ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 5
Improve the production of recombinant human factor ix in CHO cells by adding metal ions
Hong Liang, Baojin Fei*, Hu Shen, Tao Lei, Chunlei Song, Xiaojiao Li, Yantao Nie, Liheng Wu
Department of Research Management, Recombinant-factor Project, Chengdu Rongsheng Pharmaceuticals Co. Ltd., China National Pharmaceutical Group Corporation, Keyuan South Road, Hi-Tech Zone, Chengdu, Sichuan, PR China
- *Corresponding Author:
- Baojin Fei
Department of Research Management
Recombinant-factor Project Chengdu Rongsheng
Pharmaceuticals Co. Ltd. China National
Pharmaceutical Group Corporation, PR China
Accepted on October 10, 2016
Factor IX is a vitamin K-dependent coagulation factor which undergoes several post-translational modifications for its proper function. We established the Chinese hamster ovary cells expressing the recombinant human coagulation factor IX (rhFIX). In order to maximize the expression of rhFIX in serum-free suspension culture, we evaluated the effect of several metal ion concentrations including Ca2+ and Mg2+ in the cell serum-free culture medium. A high production FIX CHO cell line was screened and the highest clotting activity was obtained in 2 mM Ca2+, combined with 1 mM Mg2+ showed an excellent improvement compared with culture without any metal ion addition, the peak FIX activity was 1.36 IU/ml compared with 0.62 IU/ml. It means a higher level of fermentation process and the CHO cell line is a promising alternative for recombinant FIX manufacturing.
Keywords
Factor IX, Ca2+, Mg2+, Serum-free suspension culture, Clotting activity.
Introduction
Human Factor IX (FIX), which is synthesized in the liver, is an essential protein required in the blood coagulation cascade. Its deficiency due to expression of defective protein or lack of FIX expression causes a severe bleeding disorder, haemophilia B [1]. So, current haemophilia B management involves replacement therapy with coagulation Factor IX (FIX) concentrates, either given episodically on demand to treat bleeding episodes or prophylactically to maintain minimum FIX activity and to prevent bleeding [2-6].
We engineered Chinese Hamster Ovary (CHO) cell line clones secreting hFIX. The expression of factor IX at high levels in CHO cells results in the secretion of a mixture of uncarboxylated or carboxylated factor IX with or without propeptide, presumably due to saturation of the endogenous processing activity [7-9]. At present, there are only three commercial FIX including two commercial products just have been approved by the FDA in 2015. Therefore, at present, it will be valuable to develop culture process for the high production of a properly processed recombinant FIX [9].
Calcium ions (Ca2+), required for human FIX functional activity, are the essential components of the coagulation cascade [10], and various polyvalent metal ions including magnesium ion (Mg2+) have been shown to interact with the Ca2+-binding sites in Gla domains of vitamin K-dependent coagulation factors including FIX [11]. The FIX Gla domain has a specific Mg2+-binding site(s) and Mg2+ accelerates Ca2+- dependent activation of factor IX [12,13]. To improve FIX production from CHO cells, we evaluated the effect of several metal ion concentrations including Ca2+ and Mg2+ in the cell serum-free culture medium, and the production of FIX was greatly improved under the optimal condition. A promising CHO cell line for FIX manufacturing was obtained to alleviate the shortage of therapeutic FIX in China and around the globe.
Materials and Methods
Cell line and culture medium
The CHO cell line clone secreting hFIX was established by introducing human FIX cDNA into CHO cells (DG44) purchased from Invitrogen (America) by a cationic liposome transfection reagent, Lipofectamine2000 (Invitrogen, America) and adapting at gene amplification system using dihydrofolate reductase. The primary amino acid sequence is identical to the Ala148 allelic form of plasma-derived factor IX as same as two commercial FIX, BeneFIX (Pfizer) and Rixubis (Baxter). The stable CHO cell line clone with the highest production of FIX studied in this paper was screened out from 10000 clones by limiting dilution method. The expression vector with two cloning sites both under pcmv promoter was constructed by our own laboratory, and the human FIX cDNA was inserted in a cloning site of the vector by BssH II and XbaI (TaKaRa, Japan).
CDM4PERMAb chemically defined medium containing no animal derived components, a product of Thermo Scientific HyClone, was applied in this study. It has been developed to increase process yields in the production of recombinant proteins, and successfully tested in a variety of applications. When used, CDM4PERMAb was supplemented with 5 μg/ml vitamin K1 (Sigma) and 0.05 μM MTX (Sigma).
Cell culture
CHO cells producing FIX were cultured in 125 Erlenmeyer flasks containing 30 ml culture medium and agitated at 100 RPM with an orbital shaker in a humidified, 5% CO2 incubator at 37˚C. Cell concentration during culture was measured using a haemocytometer by tryphan blue exclusion method. For determination of Ca2+, Mg2+, Mn2+, Cu2+, and Zn2+ effects on FIX production, CDM4PERMAb with 0.1 mM, 0.5 mM, 1 mM, 2 mM, of Ca2+, Mg2+, Mn2+, Cu2+, and Zn2+ were used. Culture supernatants were aliquoted and kept frozen at -80˚C for later analyses after centrifugation. Chemicals used in this study were of analytical grade.
Measurement of FIX activity
Biophen Factor IX [5] (HYPHEN Biomed, France) was used to measure the FIX activities of the culture supernatants. In presence of thrombin, phospholipids and calcium, first Factor XIa, supplied in the assay at a constant concentration and in excess, activates FIX, present in the tested sample, into FIXa, which forms an enzymatic complex with thrombin activated factor VIII: C, also supplied in the assay at a constant concentration and in excess, Phosphplipids (PLPs) and calcium, that activates factor X, present in the assay system, into factor Xa. This activity is directly related to the amount of factor IX, which is the limiting factor. Generated factor Xa is then exactly measured by its specific activity on factor Xa chromogenic substrate (SXa-11). Factor Xa cleaves the substrate and releases pNA determined by colour development at 405 nm, after stopping the reaction by 20% acetic acid. The amount of pNA generated is directly proportional to the factor IXa activity.
Results
Cell growth in different metal ions
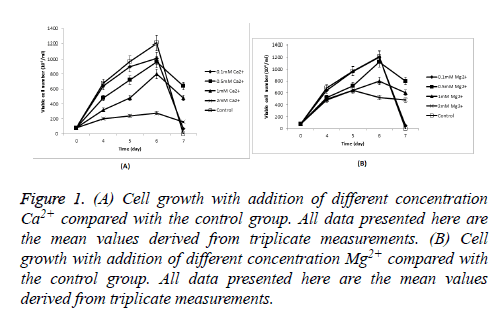
Cells producing FIX were seeded at 8 × 105 cells/ml in Erlenmeyer flasks with 30 ml CDM4PERMAb. Cell growth during culture with addition of Ca2+ and Mg2+ respectively was shown in Figure 1. Cells in Ca2+ and Mg2+ with the Lowest concentration (0.1 mM) respectively showed similar growth and viability (80% and 80.4% viability on day 6 respectively) to culture without any metal ion addition (80.8% viability on day 6, and others were below 70% on day 6), but better culture time and viability were obtained when an increase in the concentration of Ca2+ and Mg2+ respectively. Cell growth was most greatly inhibited in 2 mM Ca2+, in which the maximum viable cell density during culture was just 28 × 105 cells/ml, compared with 120.8 × 105 cells/ml when culture without any metal ion addition (Table 1).
Figure 1: (A) Cell growth with addition of different concentration Ca2+ compared with the control group. All data presented here are the mean values derived from triplicate measurements. (B) Cell growth with addition of different concentration Mg2+ compared with the control group. All data presented here are the mean values derived from triplicate measurements.
| Ion addition | Control | 0.5 mM Ca2+ | 1 mM Ca2+ | 2 mM Ca2+ | 0.5 mM Ca2+ and 0.5 mM Mg2+ | 0.5 mM Ca2+ and 1 mM Mg2+ | 1 mM Ca2+ and 0.5 mM Mg2+ | 1 mM Ca2+ and 1 mM Mg2+ | 2 mM Ca2+ and 0.5 mM Mg2+ | 2 mM Ca2+ and 1 mM Mg2+ |
|---|---|---|---|---|---|---|---|---|---|---|
| The maximum cell density (104/ml) | 1208 | 960 | 800 | 280 | 1316 | 944 | 984 | 808 | 352 | 244 |
| The maximum FIX activity (IU/ml) | 0.62 | 0.83 | 0.74 | 1.1 | 0.77 | 0.79 | 0.85 | 1.1 | 1.15 | 1.36 |
Table 1. The maximum cell density and the maximum FIX activity under several fermentation conditions. All data presented here are the mean values derived from triplicate measurements.
Clotting activities of different culture supernatant
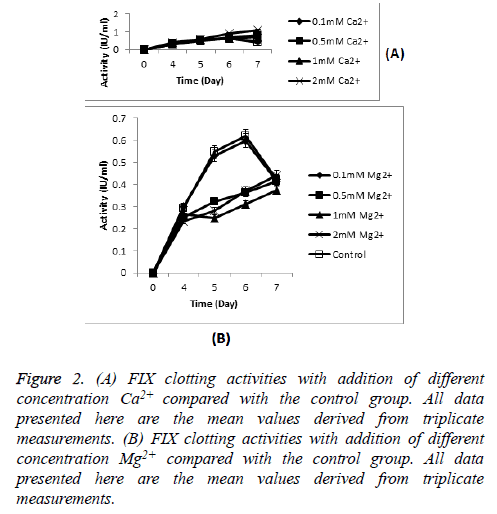
FIX clotting activities of culture supernatant containing different concentration of Ca2+ and Mg2+ were shown in Figure 2. Higher activity with more mature FIX molecules secreted was obtained when an increase in the concentration of Ca2+. The highest clotting activity was obtained in 2 mM Ca2+, showed an excellent improvement compared with culture without any metal ion addition (the peak FIX activity was 1.1 IU/ml compared with 0.62 IU/ml). But culture with Mg2+ showed an opposite effect. Culture with 0.1 mM Ca2+ and 0.1 mM Mg2+ respectively showed the similar highest activity to culture without any metal ion addition. Moreover, there was no significantly statistical difference between culture with 0.5 mM Ca2+ and 1 mM Ca2+ (0.83 IU/ml and 0.74 IU/ml on day 7, respectively), and no significantly statistical difference among culture with 0.5 mM Mg2+, 1 mM Mg2+ and 2 mM Mg2+ (0.42 IU/ml, 0.38 IU/ml and 0.44 IU/ml on day 7, respectively).
Figure 2: (A) FIX clotting activities with addition of different concentration Ca2+ compared with the control group. All data presented here are the mean values derived from triplicate measurements. (B) FIX clotting activities with addition of different concentration Mg2+ compared with the control group. All data presented here are the mean values derived from triplicate measurements.
Cell growth and clotting activities when calcium ion combined with magnesium ion addition
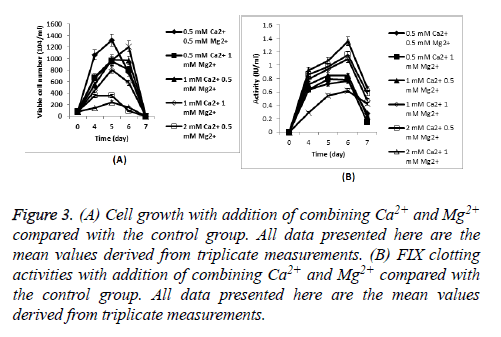
Cells producing FIX were seeded at 8 × 105 cells/ml in Erlenmeyer flasks with 30 ml CDM4PERMAb. Cell growth during culture and FIX clotting activities of culture supernatant with addition of both Ca2+ and Mg2+ were shown in Figure 3, respectively.
Figure 3: (A) Cell growth with addition of combining Ca2+ and Mg2+ compared with the control group. All data presented here are the mean values derived from triplicate measurements. (B) FIX clotting activities with addition of combining Ca2+ and Mg2+ compared with the control group. All data presented here are the mean values derived from triplicate measurements.
Similar to the case of a single ion addition, cells in the lowest ion concentration showed similar growth and viability (81% viability on day 6) to culture without any metal ion addition (80.8% viability on day 6, and others were below 70% on day 6). High ion concentration is harmful for cell growth.
The highest clotting activity was obtained in 2 mM Ca2+, combined with 1 mM Mg2+ showed an excellent improvement compared with culture without any metal ion addition (the peak FIX activity was 1.36 IU/ml compared with 0.62 IU/ml), almost doubled the supernatant activity.
Discussion
In order to investigate effects of different metal ions on animal cell culture expressing a recombinant hFIX, cells producing rhFIX were seeded at 8 × 105 cells/ml in Erlenmeyer flasks with 30 ml CDM4PERMAb, which have been used in the serum-free suspension culture for recombinant monoclonal antibody commercial production.
Mn2+, Cu2+, and Zn2+ were significantly harmful to CHO cells even in a low concentration, in which cell death occurred in early cell culture (The data were not shown here for the meaningless for FIX production). Cells in Ca2+ and Mg2+ with the lowest concentration (0.1 mM) respectively showed similar growth and viability (80% and 80.4% viability on day 6 respectively) to culture without any metal ion addition (80.8% viability on day 6, and others were below 70% on day 6). The most viable cells during culture means the rapidest depletion of nutrients so that a sharp fall in the number of viable cells in the control on day 7 by depleted nutrients. The maximum viable cell density reached over 100 × 105 cells/ml. High concentration Ca2+ and Mg2+ or combined addition also exhibited inhibitory effect on cell growth. High concentration Ca2+ improved FIX expression activity; on the other hand, High concentration Mg2+ played an opposite role. It may be due to the small number of cells under high concentration Mg2+, and addition single Mg2+ may didn’t affect FIX expression or post-translational modification without Ca2+. Cells were stretched and glued to each other under high concentration Ca2+. It may adjust the state of the cells to be more conducive to express the target protein by some intracellular or intercellular signalling. Moreover, Ca2+ could stable FIX conformation and be in favour of the posttranslational modification. Besides, another experiment was designed to exclude the impact of the addition to the detection system by adding extra Ca2+ in the detection system. The result showed Ca2+ almost has no effect on the test results.
Although addition single Mg2+ didn’t exhibit improvement in FIX expression activity that is different from a previous report [14], magnesium ion (Mg2+) has been shown to interact with the Ca2+-binding sites in Gla domains of vitamin K-dependent coagulation factors including FIX [11]. The FIX Gla domain has a specific Mg2+-binding site(s) and Mg2+ accelerates Ca2+- dependent activation of factor IX [12,13]. We evaluated the effect of combining Ca2+ and Mg2+ in the cell serum-free culture medium. We speculated that Mg2+ played a role in FIX production through Ca2+, and the mechanism of Ca2+ or Mg2+ affect the FIX production of Recombinant CHO cells is still unknown at present. Due to obvious cell growth inhibition and our fermentation process optimization experiences, we didn’t evaluate higher ion concentration here. Our following fedbatch experiments have proved that the balance between ion concentration and cell growth is important. High ion concentration didn’t mean a higher production in a longer culture due to the decreased cell number (The FIX production in 3 mM Ca2+, and 1 mM Mg2+ was absolutely lower than in 2 mM Ca2+, and 1 mM Mg2+ by a fed-batch, the data were not shown here). The optimal FIX expression activity 1.36 IU/ml was obtained under 2 mM Ca2+, and 1 mM Mg2+, almost doubled the supernatant activity compared with culture without any metal ion addition (0.62 IU/ml), exhibited a higher level of fermentation in CHO cells referring to other reports even including the reports co-expressing other processing protein, PACE/Furin or gamma-carboxylases (Kim et al. obtained 1.33 IU/ml aFIX in a coexpressing CHO line) [7,8,14-19].
Besides, the CHO cell line exhibited a stable FIX expression during long-term cell passages in another our research. It will be valuable to develop culture process for the high production of a properly processed recombinant FIX [9], and our following work has proved it.
References
- Sharathkumar A, Hardesty B, Greist A, Salter J, Kerlin B. Variability in bleeding phenotype in Amish carriers of haemophilia B with the 31008 C-T mutation. Haemophilia 2009; 15: 91-100.
- Bolton-Maggs PH, Pasi KJ. Haemophilias A and B. Lancet 2003; 361: 1801-1809.
- Mannucci PM. Hemophilia and related bleeding disorders: a story of dismay and success. Hematology Am SocHematolEduc Program 2002.
- Fischer K, Steen Carlsson K, Petrini P, Holmström M, Ljung R. Intermediate-dose versus high-dose prophylaxis for severe hemophilia: comparing outcome and costs since the 1970s. Blood 2013; 122: 1129-1136.
- Oldenburg J, Albert T. Novel products for haemostasis-current status. Haemophilia 2014; 20: 23-28.
- Franchini M, Frattini F, Crestani S, Sissa C, Bonfanti C. Treatment of hemophilia B: focus on recombinant factor IX. Biologics 2013; 7: 33-38.
- Kaufman RJ, Wasley LC, Furie BC. Expression, purification, and characterization of recombinant gamma-carboxylated factor IX synthesized in Chinese hamster ovary cells. J BiolChem 1986; 261: 9622-9628.
- Wasley LC, Rehemtulla A, Bristol JA, Kaufman RJ. PACE/furin can process the vitamin K-dependent pro-factor IX precursor within the secretory pathway. J BiolChem 1993; 268: 8458-8465.
- Lim IH, Kim JS, Lee G. The Effects of medium supplement on high-level production of recombinant human factor IX in CHO cell. Cells Culture 2010; 4: 613-618.
- Morrissey JH, Pureza V, Davis-Harrison RL. Blood clotting reactions on nanoscale phospholipid bilayers. ThrombRes 2008; 122: 23-26.
- Gopinath SCB, Shikamoto Y, Mizuno H. Snake-venom-derived factor IX-binding protein specifically blocks the γ-carboxyglutamic acid-rich-domain-mediated membrane binding of human factor IX and X. JBiochem 2007; 405: 351-357.
- Sekiya F, Yoshida M, Yamashita T, Morita T. Magnesium(II) is a crucial constituent of the blood coagulation cascade. Potentiation of coagulant activities of factor IX by Mg2+ ions. J BiolChem 1996; 271: 8541-8544.
- Shikamoto Y, Morita T, Fujimoto Z, Mizuno H. Crystal structure of Mg2+- and Ca2+-bound Gla domain of factor IX complexed with binding protein. J BiolChem 2003; 278: 24090-24094.
- Kim WH, Kim JS, Yoon Y, Lee GM. Effect of Ca2+ and Mg2+ concentration in culture medium on the activation of recombinant factor IX produced in Chinese hamster ovary cells. J Biotechnol 2009; 142: 275-278.
- Enjolras N, Dargaud Y, Perot E. Human hepatoma cell line HuH-7 is an effective cellular system to produce recombinant factor IX with improved post-translational modifications. Thromb Res 2012; 130: e266-e273.
- Turecek PL, Abbuhl B, Tangada SD, Chapman M, Gritsch H. Nonacog gamma, a novel recombinant factor IX with low factor IXa content for treatment and prophylaxis of bleeding episodes. Expert Rev ClinPharmacol 2015; 8: 163-177.
- de Castilho Fernandes A, Fontes A, Gonsales N, Swiech K, Picanco-Castro V. Stable and high-level production of recombinant Factor IX in human hepatic cell line. BiotechnolApplBiochem 2011; 58: 243-249.
- Bohm E, Dockal M, Graninger M. Expression of recombinant human coagulation factors VII (rFVII) and IX (rFIX) in various cell types, glycosylation analysis, and pharmacokinetic comparison. BMC Proc 2011; 8: 23.
- Berkner KL. Expression of recombinant vitamin K-dependent proteins in mammalian cells: factors IX and VII. Methods Enzymol 1993; 222: 450-477.