ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2016) Volume 27, Issue 4
Increased transient receptor potential canonical type 1 improves diabetic nephropathy via inhibiting NF-kB pathway
Zhi-ming Yu1#, Xiao-yan Li1, Jia-ping Li1, Shi-peng Dang1, Bing Wang2, Ying Wu1, Yue-xin Chen3, Ruxing Wang1, Ling-ling Qian1, Jie Zheng1, Meng Wang1, Feng Xu1 and Kan Hong2#*
1Department of Cardiology, Wuxi People’s Hospital Affiliated to Nanjing Medical University, Wuxi 214023, PR China
2Department of Geriatrics , Wuxi People’s Hospital Affiliated to Nanjing Medical University, Wuxi 214023, PR China
3Department of Research , Wuxi People’s Hospital Affiliated to Nanjing Medical University, Wuxi 214023, PR China
#These authors contributed equally to this work.
- *Corresponding Author:
- Kan Hong
Department of Geriatrics
Wuxi People’s Hospital Affiliated to Nanjing Medical University
PR China
Accepted date: April 17, 2016
Diabetic nephropathy (DN) is one of the complex complications of diabetes mellitus (DM). The present study has been designed to examine the possible protective effects of the transient receptor potential canonical type 1 (TRPC1) on DN. After administrated with TRPC1, the levels of blood glucose, urine protein, blood urea nitrogen (BUN) and serum creatinine (SCr) in DN rats were significantly decreased compared with the model rats. In addition, morphological studies demonstrated that TRPC1 attenuated mesangial expansion and capillary basement membrane thickening as well as glomerular structure. The treatment of TRPC1 reduced the inflammatory cytokines including transforming growth factor-β1 (TGF-β1) and nuclear factor kappa B (NF-κB) though the immunohistochemical analysis. In addition, related-mRNA and proteins of NF-κB signal pathway were investigated to determine the protective molecular mechanism of TRPC1 on the DN. The results presented here suggest that the protective mechanism of TRPC1 may be attributed partly to decreased production of pro-inflammatory cytokines via the inhibition of NF-κB signaling pathway.
Keywords
Diabetic nephropathy, TRPC1, NF-κB.
Introduction
Diabetic nephropathy (DN), as one of the most severe micro vascular complications of diabetes, has become the leading cause of end-stage renal disease in recent years. It has been reported that approximately 40% of people with diabetes develop diabetic nephropathy [1]. It has been recently reported that the change of inflammatory response plays a very important role in the progress of DN [2]. Besides, high blood glucose and fluctuations of blood glucose are the symbol of glucose metabolic disorders in the diabetic patients and also the main cause of nephropathy complications [3]. But the pathogenesis of DN has not yet been fully elucidated.
Transient receptor potential (TRP) proteins are non-selective cation channels fulfilling diverse roles as versatile cellular sensors and effectors [4]. TRPC1 (transient receptor potential canonical 1), the widely expressed in many cell types, is a Ca2+-permeable cation channel involved in diverse physiological function [5]. Because Ca2+ has been shown to play a pivotal role in insulin secretion from the islets of Langerhans and that altered cellular Ca2+ homeostasis may be involved in defective insulin release [6], it is an essential signaling molecule in the induction of dementia, and an increased risk of dementia was observed in patients with diabetes [7]. Interestingly, TRPC1 activity is positively associated with vascular smooth muscle cell (VSMC) proliferation and neointimal hyperplasia, which play essential role in progress of DN [8]. So TRPC1, reduced in the kidney of diabetic animal models, may play a vital function in the progress of DN [9]. However, the role of TRPC1 on the DN is no clear. The mechanism of TRPC1 can contribute to the development of diabetic nephropathy remains to be elucidated.
In present study, we studied whether TRPC1 exerted a protective role against DN as well as its possible protective mechanisms using in vivo diabetic rat models. The experimental results showed TRPC1 could play the renoprotective effect in DN, which indicates that TRPC1 may be developed as a candidate drug for preventing DN.
Materials and Methods
Materials and reagents
TRPC1 recombinant protein was obtained from Abnova Corp. (Taipei City, Taiwan). Streptozotocin (STZ) was purchased from Sigma–Aldrich, St. Louis, USA. Antibodies against transforming growth factor-β1 (TGF-β1), nuclear factor kappa B (NF-κB p65) and p-NF-κB p65 were purchased from Cell Signaling Technology, Inc. (Boston, USA). Antibody against β-actin was purchased from Boster Co., Ltd. (Wuhan, China).
Experimental animals for diabetic nephropathy
Male Sprague-Dawley (SD) rats were purchased from Shanghai SLAC laboratory animal Co., LTD. All animals were housed under standard conditions with unrestricted food and water. After one-week adaptive breeding, a high-fat diet was given. After 8-weeks of high-fat diet, diabetes was induced by a single intraperitoneal injection of STZ at a dose of 30 mg/kg diluted in the citrate buffer (0.1 mol/L, pH 4.0). Seventy-two hours after STZ injection, diabetes was diagnosed when their random blood glucose levels over 16.7 mmol/L for 3 days. After another 8-week of high-fat diet, the rats with maintaining blood glucose >16.7 mmol/L were classified as successful diabetes model and used in the study [10]. At this time, 24 h urine output was above 150% of the original amount of urine and urinary protein excretion was above 30 mg/24 h, indicating the model of DN [11]. Age-matched normal rats served as control. The STZ induced diabetic rats were randomly divided into 3 groups (n=8) as follows: TRPC1 for 3 weeks in which rats were induced by an intravenous injection of 20 μg of TRPC1 one time for 3 weeks; TRPC1 for 6 weeks in which rats were injected with 20 μg of TRPC1 two times for 6 weeks (one time per 3 weeks); diabetic rats in which rats were injected with an equal volume of PBS two times for 6 weeks. The normal control group was simultaneously injected with an equal volume of PBS two times for 6 weeks also. After STZ or control injections, the blood glucose and body weight were monitored weekly. The rats were housed individually in metabolic cages. The 24 hours urine samples were collected on the day before the end of the experiment and were stored at -80°C. After 3 or 6 weeks of treatment, blood samples were collected from the retro-orbital venous plexus then rats were euthanized via pentobarbital overdose (60 mg/kg) and both kidneys were removed. Serum was collected by centrifugation at 3000 g for 15 min and stored at -80°C. Urine albumin concentration was determined using the rat albumin ELISA kit (Assaypro, USA) according to the manufacturer’s instructions. The serum levels of glucose, urea nitrogen and creatinine were determined using an Automatic Biochemical Analyzer (Hitachi Co., Ltd., Japan). Kidney samples were weighed and frozen in liquid nitrogen and stored at -80°C or fixed in 10% neutralbuffered formalin.
Morphological and immunohistochemical analysis
The kidneys were harvested and fixed with 4% formaldehyde for paraffin embedding and were sliced into 4 μm sections. The tissue slices were sliced for periodic acid-Schiff (PAS) staining. For immunohistochemical analysis, the paraffin sections were deparaffinized, rehydrated and incubated with 3% H2O2 followed by incubation with pancreatic enzyme at 37°C for 15 min. The tissue slices were incubated with primary antibodies (TGF-β1, NF-κB p65, diluted 1:200) at 4°C overnight. The slices were then washed with PBS and incubated with a peroxidase-labeled second antibody (diluted 1:200) at room temperature for 30 min. The slices were washed again with PBS and incubated with a Vectastain ABC kit (Vector, UK) according to the manufacturer’s instructions. The slides were washed and visualized using 3, 3-diaminobenzidine (DAB kit, MaiXin, China). The slices were counterstained with hematoxylin, dehydrated and mounted. Brown areas were judged as positive staining. The results were viewed under a confocal FV1000 SPD laser-scanning microscope (Olympus).
Quantitative real-time RT-PCR
Total RNA was isolated and purified from the kidney tissue and the concentrations were determined by using a microspectrophotometer. The cDNA was prepared by using these samples, according to the protocols provided by a commercially available QuantiTect Reverse Transcription kit (Qiagen, Germany). Real-time PCR was carried out using the SYBR Green PCR Master Mix and the 7500 FAST Real-time PCR System and specific primers as reported previously [12]. All reactions were carried out at least in triplicate and primer sequences used in the PCR were listed as followed:
β-actin forward 5’- AACTCCATCATGAAGTGTGA -3’
Reverse 5’- ACTCCTGCTTGCTGATCCAC-3’
TGF-β1 forward 5’- GAGAGCCCTGGATACCAACTA -3’
Reverse 5’- CTGGTGTGTCCAGGCTCCAAATGT-3’
Western blot
Proteins were extracted from the kidney tissue by using RIPA buffer (containing 0.1% PMSF). Equal amounts of protein from each sample (50 μg) were electrophoresed on 10% sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) gels and transferred to polyvinylidene fluoride (PVDF) membranes. The membranes were blocked by 5% nonfat milk for 30 minutes and then incubated with the following antibodies: TGF-β1 (1:1000), NF-κB (1:800), p-NF- κB (1:1000), β-actin (1:1000) at 4°C overnight, and horseradish peroxidase (HRP) rabbit anti-rat secondary antibody (1:5000) for 2 h. The blots were detected by chemiluminescent substrate. The densitometric analysis was conducted with ChemiDoc MP Imaging System (BIO-RAD). Each band normalized to β-actin presented the relative density values.
Statistical analysis
All of the results were expressed as the means ± SEM. Statistical analyses were performed using IBM SPSS Statistics 19.0 software. Statistical significance among multi-groups was determined using one way analysis of variance and Tukey’s test post hoc analysis. Statistical significance between two groups was determined using the unpaired Student’s t-test. P<0.05 was considered statistically significant.
Results
TRPC1 ameliorated biochemical parameters and kidney hypertrophy in STZ-induced diabetic rats
As showed in Table 1, the STZ-induced diabetic rats had significantly higher blood glucose, urine albumin, urea nitrogen and creatinine compared with the normal control group (P<0.01 or P<0.001, respectively). Besides, the body weight of rats markedly decreased in diabetic rats compared with the normal control group (P<0.01) (Table 1). However, the diabetic rats treated with TRPC1 significantly ameliorated biochemical parameters compared with untreated diabetic rats, especially in rats treated with TRPC1 for 6 weeks. The kidney hypertrophy index (kidney/body weight ratio) increased significantly (P<0.01) in the diabetic rats compared to the control rats. However, the degree of kidney hypertrophy index decreased after treatment with TRPC1.
| Groups | Ctrl | DM | DM+TRPC1 | |
|---|---|---|---|---|
| 3 wk | 6 wk | |||
| Body weight (g) | 448 ± 27.8 | 337 ± 14.2## | 364 ± 15.6 | 382 ± 14.7* |
| Kidney/body weight ratio (mg/g) | 4.3 ± 1.2 | 6.3 ± 1.4## | 6.1 ± 1.2 | 5.7 ± 1.4* |
| Blood glucose (mmol/L) | 7.23 ± 1.32 | 25.45 ± 5.25### | 22.72 ± 4.56* | 20.88 ± 5.29* |
| Serum creatinine (μmol/L) | 55.4 ± 11.2 | 82.3 ± 12.6## | 75 ± 10.5 | 67 ± 9.8** |
| Blood urea nitrogen (mg/dl) | 16 ± 4.5 | 38 ± 7.2## | 30 ± 5.2* | 26 ± 6.3* |
| Urine protein (μg/24 h) | 1.4 ± 0.5 | 11.2 ± 6.5## | 8.9 ± 3.8 | 6.2 ± 2.6* |
| Ctrl: Normal Control; DM: Diabetic Model Control; TRPC1: 20 μg TRPC1; Ctrl and DM were treated with equal volumes of PBS. All the mice were administered via intravenous injection. ##P<0.01, ###P<0.001 vs. Ctrl group. *P<0.05, *P<0.01 vs. DM group. | ||||
Table 1. The effects of TRPC1 on the biochemical parameters and renal function of STZ-induced diabetic rats at the end of the experimental period. Data are expressed as the mean ± S.E.M. (n=8 for each group).
TRPC1 improved glomerular morphological changes in STZ-induced diabetic rats
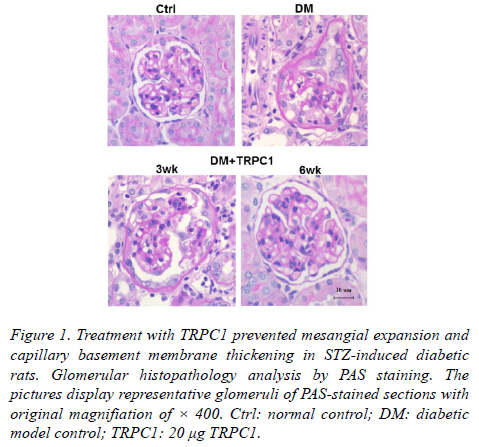
The diabetic model group showed the thicken mesangial expansion and capillary basement membrane by PAS staining (Figure 1).
Figure 1. Treatment with TRPC1 prevented mesangial expansion and capillary basement membrane thickening in STZ-induced diabetic rats. Glomerular histopathology analysis by PAS staining. The pictures display representative glomeruli of PAS-stained sections with original magnifiation of × 400. Ctrl: normal control; DM: diabetic model control; TRPC1: 20 μg TRPC1.
As treatment with TRPC1 time progressed, there was decreased mesangial expansion and capillary basement membrane thickening. Particularly, the PAS staining showed nearly normal glomerular structure following a 6-weeks treatment of TRPC1.
TRPC1 decreased the excessive accumulation of inflammatory cytokines in STZ-induced diabetic rats
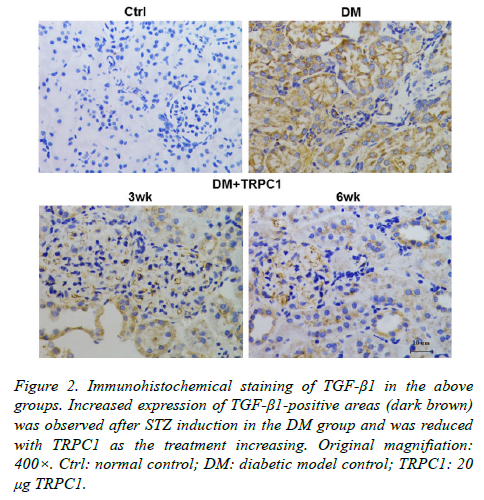
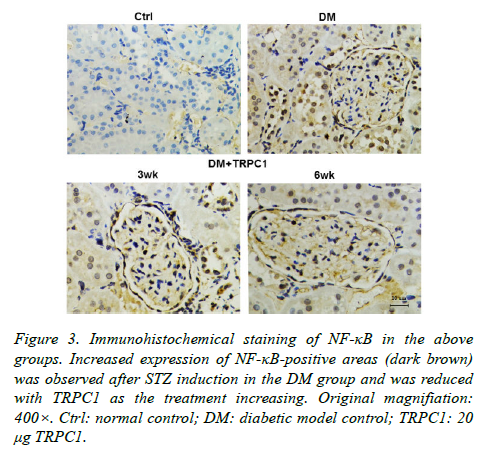
The STZ-induced diabetic rats exhibited an increase in positive areas of TGF-β1 (Figure 2) and NF-κB (Figure 3) accumulated in the mesangial matrix. After 3 weeks treatment with TRPC1, the positive areas of TGF-β1 and NF-κB decreased slightly compared with the diabetic control group. However, 6 weeks treatment with TRPC1 exhibited significantly less positive areas of TGF-β1 and NF-κB.
Figure 2. Immunohistochemical staining of TGF-β1 in the above groups. Increased expression of TGF-β1-positive areas (dark brown) was observed after STZ induction in the DM group and was reduced with TRPC1 as the treatment increasing. Original magnifiation: 400×. Ctrl: normal control; DM: diabetic model control; TRPC1: 20 μg TRPC1.
Figure 3. Immunohistochemical staining of NF-κB in the above groups. Increased expression of NF-κB-positive areas (dark brown) was observed after STZ induction in the DM group and was reduced with TRPC1 as the treatment increasing. Original magnifiation: 400×. Ctrl: normal control; DM: diabetic model control; TRPC1: 20 μg TRPC1.
TRPC1 inhibited the activation NF-κB of in STZinduced diabetic rats
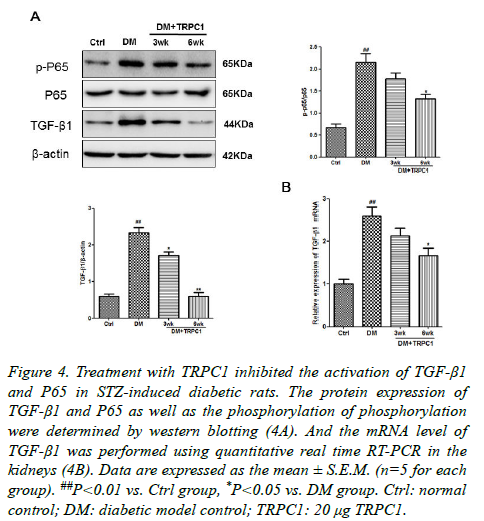
In order to further explore the mechanism of TRPC1 on the DN. We determined the expression of NF-κB and TGF-β1 in the kidney tissues. There were a significantly increase in the phosphorylation of NF-κB as well as TGF-β1 in the diabetic group compared with the normal control group (Figures 3A and 3B). Although TGF-β1 expression was higher than the normal control group after treatment, they were lower than the diabetic group. Meanwhile, the TRPC1 treated diabetic rats had decreased the both TGF-β1 and the phosphorylation of NF-κB protein expression compared with the diabetic and normal control groups, especially in the diabetic rats treated with TRPC1 for 6 weeks. It was also noted that the level of TGF-β1 in treated diabetic rats was lower than the normal control group.
Discussion
In this present study, we demonstrated that diabetic rats induced by a high-fat diet and STZ exhibited a number of early clinical and pathological characteristics of DN. Treatment with TRPC1 improved the above changes with time dependently, indicating its renoprotective effect during the progress of DN. In addition, we also determined that TGF-β1 expression was significantly increased in STZ-induced diabetic rats, which was consistent with previous reports. In addition, treatment with TRPC1 effectively decreased TGF-β1 and NF-κB expression, indicating that TRPC1 could improve kidney in the release of inflammatory cytokines. After treatment of TRPC1, it showed a significant decrease, particularly in the diabetic rats treated with TRPC1 for 6 weeks. In conclusion, the results from the experiment showed that TRPC1 could ameliorate the diabetic rat though inhibiting the release of NF-κB, which is the central inflammatory factor, suggesting that TRPC1 is a potential function proteasome which may play an effective role in the development of DN (Figure 4).
Figure 4. Treatment with TRPC1 inhibited the activation of TGF-β1 and P65 in STZ-induced diabetic rats. The protein expression of TGF-β1 and P65 as well as the phosphorylation of phosphorylation were determined by western blotting (4A). And the mRNA level of TGF-β1 was performed using quantitative real time RT-PCR in the kidneys (4B). Data are expressed as the mean ± S.E.M. (n=5 for each group). ##P<0.01 vs. Ctrl group, *P<0.05 vs. DM group. Ctrl: normal control; DM: diabetic model control; TRPC1: 20 μg TRPC1.
TGF-β1, as an important inflammatory cytokine, has been reported to effectively delay the development of DN though inhibiting mesangial cell proliferation and excessive secretion of extracellular matrix [13]. The abnormal production of TGF- β1 induced by hyperglycemia causes an excessive accumulation of extracellular matrix (ECM) proteins, which ultimately leads to mesangial expansion and glomerular basement membrane thickening. Therefore, TGF-β1 has been proposed as an intervention target for DN treatment. Moreover, NF-κB has been recognized as an important inflammatory cytokine in DN as previously mentioned. The inhibition of NF- κB expression enhances DN treatment [14]. Therefore, NF-κB has been proposed as an intervention target for DN treatment. In the present study, we determined that the STZ-induced diabetic rats had increased TGF-β1 and NF-κB expression in the renal tissue. However, treatment with TRPC1 could effectively inhibit the secretion of TGF-β1 and decrease the phosphorylation of NF-κB in STZ-induced diabetic rats.
The function of the TRPC1 protein in vascular smooth muscle cells has been well-studied over the past few years. The role of this function plays a core role in the progress of DN. An earlier study by Zhang et al. [8] had been demonstrated that TRPC1 genetic polymorphism may not fundamentally contribute to the development of DN, while reduction of the gene expression in kidney may be a late phen. TRPC1 expression is reduced in kidney tissues from patients with diabetic nephropathy or from Zucker diabetic rats, compared with controls [15]. TRPC1 dysfunction might contribute to the development of diabetic nephropathy. Thus, in this study, we demonstrated the role of TRPC1 in DN, indicating that TRPC1 could play the renoprotective and anti-inflammation effects after given TRPC1’s high concentration in plasma.
In conclusion, we demonstrated that TRPC1 could ameliorate the body weight, blood glucose, urine albumin, urea nitrogen and creatinine in the diabetic rat. In addition, TRPC1 also improved renal dysfunction and kidney inflammatory associated with suppressing the activation of TGF-β1 and NF- κB expression in STZ-induced diabetic rats. Currently, the function of TRPC1 has been poorly understood, the definitive molecular mechanism of TRPC1 inhibition of the NF-κB pathway in DN needs to be investigated in future. According to the American Diabetes Association (ADA), the pre-diabetic condition is related to increased cardiovascular risk profile of affected individuals, as demonstrated by several studies which revealed the impairment in early markers of atherosclerosis due to pre-diabetes. Family history of diabetes, for example, account for an increase in cardiovascular risk of individuals even if such subjects have no sign of pre-diabetes or diabetes: the demonstration of an insulin resistance condition can account for the induction of an endothelial impairment able to enhance atherosclerotic disease [16]. Hence, it is very important to identify the related gene contributed to DN pathology development, and TRPC1 plays a critical role in renoprotection through specific gene modification, but the whole picture of contextual regulation is indistinct and need an intensive study. As the microRNAs have been shown to play a more and more important role in coupling lipid metabolism and atherosclerosis to ameliorate diabetes mellitus, it indicates a certain therapeutic potential on the DN [17,18]. Together with those findings, in the further study, we may use the miRNAs to research the role of TCR1 on the progress of DN, which could help us to understand deeply its protective molecular mechanism.
Acknowledgements
This study was supported by the Natural Science Foundation of Jiangsu Province of China (BK20131092).
References
- Mahmoud RG, Youssef F. Predictors of diabetic nephropathy. Central European Journal of Medicine 2013; 8: 287-296.
- Yang M, Xu J, Yu J, Yang B, Gan H, Li S, Li X. Anti-inflammatory effects of 1,25-dihydroxyvitamin D3 in monocytes cultured in serum from patients with type 2 diabetes mellitus and diabetic nephropathy with uremia via Toll-like receptor 4 and nuclear factor-κB p65. Mol Med Rep 2015; 12: 8215-8222.
- Zhou L, Xu DY, Sha WG, Shen L, Lu GY, Yin X. Long Non-Coding MIAT Mediates High Glucose-Induced Renal Tubular Epithelial Injury. Biochem Biophys Res Commun 2015.
- Vassort GF. Transient receptor potential, TRP channels: a new family of channels broadly expressed. Med Sci (Paris) 2008; 24: 163-168.
- Kwan HY, Shen B, Ma X, Kwok YC, Huang Y, Man YB, Yu S, Yao X. TRPC1 associates with BK (Ca2+) channel to form a signal complex in vascular smooth muscle cells. Circ Res 2009; 104: 670-678.
- Santulli G, Pagano G, Sardu C, Xie W, Reiken S, D'Ascia SL, Cannone M, Marziliano N, Trimarco B, Guise TA, Lacampagne A, Marks AR. Calcium release channel RyR2 regulates insulin release and glucose homeostasis. J Clin Invest 2015; 125: 1968-1978.
- Santulli G, Marks AR. Essential Roles of Intracellular Calcium Release Channels in Muscle, Brain, Metabolism, and Aging. Curr Mol Pharmacol 2015; 8: 206-222.
- Xu SZ, Beech DJ. TrpC1 is a membrane-spanning subunit of store-operated Ca (2+) channels in native vascular smooth muscle cells. Circ Res 2001; 88: 84-87.
- Zhang D, Freedman BI, Flekac M. Evaluation of genetic association and expression reduction of TRPC1 in the development of diabetic nephropathy. Am J Nephrol 2009; 29: 244-251.
- Chen P, Chen JB, Chen WY, Zheng QL, Wang YQ, Xu XJ. Effects of quercetin on nuclear factor-κB p65 expression in renal ubiquitin-proteasome system of diabetic rats. Zhonghua Nei Ke Za Zhi 2012; 51: 460-465.
- Fang D, Wan X, Deng W, Guan H, Ke W, Xiao H, Li Y. Fufang Xue Shuan Tong capsules inhibit renal oxidative stress markers and indices of nephropathy in diabetic rats. Exp Ther Med 2012; 4: 871-876.
- Yunusi K, Zhang J, Zhong L, Mosha G, Nuermaimaiti A, Abudula M, Upur H. Uygur Medicine Xipayi Kui Jie'an Affects Gene Expression profiles in intestinal tissue Lesions in a Rat Model of Ulcerative Colitis. BMC Complement Altern Med 2015.
- Valladares-Salgado A, Angeles-Martínez J, Rosas M, García-Mena J, Utrera-Barillas D, Gómez-Díaz R, Escobedo-de la Peña J, Parra EJ, Cruz M. Association of polymorphisms within the transforming growth factor-β1 gene with diabetic nephropathy and serum cholesterol and triglyceride concentrations. Nephrology (Carlton) 2010; 15: 644-648.
- Wada J, Makino H. Innate immunity in diabetes and diabetic nephropathy. Nat Rev Nephrol 2016; 12: 13-26.
- Ito D, Cao P, Kakihana T, Sato E, Suda C, Muroya Y, Ogawa Y, Hu G, Ishii T, Ito O, Kohzuki M, Kiyomoto H. Chronic Running Exercise Alleviates Early Progression of Nephropathy with Upregulation of Nitric Oxide Synthases and Suppression of Glycation in Zucker Diabetic Rats. PLoS One 2015; 10: e0138037.
- Ciccone MM, Scicchitano1P, Cameli M, Cecere A, Cortese F. Endothelial Function in Pre-diabetes, Diabetes and Diabetic Cardiomyopathy: A Review. J Diabetes Metab 2014; 5: 364.
- Santulli G. MicroRNAs and Endothelial (Dys) Function. J Cell Physiol 2015.
- Novák J, Olejníčková V, Tkáčová N, Santulli G. Mechanistic Role of MicroRNAs in Coupling Lipid Metabolism and Atherosclerosis. Adv Exp Med Biol 2015; 887: 79-100.