ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
- Biomedical Research (2016) Volume 27, Issue 1
In vitro genotoxicity evaluation of tungsten (VI) oxide nanopowder using human lymphocytes.
| Bugra G. Akbaba1*, Hasan Turkez2, Erdal Sonmez3, Ugur Akbaba4, Elanur Aydin5, Abdulgani Tatar6, Guven Turgut7, Salim Cerig5 1Bioengineering Department, Faculty of Engineering and Architecture, Kafkas University, Kars, Turkey 2Department of Molecular Biology and Genetics, Faculty of Science, Erzurum Technical University, Erzurum, Turkey 3Graduate School of Natural and Applied Sciences, Department of Nanoscience & Nanoengineering, Advanced Materials Research Laboratory, Atatürk University, Erzurum, Turkey 4Department of Elementary Mathematics Education, Education Faculty, Kafkas University, Kars, Turkey 5Atatürk University, Faculty of Science, Department of Biology, 25240, Erzurum, Turkey 6Medical Genetics Department, Faculty of Medicine, Ataturk University, Erzurum, Turkey 7Department of Physics. K. K Education Faculty, Atatürk University, Erzurum, Turkey |
| Corresponding Author: Dr. Giray Bugra Akbaba, Kafkas University, Faculty of Engineering and Architecture, Bioengineering Department, 36100, Kars/Turkey. |
| Accepted December 24, 2015 |
Tungsten (VI) oxide (or tungsten trioxide) (WO3, <100 nm particle size) nanoparticles (NPs) are used for many purposes including production of electrochromic windows, or smart windows, x-ray screen and gas sensors in everyday life. However, their carcinogenicity and genotoxicity have not been sufficiently evaluated. Therefore, the genotoxic potential of WO3 nanoparticle was examined in cultured human lymphocytes by the use of the micronucleus (MN) test and the comet (SCGE) assay. Freshly isolated human lymphocytes were exposed to WO3 nanoparticle at concentrations ranging from 0 to 500 μM for 72 hours at 37°C. Our results indicated that 400 and 500 μM of WO3 nanoparticle treatment caused slight increases of the MN frequencies in cultured human lymphocytes. Likewise, WO3 nanoparticle (at concentrations above 200 μM) led to increases of DNA damage (estimated with the comet assay) in human lymphocytes. The observed alterations in the MN and the comet assay parameters revealed that WO3 nanoparticles have genotoxic potential and could pose environmental and human health risk.
Keywords |
| Genotoxicity, Health risk, Human lymphocytes, Nanoparticle, Tungsten trioxide |
Introduction |
| Nanoparticles (NPs) are identified as particles with diameters under 100 nm, are unique in that their electronic, chemical, and physical properties enable many promising technical and medicinal applications [1,2]. Thanks to their unique features, NPs have been the focus of much research such as in industrial applications, environmental toxicity studies and human health impacts. Various industrial NPs are made from titanium oxide, silver, gold, cadmium selenite, other carbon NPs, cerium oxide and hydroxyapatite NPs [3-6]. Together with the fast development of nanotechnology today, NPs are used for various biomedical applications such as targeted delivery/ imaging, hyperthermia, cell therapy and stem cell tracking [7-14]. In recent years, many efforts were made to investigate the toxicity of micro sized natural and man-made mutagens to human life and the ability of therapeutic substances to reduce the toxicity of these chemicals [15-20]. But the toxic effects of NPs were not fully detailed except for some inorganic and organic NPs. In fact, the most recent report indicated that there was a lack of systematic assessment of the DNA damaging and carcinogenic potential of NPs in spite of their extensive use in nanotechnological applications. People are exposed to NPs from various sources and in many pathways, including inhalation, dermal absorption, eye contact and oral ingestion [21,22]. Therefore, the evaluation of NPs toxicity has become very important for public health and the environment [23-25]. |
| Tungsten trioxide contains oxygen and the transition metal tungsten. It is gained as an intermediate in the recovery of tungsten from its minerals. To produce tungsten products tungsten ores are treated with alkalis. Tungsten trioxide can be prepared in several different ways. Scheelite (CaWO4) is allowed to react with HCl to produce tungstic acid, which decomposes to WO3 and water at high temperatures. Another common way to synthesize WO3 is by calcination of ammonium paratungstate (APT) under oxidizing conditions. There are many applications of the WO3 in everyday life. It is used in industry to manufacture tungstates for fireproofing fabrics, for x-ray screen, in gas sensors, automobile glass and as a pigment in ceramics and paints because of its rich yellow color [26-30]. In recent years, WO3 has been employed in biomedical applications as an endovascular coil, endovascular catheter and bone cement [31-33]. Although tungsten had been considered a relatively inert and toxicologically safe material, recent research findings have raised concerns about possible deleterious health effects after acute and chronic exposure to this metal [34, 35]. It was reported that soluble tungsten compounds were absorbed after oral exposure both in humans and in laboratory rats. It has been shown that the embedded tungsten alloy pellets caused metastatic tumors in rats. Tungsten was found to accumulate in several organs and/or tissues such as kidneys, liver, ovaries, prostate, pancreas, lung, heart, muscle, spleen and bone following a single oral dose [36]. In addition, a previous report indicated the potential for tungsten alloy-induced immunotoxicity [37]. The genotoxic potential of tungsten and tungsten compounds has not been extensively assessed [38]. Considering the latest information, the mutagenic potential of WO3 nanopowder has not been accurately perused. Thus, in this paper, we thoroughly investigate the cytotoxic and genotoxic potentials of WO3 nanoparticles in human lymphocytes culture by using the micronucleus (MN) test and the comet (SCGE) assay. |
| Materials and Methods |
Synthesis of tungsten trioxide nanoparticles |
| Metal oxide based semiconductors such as SnO2, ZnO, TiO2, CuO and WO3 etc. have been used in many application areas [39]. Among these, WO3 is one of the most valuable materials for electrochromic devices, information displays, smart windows and rechargeable lithium batteries [40]. WO3 as transitional metal oxide not only has reversible electrochromism property and special catalysis property [41]; at one time because of its big surface area, WO3 can be used excellent solar absorb material and contact material: but also WO3 belonging to an n-type semiconductor has excellent gas sensing property [42]. In the recent years, nanopowder WO3 materials have gained much attention due to its surface to volume ratio, which is much greater than that of coarse-grained materials [43]. There is a wide range of techniques for preparation of the powders such as the sol-gel process [44], the micro emulsion method [42], the inert gas condensation method and the chemical vapor condensation process [45]. Among these techniques, the sol-gel technique is attractive due to its easy manipulation of the samples, simplicity, safety, low cost [46], and easy control of chemical components [47]. The sol-gel method involves the dispersion of metallic salts in solutions. The sol is later ?solidified? through stages of stiffening and polymerization to give a gel (gelation) [48]. The gel so obtained is thoroughly washed with distilled water or alcohol, filtered, dried and finally heated to high temperatures to obtain the required material [49]. Therefore, in present study, WO3 nanopowders were prepared via the sol-gel process. The experimental construction is shown in Figure 1. Firstly, nitric acid (HNO3) solution was added drop by drop to sodium tungstate (Na2WO4.2H2O), so tungstic acid deposit was formed. Oxalic acid (H2C2O4) and citric acid (C6H8O7) were used as complex forming agents in the sol solution. The precipitate obtained from this solution was washed several times with absolute ethyl alcohol and then dried at 50°C. In this manner yellow precipitates were formed and WO3 powder was produced with calcination at 550°C for 3h. |
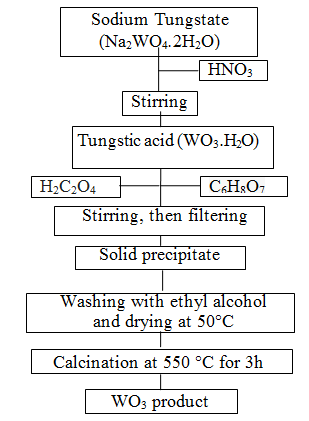 |
| Figure 1: Schematic diagrams of steps involved in obtaining WO3 powders |
Lymphocyte cultures |
|
Typically, two or three donors have been used in toxicity studies [18,19]. In this study, four donors were used to increase the statistical reliability. Human peripheral blood samples were drawn from healthy volunteers (age = 30 years), by venipuncture in heparinized tubes. Leukocytes (lymphocytes +monocytes) were isolated on a Ficoll-Paque gradient, washed with Phosphate Buffered Saline (PBS) and resuspended in Ham?s F10 medium containing 15% foetal calf serum (FCS). Lymphocytes were stimulated to divide by 2% phytohaemagglutinin (PHA). Cultures were set up at a concentration of 0.5 Ã References
|