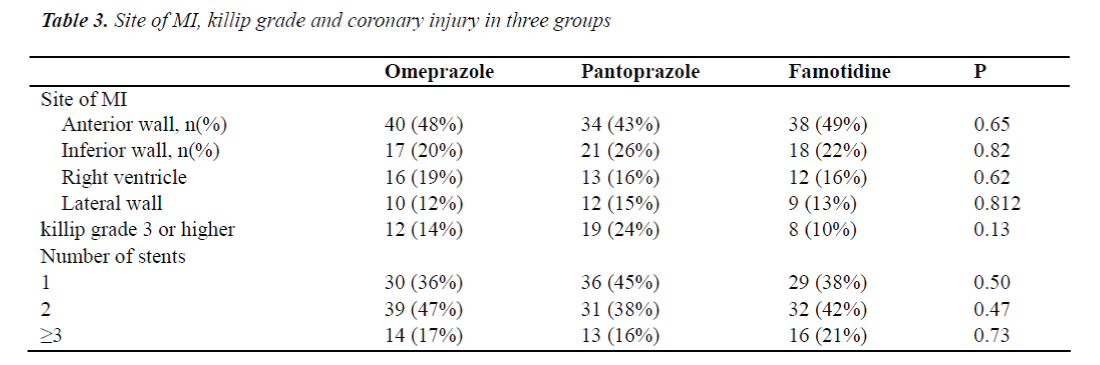
Patients were randomly assigned into three groups with a random number table: omeprazole (40 mg/d; n=83) group, pantoprazole (40 mg/d; n=80) group and famotidine (40 mg/d; n=77) group. Treatment was initiated immediately after surgery and continued for 3 days. All patients were treated with two anti-platelet drugs. In brief, 300 mg of aspirin and 600 mg of clopidogrel were administered on the first day, and then patients were treated with aspirin at 100 mg/d, clopidogrel at 75 mg/d for maintenance and subcutaneous injection of heparin. Patients were followed up for 30 days after surgery via hospital visit, hospitalization and/or telephone. The endpoints included gastrointestinal bleeding; in-stent restenosis; acute or subacute thrombosis. The gastrointestinal bleeding was defined when the haematemesis and positive vomit occult blood test or hematochezia and positive fecal occult blood test were present. In-stent restenosis was defined when the coronary angiography after recurrence of ACS confirmed in-stent restenosis (≥ 50% luminal stenosis). Acute thrombosis was defined when the in-stent thrombotic occlusion was present within 24 h after stent placement. Subacute thrombosis was defined when the coronary angiography confirmed the in-stent thrombotic occlusion at 24 h to 30 days after stent placement.
Statistical analysis
SPSS version 12.0 for Windows was used for statistical analysis. Qualitative data were compared with chi square test. Quantitative data were expressed as means ± standard deviation ( X ±S) and analysis of variance was employed to compare the means among different groups. A value of P<0.05 was considered statistically significant.
Results
Characteristics of patients in three groups
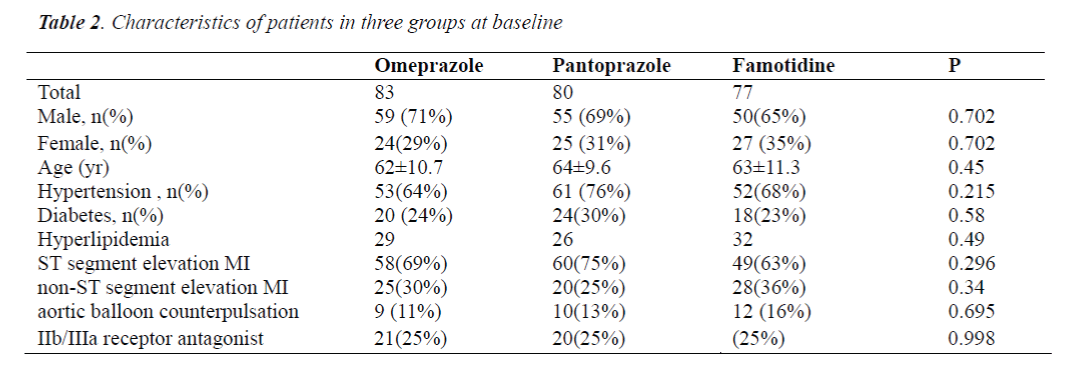
There were no marked differences in the age, gender, body mass index, proportion of patients with hypertension, diabetes or hyperlipidemia, proportion of patients with ST segment elevation MI or non-ST segment elevation MI, proportion of patients with aortic balloon counterpulsation and proportion of patients treated with platelet membrane glycoprotein IIb/IIIa receptor antagonist among three groups (P>0.05) (Table 2).
Incidence of gastrointestinal bleeding in three groups
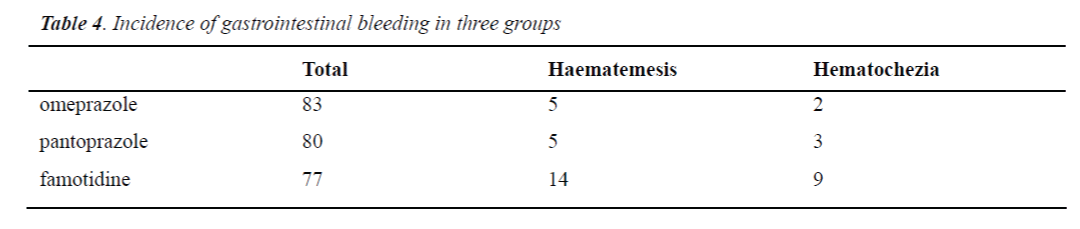
Gastrointestinal bleeding was observed in 7 patients in the omeprazole group (haematemesis: n=5; hematochezia: n=2) in 8 patients in the pantoprazole group (haematemesis: n=5; hematochezia: n=3) and 16 patients in the famotidine group (haematemesis: n=10; hematochezia: n=9). All patients with gastrointestinal bleeding were positive in fecal or vomit occult blood test. There was no significant difference in the incidence of haematemesis and hematochezia between pantoprazole group and omeprazole group (P>0.79 and P>0.95, respectively). The incidence of haematemesis and hematochezia in the omeprazole group was significantly lower than that in the famotidine group (P<0.006 and P<0.01, respectively). The incidence of haematemesis and hematochezia in the pantoprazole group was markedly lower than that in the famotidine group (P<0.008 and P<0.04, respectively) (Table 4).
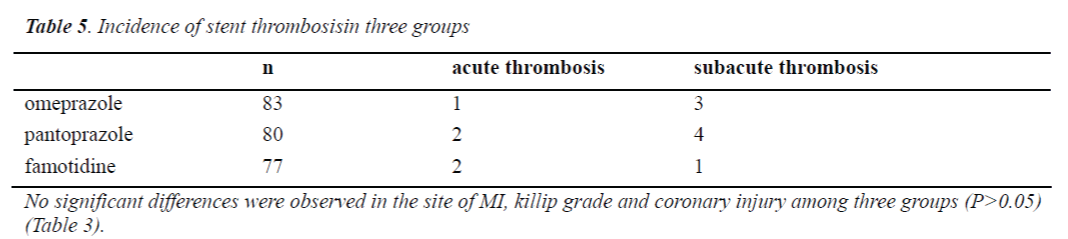
Incidence of stent thrombosis in three groups
There were 6 patients with acute thrombosis and 6 patients with subacute thrombosis in the omeprazole group; 4 patients with acute thrombosis and 7 patients with subacute thrombosis in the pantoprazole group; 3 patients with acute thrombosis and 5 patients with subacute thrombosis in the famotidine group. There was no marked difference in the incidence of stent thrombosis among three groups (P>0.05) (Table 5).
Discussion
A lot of clinical studies have shown that anti-platelet therapy with both aspirin and clopidogrel can significantly reduce the incidence of adverse cardiovascular events after PCI and has been a standardized therapy after PCI. However, Derry et al [5] found the combined use of aspirin and clopidogrel could markedly increase the risk for gastrointestinal bleeding. To date, PPIs have been the most effective acid-blocking agents and mucosal protective agents. PPIs are highly specific to persistently and potently inhibit the acid production. Thus, they are usually used to prevent the complications such as gastrointestinal bleeding secondary anti-platelet therapy. However, there is controversy on the adverse consequence of combined use of aspirin and clopidogrel. Sibbing et al [7] found that the degree of platelet aggregation in omeprazole treated patients was significantly higher than that in patients without PPI treatment after PCI and stent placement. On the contrary, the degree of platelet aggregation was comparable between patients with pantoprazole treatment and those without PPI treatment. The study of Ho et al [8] revealed that combined use of clopidogrel and omeprazole could increase the risk for post-PCI mortality and re-hospitalization. This might be attributed to the inhibition of cytochrome P450 2C19 activity. The antiplatelet activity of clopidogrel is dependent on the activation of cytochrome P450 after oxidation in which CYP2C19 plays an important role. A majority of PPIs can inhibit the CYP2C19 activity, which may lead to the reduction of anti-platelet effect of clopidogrel. There is evidence showing that omeprazole is more potent to increase the risk for the recurrence of acute MI than pantoprazole, which may be attributed to the inhibition of CYP2C19 activity by omeprazole but not by pantoprazole.
On the contrary, Schreiner et al [9] and O’Donoghue et al [10] found that the combined use of PPI and clopidogrel failed to increase the risk for adverse cardiovascular events, but could markedly reduce the incidence of gastrointestinal bleeding when compared with placebo. This might be multifactorial. The oxidation of clopidogrel in the liver involves several CYP isoforms such as CYP3A4, CYP3A5, CYP1A and CYP2B besides CYP2C19. Clopidogrel amy be also metabolized into active product when the bypass metabolism is active. In addition, the liver is rich in CYP2C19, and omeprazole at routine blood concentration is insufficient to saturate CYP2C19. Thus, it fails to competitively inhibit the anti-platelet effect of clopidogrel.
Our results showed there was no marked difference in the incidence of stent thrombosis between omeprazole group and pantoprazole group (pantoprazole is unable to inhibit the cytochrome P450 2C19), which was consistent with findings in the randomized studies of Gremmel et al [11] and Cai et al [12]. This suggests that short term use of omeprazole or pantoprazole has no influence on the antiplatelet effect of clopidogrel and aspirin in patients receiving PCI and stent placement, and combined use of PPI and clopidogrel fails to increase the risk for thrombosis. The results from the current study suggest that the short-term application of omeprazole does not increase thrombus events and can reduce bleeding complications, providing a basis for clinical medication. However, some limitations exist in our study due to the small sample size, short time to taking PPI inhibitor and following up and other reasons. Further studies are required to confirm the influence of long term use of omeprazole or omeprazole at higher dose on the incidence of adverse cardiovascular events in MI patients receiving PCI.
Conflict of interest
None of the authors declare that there exist any conflicts of interest.
References
- Bhatt DL, Scheiman J, Abraham NS, Antman EM, Chan FK, Furberg CD,, et al: ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force of Cinical Expert Consensus Documents. Circulation 2008; 118: 1894-1909
- Aubert RE, Epstein RS, Teagarden JR, et al: Proton pump inhibitors effect on clopidogrel effectiveness: the clopidogel Medco outcome study(abstract 3998). Circulation 2008;118: S815
- Simon T, Verstuyft C, Mary-Krause M, et al: Genetic determinants of response to clopidogrel and cardiovascular events. N Engl J Med 2009;360: 363-375.
- Ramirez JF, Selzer F, Chakaprani R, et al: Proton pump inhibitor and clopidogrel combination is not associated with adverse clinical outcomes after PCI: the NHLBI dynamic registry. JACC 2009;53 (Suppl 1): A27 (Abstract)
- Derry S and Loke YK. Risk of gastrointestinal haemorrhage with long term use of aspirin: meta-analysis. BMJ 2000, 321: 1183-1187
- Thygesen K, Alpert JS, White HD, Jaffe AS, Apple FS, Galvani M, Katus HA, Newby LK, Ravkilde J, Chaitman B, et al: Universal definition of myocardial infarction. Circulation 2007;116:2634–2653.
- Sibbing D, Morath T, Stegherr J, et al: Impact of proton pump inhibitors on the antiplatelet effects of clopidogrel. J Thromb Haemost 2009;101:714-719
- Ho PM, Maddox TM, Wang L, Fihn SD, Jesse RL, Peterson ED and Rumsfeld JS: Risk of adverse outcomes associated with concomitant use of clopidogrel and proton pump inhibitors following acute coronary syndrome. JAMA 2009; 301: 937-944
- Schreiner GC, Laine L, Murphy SA and Cannon CP: Evaluation of proton pump inhibitor use in patients with acute coronary syndromes based on risk factors for gastrointestinal bleed. Crit Path Cardiol 2007; 6: 169-172
- O'Donoghue ML, Braunwald E, Antman EM, et al: Pharmacodynamic effect and clinical efficacy of clopidogrel and prasugrel with or without a proton-pump inhibitor: an analysis of two randomised trials. Lancet 2009;374: 989-997
- Gremmel T, Steiner S, Seidinger D, Koppensteiner R, Panzer S and Kopp CW. The influence of proton pump inhibitors on the antiplatelet potency of clopidogrel evaluated by 5 different platelet function tests. J Cardiovasc Pharmacol 2010; 56: 532-539
- Cai J, Wu Q, Fan L, Liu CF, Wang ZG and Sun J. [Im pact of different proton pump inhibitors on the antiplatelet activity of clopidogrel in combination with aspirin for patients undergoing coronary stent implantation]. [Article in Chinese] Zhongguo Ying Yong Sheng Li Xue Za Zhi 2010; 26: 266-269,