ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 8
Implementation of a protocol to control pain, agitation and delirium in the patients admitted to intensive care unit with opioid drug dependency: A feasibility study
Golnar Sabetian1, Farid Zand2, Fatemeh Khalili2, Ramin Afshari3, Mansoor Masjedi2, Behzad Maghsoodi2, Hossein Haddad Bakhodaei4*, Shohreh Javadpour5, Soheila Nasimi2, Peyman Petramfar6, Afsaneh Vazin7 and Alireza Ghanbari8
1Trauma Research Center, Shiraz University of Medical Sciences, Shiraz, Iran
2Anaesthesiology and Critical Care Research center, Shiraz University of Medical Sciences, Shiraz, Iran
3Department of Drug Abuse Control, Shiraz University of Medical Sciences, Shiraz, Iran
4Department of Neurosurgery, Shiraz University of Medical Sciences, Shiraz, Iran
5Department of Critical Care Nursing, Jahrom University of Medical Sciences, Jahrom, Iran
6Department of Neurology, Shiraz University of Medical Sciences, Shiraz, Iran
7Department of Clinical Pharmacy, School of Pharmacy, Shiraz University of Medical Sciences, Shiraz, Iran
8Student of Research Committee, Anesthesiology and Critical Care Research Center, Shiraz University of Medical Sciences, Shiraz, Iran
- *Corresponding Author:
- Hossein Haddad Bakhodaei
Department of Neurosurgery
Shiraz University of Medical Sciences,Iron
Accepted on January 10, 2017
Background: Pain, Agitation and Delirium (PAD) are common in critically ill patients admitted in Intensive Care Units (ICU) being reported in 15-80% of the patients. Control of agitation and delirium in critically ill patients is somehow different and hard to achieve in patients with drug abuse and opium addiction.
Aim: We hypothesized if a protocol could be designed and implemented to concomitantly control Pain, Agitation and Delirium (PAD) and prevent withdrawal signs in this population during ICU admission.
Methods: This prospective cross-sectional study. A multidisciplinary team designed the protocol. We included a total number of 30 critically ill patients during the study period. We included adult patients (>18 years) who were opium dependent and used drugs daily and had uncontrolled behaviors of drug consumption and reportedly had withdrawal symptoms. Methadone was used to prevent withdrawal syndrome and pain was assessed hourly, by Behavioural Pain Scale and controlled by morphine or fentanyl. Level of sedation was also assessed hourly, by Richmond Agitation-Sedation Scale and controlled by midazolam or propofol, according to the protocol. Delirium was checked by Confusion Assessment Method for ICU, once in every working shift.
Results: Patients were recruited during an eight months period in 2 mixed medical-surgical ICU’s. The protocol was effective to completely prevent the withdrawal syndrome in 24 patients (80%). The average need to methadone was 14.5 ± 22.2 mg in the patients. The pain, sedation and delirium were evaluated and documented by the staff in 97%, 98% and 56% of situations, respectively. Pain and sedation scores were within acceptable limits in 93% and 98% of occasions, respectively. Delirium occurred in 2 patients during the ICU stay.
Keywords
Analgesia, Delirium, Sedation, Opium, Intensive care unit (ICU), Critically ill patients.
Introduction
Pain, Agitation and Delirium (PAD) are common in critically ill patients admitted in Intensive Care Units (ICU) being reported in 15-80% of the patients [1-5]. Control of agitation and delirium in critically ill patients is somehow different and hard to achieve in patients with drug abuse and opium addiction. These groups of patients develop withdrawal symptoms on abstinence from the drug [6,7]. There are several kinds and routes of administration in eastern countries for opioid drug usage including inhalational, oral and intravenous methods. This diversity in drug consumption makes it difficult to estimate the need of the critically ill patients to basal opioid medications to prevent withdrawal symptoms and pain control [8]. The symptoms of withdrawal peak during the first 48 h and are sometimes persistent for several days, and then gradually decrease to baseline levels [7].
Several sedation protocols have been introduced during the past two decades for critically ill patients on mechanical ventilation including bolus sedation and daily sedation interruptions. These sedation protocols have shown to improve the patient outcome by decreasing the hospital and ICU length of stay and by decreasing the number of days on mechanical ventilation [1,5,9-11]. Most of these protocols are based on frequent sedation and analgesia assessment and protocolized weaning of sedation [4,12-14]. Most of the previous studies are focused on the implementation of analgesia-sedation-delirium protocols in the patients without history of opioid dependency and data regarding implementation of PAD protocol in these patients are scarce in the literature.
In the current study we developed and implemented an analgesia-sedation-delirium protocol using methadone in opium dependent critically ill patients and evaluated the outcomes. We hypothesized that implementing this protocol is feasible and would control the withdrawal symptoms leading to decreased agitation and delirium.
Materials and Methods
Study population
This prospective cross-sectional study was conducted in 2 intensivist driven mixed medical-surgical intensive care units (ICU) in Nemazee hospital, a tertiary healthcare center affiliated to Shiraz University of Medical Sciences during an eight months period from May to November 2014. We included a total number of 30 critically ill patients during the study period with average age of 55.3 ± 15.4. We included adult patients (>18 years) who were opium dependent and used drugs daily and had uncontrolled behaviors of drug consumption and reportedly had withdrawal symptoms. We excluded the patients who were expected to die within 48 h of admission, those on anticonvulsants, those with best motor response of less than 6 in Glasgow coma scale score and those who had to receive muscle relaxant during the ICU course. We also excluded those patients who could not move both upper extremities and those with untreated psychosis or neurosis. The Patients with prolonged QT-interval (seconds) in ECG were also excluded from the study. The study protocol was approved by Institutional Review Board (IRB) and medical ethics committee of Shiraz University of Medical Sciences. All the patients or their next of kin provided the informed written consents before inclusion in the study. A urine stick (Drug of Abuse Test, Core Technology Co. Ltd. China) was also used to confirm the history of drug abuse in all patients.
Study protocol
Complete history was determined according to the patients or their next of kin and a urine toxicology screening test was performed on admission for all the individuals. A protocol was developed by a multidisciplinary team including critical care physicians, pharmacist, psychiatrist and neurologist and expert nurses. The protocol was focused on the control of pain, delirium, and agitation of opium addicted patients based on validated assessment tools. The goal of the current protocol was to maintain a comfortable, awake patient by pharmacologic titration via objective assessments, limit the use and duration of continuous infusion sedation, and increasing the awareness and control of delirium.
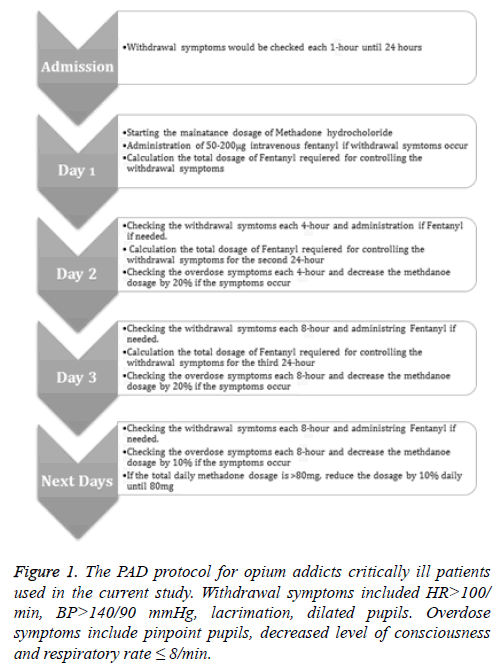
We used methadone hydrochloride in the current protocol for prevention of opium withdrawal symptoms. Four determinants of the abused drug by the patients (according to the best available history) were used for calculation of methadone dosage for each patient which included purity of the abused substance, bioavailability of the route of abuse (oral, inhalational, intravenous), conversion ratio of morphine to methadone (Fudin 2012 formula) and cross tolerance. The type and dose of the abused drug (according to the popular language of the patients or their family) were entered to software that was specially designed for this study. The maintenance dosage of methadone (Methadone Hydrochloride®, Eksir Pharmaceuticals Ltd., Tehran, Iran) was calculated accordingly for each patient and was divided to two doses and was given subcutaneously or intravenously (for ease of administration, dosages less than 2.5 mg or more than 20 mg were given intravenously). Patients were checked hourly by an ICU nurse for withdrawal signs which included tachycardia (heart rate>100/min or 20% increase in heart rate from baseline), hypertension (blood pressure>140/80 mmHg or 20% increase in blood pressure from baseline), tachypnea (respiratory rate>30/min), sweating, lacrimation, diarrhoea, vomiting, fever and pupillary dilation. Patients with withdrawal symptoms were given bolus dosages of 50 μg Fentanyl (Hameln- Fentanyl®, Hameln Pharmaceuticals Ltd., Hameln, Germany) with 5-10 minutes intervals to a maximum dosage of 200 μg.
The pain intensity and agitation were also evaluated in each patient by the ICU nurse hourly and delirium every 8 h. To assess pain intensity, we used the Persian versions of Numerical Rating Scale (NRS) and Behavioural Pain Scale (BPS). These tools were previously translated and validated in another study by our team [13]. BPS is used after observation of the patient for about 1 minute and has been exclusively designed and validated in critically ill sedated patients [15,16]. The BPS is based on the sum of three subscales: facial expression, upper limb posture and compliance with mechanical ventilation. The NRS is based on a scale from 0 to 10; 0 represents no pain and 10 represents the worst imaginable pain [17]. The goal of pain control was to maintain BPS between 3 and 5 and NRS between 0 and 3. Pain scores above the mentioned goal were treated with morphine sulphate according to the protocol. The total needed dose of fentanyl to control withdrawal signs and morphine to control pain were registered.
The agitation was evaluated by the ICU nurse according to the Richmond Agitation-Sedation Scale (RASS) each hour [18]. RASS is mostly used in order to avoid over and under-sedation. RASS is a 10-sacle measure ranging from -5 (unarousable) to +4 (combative). Obtaining a RASS score is the first step in administering the Confusion Assessment Method in the ICU (CAM-ICU), a tool to detect delirium in intensive care unit patients [19]. Our goal was to maintain RASS between -1 and +1. Those with RASS of more than +1 received 2-5 mg of midazolam intravenously (Midazolam Injection®, Eksir Pharmaceuticals Ltd., Tehran, Iran). The delirium was assessed three times a day in those with RASS score of more than -4 based on CAM-ICU by the nurses. Those with hyperactive delirium (RASS ≥ +1) received 2.5-5 mg intravenous injection of haloperidol (Janssen Haloperidol Injection®, Janssen Pharmaceutical Ltd., Beerse, Belgium).
The overdose symptoms were also checked every 4 h which included pinpoint pupils, deceased level of consciousness and RR<8/min. The daily methadone dose for the next day was adjusted according to the total dose of fentanyl required for control of withdrawal syndrome during the previous day. If the patients showed any sign of overdose the methadone dose was reduced accordingly. The study protocol is demonstrated in Figure 1. The parenteral methadone was converted to enteral form upon discharge to ward.
Statistical analysis
All recorded data were prospectively entered into a computerbased database and were further analysed by statistical package for social sciences (SPSS Inc., Chicago, USA) version 19.0. Data are presented as mean ± SD and proportions as appropriate. Kolmogorov Smirnov test was used to test the normal distribution of the data. The repeated measure test was used to compare the parametric variables before and after the study. Paired t-test was also used to compare the results before and after the protocol.
Results
Overall we assessed 40 patients for eligibility of whom 30 fulfilled the study criteria and were included in the study. The mean age of the patients was 55.3 ± 15.4 years and there were 28 (93.3%) men and 2 (6.7%) women among the participants. The baseline characteristics of the patients are summarized in Table 1. The calculated daily dosage of methadone was 14.46 ± 22.2 mg (Table 2). Only 2 (6.7%) patients required increase in the daily dose of methadone. Withdrawal symptoms were recorded in 6 (20%) patients during the study period. In these patients, withdrawal symptoms were more frequent in the second day (33% of the observation period) than in the first day (10% of the observation period). None of the patients developed overdose symptoms during the study period. Only 2 (6.7%) patients developed delirium during the study that was treated by haloperidol. The duration of acceptable RASS was 98 percent of total ICU stay hours. The number of hours with acceptable BPS and NRS increased from 79% in the first day to 97% in the third day. Overall the duration of acceptable BPS and NRS was about 93% of total ICU stay hours. The administered dosages of morphine for pain control was 16.3 ± 14.7 mg during the first day which was decreased to 9.23 ± 10.2 mg in the second day and 5.03 ± 7.6 mg in the third day. In the same way, the amount of administered midazolam decreased significantly from 1.59 ± 4.9 mg in the first day to zero in the third day. The nurses’ compliance to check the pain, agitation and delirium scores was 97, 98 and 56 percent, respectively. The response of the nurses to the out of range scores, according to the protocol, was more than 97%.
| Variable | P-value |
|---|---|
| Age, y | 55.3 ± 15.4 |
| Gender, no. (%) | |
| Men | 28 (93.3) |
| APACHE IV score, mean ± SD | 83.9 ± 21.7 |
| Type of abused drug, no. (%) | |
| Morphine | 27 (90) |
| Methadone | 2 (6.3) |
| Meperidine | 1 (3.3) |
| Route of consumption, no. (%) | |
| Inhalation with pipe | 6 (20) |
| Inhalation without pipe | 15 (50) |
| Oral | 8 (26.7) |
| Intravenous | 1 (3.3) |
| Equivalent morphine dosage, mg | 46.7 ± 53.1 |
| Urine toxicology, No. (%) | |
| Morphine sulphate | 28 (93.3) |
| Methadone hydrochloride | 2 (6.7) |
| Benzodiazepines | 25 (83.3) |
| Barbiturates | 23 (76.6) |
| Cause of admission, No. (%) | |
| Non-surgical | 5 (16.7) |
| Surgery | 25 (83.3) |
| Comorbidities, No. (%) | 16 (53.3) |
Table 1. The baseline characteristics of the 30 opium dependent critically ill patients.
| Variable | P-value |
|---|---|
| ICU admission length, day | 3.7 ± 3.47* |
| Calculated daily methadone, mg | 14.46 ± 22.2* |
| Route of administration, no. (%) | |
| Subcutaneous | 24 (80) |
| Intravenous | 6 (20) |
| Delirium, no. (%) | 2 (6.7) |
| Withdrawal symptoms, no. (%) | 6 (20) |
| Mortality, no. (%) | 2 (6.7) |
Table 2. The outcome of 30 opium addicted critically ill patients managed with study protocol.
Discussion
In this study we developed and implemented a PAD protocol for critically ill patients’ with history of opium abuse. We demonstrated that this protocol can successfully control pain and agitation in the majority of these patients. Twenty percent of the patients showed short periods of withdrawal signs and two (6.7%) patients developed delirium. This protocol was based on the meticulous patient assessment and protcolized titration of sedative and analgesic medications. During the study period 368 patients were admitted to our ICU among whom 40 (10.9%) patients were opium dependent which is higher than previously reported rates of opium addiction in general population in Iran [20]. This finding may show higher risk of acute illness in this group of the community. Meanwhile the majority of our patients were male which is in accordance with previous demographic studies [20]. The most frequent type of addiction was opium and the most prevalent route of use was by inhalation (70%), and oral route was used in 26.7% of our patients which is again in accordance with previous studies in Iran as opposed to western countries where heroin is more frequent and intravenous route is more popular [21]. The mean calculated equivalent of daily abused morphine was 46.7 ± 53.1 mg in our patients indicating a moderately high intensity of opium abuse in our patients. The calculated daily need to methadone was 14.46 ± 22.2 mg which is relatively high. As opioid medications are supplied and distributed by the hospital pharmacy under special regulations in most of countries, this increased need to methadone should be anticipated and communicated with respective authorities. Actually at the beginning, we encountered some problems for providing the needed methadone in our unit.
The results of urine toxicology of the patients on arrival to ICU showed that most of the exams were positive for morphine (93.3%), and in two other patients the urine study was positive for methadone (Table 1). This finding shows that most probably all of our patients were true opium addict patients and patient selection has been appropriately done in this study. However, considering that benzodiazepines and barbiturates were also positive in 83 and 77 percent of urine study results, one may assume that presence of opioids in the urine is just the result of use of these medications before arrival to ICU especially for general anesthesia. This theory seems rational considering that the dominant type of admission in this study was surgical (83.3%) and use of midazolam and sodium thiopental is routine during general anesthesia in our hospital. Overall, we should admit that although urine toxicology may confirm acceptable patient inclusion, patient history was the main method of diagnosis of opium abuse in our study. This is among the first studies to introduce and implement a PAD protocol for opium addict critically ill patients. We used methadone for maintaining the baseline need of the patients to opioids. Methadone is a synthetic opioid which is frequently administrated to prevent acute opioid withdrawal symptoms [22]. Due to desirable pharmacologic properties such as long duration of action regardless of delivery method and low risk of central sensitization and tolerance methadone therapy is one of the most common methods of long-term treatment of illicit opioid abuse in Iran. Some studies have also demonstrated that tapering doses of methadone could successfully be used for prevention of opioid abstinence symptoms in non-critically ill opioid addicted patients [23].
Jeffries et al. examined a protocol using methadone hydrochloride for prevention of acute opioid withdrawal in 43 critically ill paediatric patients [24]. That protocol was not followed consistently and withdrawal symptoms were more frequent (42%). There was a need to increase the dose of methadone in 26% of the patients and over and under sedation was more prevalent than our study [24]. These differences could be easily justifiable because of different types of drug dependency between our patients (chronic) and Jeffries study (acute) and different patient population and protocols. In another study, Lugo and colleagues also used a protocol based on enteral methadone to prevent withdrawal symptoms in 22 paediatric patients after prolonged infusion of fentanyl in a critical care setting [23,25]. They could successfully facilitate discontinuation of fentanyl and prevent withdrawal symptoms in almost all of the patients. Similarly, in this study, one patient needed to restarting the infusion of fentanyl and increasing the dose of methadone [25]. These finding are comparable to ours, emphasizing the importance of observing the implemented protocol to obtain acceptable clinical results.
Methadone has a variable half-life, ranging from 15 to 50 h. As a result, the drug level will increase over time to a steady state level [22]. This effect results in an increase in the clinical effect of methadone over days. This property makes titration of methadone difficult, raising the concern over possible overdose [23]. Most of the protocols use oral methadone in patients requiring mechanical ventilation as it has shorter half-life and associated with fewer side effects. However we could not use oral methadone in the current study as most of our patients had undergone abdominal surgeries and could not receive anything orally during the postoperative period [26]. We did not encounter any sign of overdose in our patients. On the contrary, 20 percent of our patients showed withdrawal symptoms during first and second days of ICU admission. This finding although emphasis the good safety margin of our protocol may demonstrate a need to revision of the protocol to calculate the needed methadone dose if more patient comfort is targeted. However, this observation could be simply explained by underreporting of the amount of opioid consumption by the patients or their family that is not unusual in critical care and drug abuse settings.
Diagnosis and treatment of delirium in critically ill patient on mechanical ventilation is important to improve the patients’ outcome. Ely and co-workers demonstrated that patients who developed delirium in the ICU had a 6-monthmortality rate of 34% versus 15% in those who did not. Furthermore, this development of delirium was associated with an increase in length of hospital stay by 10 days [19,27]. Assessment of delirium was performed by use of the validated CAM-ICU. The incidence of delirium in our patients was quite low compared to similar studies by our team and others [12,14,19,28]. This finding may underscore either the ability of the PAD protocol to decrease the incidence of delirium in our patients or some unknown effects of methadone itself. The possibility of underestimation of this problem by our nurses due to modest level of compliance (56%) could not be excluded. Overall our PAD protocol resulted in more than 90 percent of acceptable pain and sedation scores during the ICU period. This finding is rarely debatable as we used very accurate tools to monitor pain and sedation in our patients [13-16] and our nurses were already familiar and competent to use these tools, as is reflected in high compliance and appropriate response by them (97 to 98 percent).
Some limitations of our study should be declared. As the source of information for the previous opioid consumption was the patients themselves or their family members, the information was not complete and sometimes not reliable. The baseline information is critical and lack of that information may lead to miscalculation of the needed methadone. However the dosage was adjusted during the following days based on the patients’ response. The other limitation was the limited number of patients included in the study. This was just a feasibility study and the results should be re-evaluated in large and multicenter investigations. The other limitation of the study was limited evaluation of the delirium in the patients. The delirium was evaluated using CAM-ICU by trained nurses. The process include several stages interview with the patient and thus most of the patients could not finish the interview properly which led to lack of information on delirium in our series. In addition the CAM-ICU has low sensitivity and high specificity for detection of delirium in ICU patients [29]. Further studies with more reliable indices are required to overcome this pitfall. In conclusion, implementation of a multidisciplinary PAD protocol is possible in opioid addicted critically ill patients and is accompanied by favourable targeted pain and sedation scores during the ICU period. Further research in this difficult to manage subgroup of patients is needed to verify the impact of such protocols on significant ICU outcome measures like length of stay and mortality.
Acknowledgements
We would like to thankfully acknowledge the kind help of all the ICU staff of General ICU of Nemazee hospital for their help during the conduct of this study. This manuscript has been extracted from the thesis no. 91/01/01-4442 which has been submitted to the Shiraz Medical School as a requirement for graduation in anesthesia residency program by Khalili. The financial support was solely provided by Vice-Chancellery of Research, Shiraz University of medical Sciences.
Conflict of Interest
There isn’t any conflict of interest to be declared regarding the manuscript.
References
- Girardis M, Cantaroni C, Savoia G, Melotti R, Conti G. A critical appraisal of the quality of analgosedation guidelines in critically ill patients. Minerva Anestesiol 2016; 82: 230-235.
- Chevrolet JC, Jolliet P. Clinical review: agitation and delirium in the critically ill--significance and management. Crit Care 2007; 11: 214.
- Treggiari MM, Romand JA, Yanez ND, Deem SA, Goldberg J, Hudson L, Heidegger CP, Weiss NS. Randomized trial of light versus deep sedation on mental health after critical illness. Crit Care Med 2009; 37: 2527-2534.
- Jackson DL, Proudfoot CW, Cann KF, Walsh T. A systematic review of the impact of sedation practice in the ICU on resource use, costs and patient safety. Crit Care 2010; 14: 59.
- Robinson BR, Mueller EW, Henson K, Branson RD, Barsoum S, Tsuei BJ. An analgesia-delirium-sedation protocol for critically ill trauma patients reduces ventilator days and hospital length of stay. J Trauma 2008; 65: 517-526.
- Hatsukami DK, Stead LF, Gupta PC. Tobacco addiction. Lancet 2008; 371: 2027-2038.
- Hughes JR. Effects of abstinence from tobacco: valid symptoms and time course. Nicotine Tob Res 2007; 9: 315-327.
- Imani S, Atef Vahid MK, Gharraee B, Habibi M, Bowen S, Noroozi A. Comparing mindfulness-based group therapy with treatment as usual for opioid dependents: a pilot randomized clinical trial study protocol. Iran J Psychiatry BehavSci 2015; 9: 216.
- Dale CR, Kannas DA, Fan VS, Daniel SL, Deem S, Yanez ND, Hough CL, Dellit TH, Treggiari MM. Improved analgesia, sedation, and delirium protocol associated with decreased duration of delirium and mechanical ventilation. Ann Am ThoracSoc 2014; 11: 367-374.
- Girard TD, Kress JP, Fuchs BD, Thomason JW, Schweickert WD, Pun BT, Taichman DB, Dunn JG, Pohlman AS, Kinniry PA, Jackson JC, Canonico AE, Light RW, Shintani AK, Thompson JL, Gordon SM, Hall JB, Dittus RS, Bernard GR, Ely EW. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet 2008; 371: 126-134.
- Barr J, Fraser GL, Puntillo K, Ely EW, Gelinas C, Dasta JF, Davidson JE, Devlin JW, Kress JP, Joffe AM, Coursin DB, Herr DL, Tung A, Robinson BR, Fontaine DK, Ramsay MA, Riker RR, Sessler CN, Pun B, Skrobik Y, Jaeschke R. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med 2013; 41: 263-306.
- Mehta S, Burry L, Cook D, Fergusson D, Steinberg M, Granton J, Herridge M, Ferguson N, Devlin J, Tanios M, Dodek P, Fowler R, Burns K, Jacka M, Olafson K, Skrobik Y, Hebert P, Sabri E, Meade M. Daily sedation interruption in mechanically ventilated critically ill patients cared for with a sedation protocol: a randomized controlled trial. JAMA 2012; 308: 1985-1992.
- Blackwood B, Burns KE, Cardwell CR, OHalloran P. Protocolized versus non-protocolized weaning for reducing the duration of mechanical ventilation in critically ill adult patients. Cochrane Database Syst Rev 2014; 6904.
- Mansouri P, Javadpour S, Zand F, Ghodsbin F, Sabetian G, Masjedi M, Tabatabaee HR. Implementation of a protocol for integrated management of pain, agitation, and delirium can improve clinical outcomes in the intensive care unit: a randomized clinical trial. J Crit Care 2013; 28: 918-922.
- Payen JF, Bru O, Bosson JL, Lagrasta A, Novel E. Assessing pain in critically ill sedated patients by using a behavioral pain scale. Crit Care Med 2001; 29: 2258-2263.
- Aissaoui Y, Zeggwagh AA, Zekraoui A, Abidi K, Abouqal R. Validation of a behavioral pain scale in critically ill, sedated, and mechanically ventilated patients. AnesthAnalg 2005; 101: 1470-1476.
- Chauny JM, Paquet J, Lavigne G, Marquis M, Daoust R. Evaluating acute pain intensity relief: challenges when using an 11-point numerical rating scale. Pain 2015.
- Sessler CN, Gosnell MS, Grap MJ, Brophy GM, ONeal PV. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J RespirCrit Care Med 2002; 166: 1338-1344.
- Ely EW, Inouye SK, Bernard GR, Gordon S, Francis J, May L, Truman B, Speroff T, Gautam S, Margolin R, Hart RP, Dittus R. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA 2001; 286: 2703-2710.
- Mokri A. Brief overview of the status of drug abuse in Iran. Arch Iranian Med 2002; 5: 184-190.
- Jones CM, Logan J, Gladden RM, Bohm MK. Vital signs: demographic and substance use trends among heroin users-United States 2002-2013. MMWR Morb Mortal Wkly Rep 2015; 64: 719-725.
- Trafton JA, Ramani A. Methadone: a new old drug with promises and pitfalls. Curr Pain Headache Rep 2009; 13: 24-30.
- Amato L, Davoli M, Minozzi S, Ferroni E, Ali R, Ferri M. Methadone at tapered doses for the management of opioid withdrawal. Cochrane Database Syst Rev 2013; 2: 3409.
- Jeffries SA, McGloin R, Pitfield AF, Carr RR. Use of methadone for prevention of opioid withdrawal in critically ill children. Can J Hosp Pharm 2012; 65: 12-18.
- Lugo RA, MacLaren R, Cash J, Pribble CG, Vernon DD. Enteral methadone to expedite fentanyl discontinuation and prevent opioid abstinence syndrome in the PICU. Pharmacotherapy 2001; 21: 1566-1573.
- Byford S, Barrett B, Metrebian N, Groshkova T, Cary M. Cost-effectiveness of injectable opioid treatment v. oral methadone for chronic heroin addiction. Br J Psychiatry 2013; 203: 341-349.
- Ely EW, Shintani A, Truman B, Speroff T, Gordon SM. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA 2004; 291: 1753-1762.
- Pisani MA, Murphy TE, Araujo KL, Van Ness PH. Factors associated with persistent delirium after intensive care unit admission in an older medical patient population. J Crit Care 2010; 25: 541-547.
- Nishimura K, Yokoyama K, Yamauchi N, Koizumi M, Harasawa N, Yasuda T, Mimura C, Igita H, Suzuki E, Uchiide Y, Seino Y, Nomura M, Yamazaki K, Ishigooka J. Sensitivity and specificity of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) and the Intensive Care Delirium Screening Checklist (ICDSC) for detecting post-cardiac surgery delirium: A single-center study in Japan. Heart Lung 2016; 45: 15-20.