ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
- Biomedical Research (2012) Volume 23, Issue 4
Glucose uptake and serine phosphorylation in differentiated adipocytes co-cultured with macrophages
1Julius Center, Department of Social and Preventive Medicine, Faculty of Medicine, University Malaya
2Department of Medicine, Faculty of Medicine, University Malaya, Malaysia
- *Corresponding Author:
- Karuthan Chinna
Julius Center
Department of Social and Preventive Medicine
Faculty of Medicine, University Malaya
Malaysia.
Accepted date: August 13 2012
The association of obesity with type 2 diabetes and insulin resistance has been recognized for decades. However, the mechanism that links obesity and insulin resistance has not been fully elucidated. Recent studies have suggested that the macrophages in the adipose tissue are involved in the association between insulin resistance and obesity. In the present study we co-cultured adipocytes with macrophages and investigated the effect of macrophages on basal and insulin-dependent glucose uptake and serine phosphorylation. An in-vitro coculture system was developed using differentiated 3T3-L adipocytes and macrophages. Confluent preadipocytes were stimulated with 1μM dexamethazone, 0.5Mm isobutylmethylxanthine and 10μg of insulin. The differentiated adipocytes were identified by lipid accumulation in the form of droplets in the cells . The success of the co-culture was determined by the morphology of the macrophages, which became elongated. Our results demonstrated an increase in the basal uptake of glucose and a decrease in the insulindependent uptake of glucose in the co-cultured adipocytes. We also showed a significant increase in serine-307 phosphorylation which prevents the interaction of IRS-1 and IR, resulting in insulin resistance. In order to validate our results, we determined the differential gene expression of the insulin signaling pathway in differentiated adipocytes co-cultured with macrophages, as compared to the differentiated adipocytes cultured alone. The IRS-1 and GLUT-4 genes were down regulated, while GLUT-1 gene was up regulated. : Macrophages that accumulate in the adipose tissue of obese subjects may be one of the risk factors for the development of insulin resistance and type 2 diabetes.
Keywords
Adipocytes, Macrophages, Glucose uptake, Serine 307 phosphorylation.
Introduction
Obesity is becoming a major health problem globally and is often accompanied by a variety of adverse health outcomes such as insulin resistance, type 2 diabetes, dyslipidemias and cardiovascular diseases [1]. The association of obesity with type 2 diabetes and insulin resistance has been recognized for decades, but the mechanism that links obesity and insulin resistance has not been fully elucidated. It has been shown that adipose dysfunction [2] plays a prominent role in the development of insulin resistance and type2 diabetes.
Insulin is the primary hormone involved in glucose homeostasis and the stimulation of glucose transport. Circulating insulin rapidly reaches its target tissues, mainly liver, muscle and adipose tissue, where it interacts with its receptors and carries out various cellular functions [3].
Insulin receptor (IR) belongs to a family of cell surface receptors, possessing intrinsic tyrosine kinase activity. Insulin binds to the alpha subunit of the receptor to induce tyrosine autophosphorylation of the beta subunit. The activated IR subsequently phosphorylates its substrates IRS-1 and IRS-2 [4]. In cell-free systems and cell cultures, phosphorylation of IRS-1 in serine residues has been reported to block IRS-1 interaction with the insulin receptor [5] or to enhance its proteolytic degradation in the cell [6]. The earliest abnormality observed in insulin resistance is a decrease in insulin-induced glucose uptake in skeletal muscle and a reduced ability of the hormone to suppress glucose production by the liver [7].
Obesity has also been shown to be associated with increased accumulation of macrophages in adipose tissue [8]. Lumeng and coworkers [9] explored the interaction of macrophages and adipocytes to study insulin signaling and glucose transport. Indirect culture by using conditioned medium from activated macrophages, and direct co-culture by mixing the adipocytes with the macrophages,were explored in that study. In the indirect system, the macrophages secreted factors that induced an inflammatory response in adipocytes and influenced insulin sensitivity [10].When the macrophages were cultured with the adipocytes, the macrophages produced long cellular processes that interacted with the surface of the adipocytes, suggesting that adipocytes may modify macrophage properties. This is consistent with a model in which macrophages are activated upon their recruitment into adipose tissue. Lumeng and coworkers [9] also provided evidence that both secreted and cell contactmediated signals participate in macrophage–adipocyte interactions that contribute to the impairment of adipocyte function.
We set up an in vitro culture system to study the effect of cell-to-cell contact in the induction of insulin resistance. While the role of IRS-1 tyrosine phosphorylation in insulin resistance has been largely elucidated, the mechanism involved in serine/threonine phosphorylation has not been fully understood. Therefore, we examined the effect of macrophage–adipocyte interactions in glucose uptake and serine phosphorylation. At the same time we examined the differential expression of selected genes involved in the insulin signaling pathway of differentiated adipocytes cultured alone and adipocytes cultured with macrophages.
Materials and Methods
Cell Culture
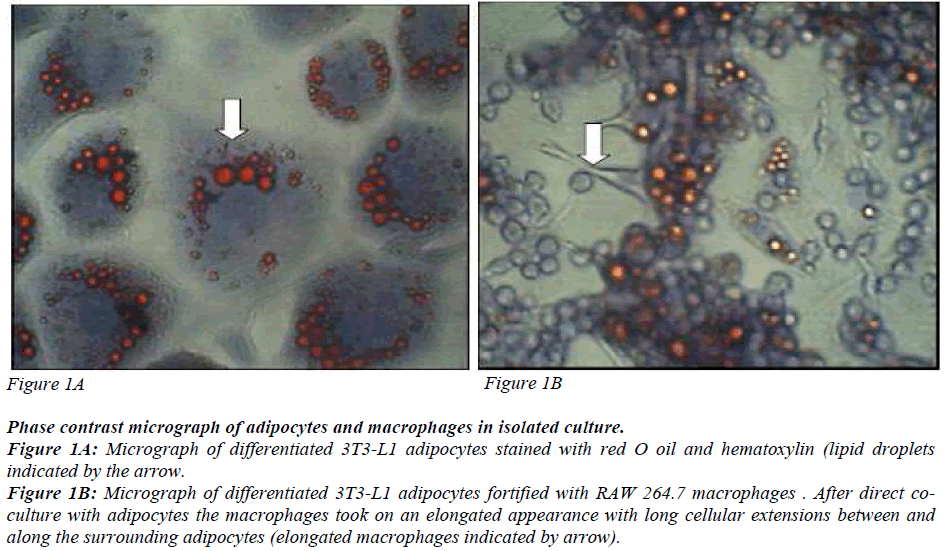
Preadipocytes 3T3-L1 derived from mouse embryos were used. They are aneuploid cells with the ability to differentiate into matured adipocytes with a dramatic change in morphology and gene expression. 3T3-L1 preadipocytes and RAW 264.7 macrophages were obtained from ATCC, Manassas, USA. They were cultured and maintained as described in American Type Culture Collection Catalogue with some modifications. Confluent preadipocytes were stimulated to differentiate into matured adipocytes as described by Miyazawa- Hoshimoto and coworkers [12] with some modifications. The cells were incubated in 1μM dexamethazone (Sigma- Aldrich, Missouri, USA). 0.5 mM isobutylmethylxanthine (Sigma Aldrich, Missouri, USA) and 10μg/ml insulin (Sigma-Aldrich, Missouri, USA) for three days followed by 10μg/ml of insulin for another three days. The cells were maintained in complete medium until 80% of the cells differentiated into matured adipocytes. The differentiated cells were identified by lipid accumulation in the form of droplets in the cells (Figure 1) and the elongated macrophages are seen in the co-culture (Figure 2).
Phase contrast micrograph of adipocytes and macrophages in isolated culture.
Figure 1A: Micrograph of differentiated 3T3-L1 adipocytes stained with red O oil and hematoxylin (lipid droplets
indicated by the arrow.
Figure 1B: Micrograph of differentiated 3T3-L1 adipocytes fortified with RAW 264.7 macrophages . After direct coculture
with adipocytes the macrophages took on an elongated appearance with long cellular extensions between and
along the surrounding adipocytes (elongated macrophages indicated by arrow).
Red O Oil Staining
Red O oil staining was performed to determine adipogenesis [13]. Differentiated adipocytes were rinsed with phosphate buffered saline (PBS) and fixed in 10% formaldehyde. After one hour, formaldehyde was withdrawn and the cells were rinsed three times with PBS. Then the cells were incubated with red O oil for one hour and after which the red O oil was removed and the cells were again rinsed three times with PBS. The stained lipid droplets were visualized and photographed (Figure 1).
Co-culture of differentiated adipocytes with macrophages
Differentiated adipocytes and macrophages were cocultured in serum-free media (0.1% BSA-DMEM) for two hours. The co-cultured adipocytes were incubated for another two days in complete medium as described by Suganami and coworkers [14]. The success of the coculture was determined by the morphology of the macrophages, which became elongated as described by Lumeng [9].
Glucose Uptake
Glucose uptake was determined by using tritium labelled 2-deoxy glucose as described by Kozma and co-workers [15]. Glucose uptake was measured using a microplate scintillation and luminescence counter (TopCount NXT). Nonspecific glucose uptake was measured by using 20μM of cytochalasin B. Counts per minute (CPM) were converted to pmol/min/mg of protein using the formula described by Lakshmanan and coworkers [16].
Serine-307 phosphorylation
Serine 307 IRS-1 phosphorylation was measured using a Millipore 17-459 Kit (Millipore, USA) according to the manufacturer’s instructions. Twelve μg of total protein was used for this assay. A standard curve was prepared from the standards provided in the kit. Standards and samples were added into each well in a 96- well plate that was coated with specific antibody and incubated for 2 hours at room temperature. After three washings the plate was incubated with anti-rabbit IgG conjugate horse radish peroxidase and the colour was developed with tetramethylbenzidine. Then the reaction was stopped with the stop solution and the readings were measured at an absorbance of 450 nm.
Real Time Reverse Transcription PCR
Real-Time PCR was performed using RT2PCR arrays from SABiosciences (Frederick, USA). Total RNA was isolated from differentiated adipocytes and differentiated adipocytes co-cultured with macrophages as described by the manufacturer [17]. For each experiment, isolated RNA was converted into cDNA and used as a template for the real-time reverse transcriptase PCR assay. Real- Time cocktails were prepared by mixing the synthesized cDNA with SYBR green master mix. Twenty five μl of the cocktail was added into each well of a 96-well array plate that was coated with specific primers for genes involved in insulin signaling. The array was sealed with optical thin wall 8-cap strips and loaded into the thermal cycler with the appropriate program. The instrument program was set up according to ‘setup guide manual’ that was downloaded from: http://sabiosciences.com/pcarraydataanalysis.php. The cDNA was subjected to RNA quality control PCR amplification before performing real-time reverse transcription PCR to determine any possible DNA contamination in the sample, reverse transcription efficiency, PCR efficiency and high or low housekeeping gene expression level.
Statistical analysis
Ct values that were obtained from all the wells were analyzed using templates that were provided by SABiosciences. A p value < 0.05 was considered to be statistically significant. The template was downloaded from http://sabiosciences.com/pcarraydataanalysis.php.
Results
Differentiation of mature adipocytes and macrophages in co-culture
Figures 1A and 1B are phase contrast micrographs of differentiated adipocytes (400x), and differentiated adipocytes co-cultured with macrophages, respectively (400x). Figure 1 shows that the mature adipocytes are spherical in shape with accumulated lipid droplets. Figure 2 shows that macrophages which have infiltrated the adipose tissue, interact with adipocytes and take on an elongated phenotype with long cellular processes.
Glucose Uptake
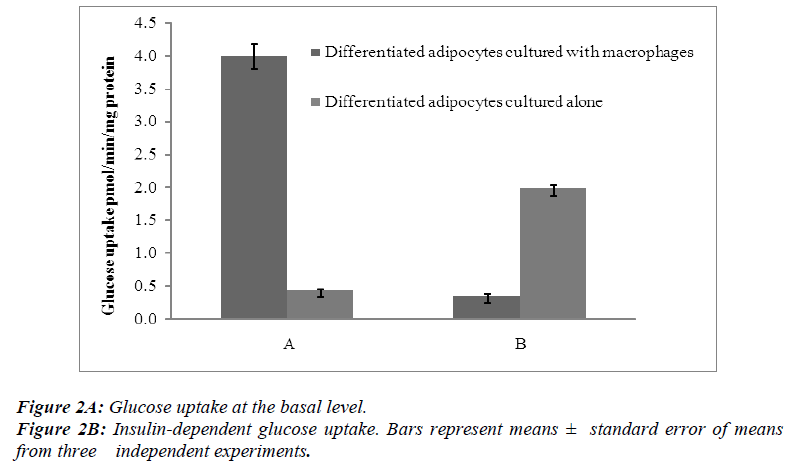
Radiometric assays were performed to determine the uptake of basal glucose and insulin-dependent glucose in both differentiated adipocytes cultured alone and those co-cultured with macrophages. In one series of experiments, the cells were incubated for 30 min without insulin and in other series the cells were treated for 30 min with insulin at a concentration of 100 nM per flask of confluent cells.
The results showed that the basal levels of glucose uptake were significantly higher (p< 0.05 ) when the differentiated adipocytes were co-cultured with macrophages than when the differentiated adipocytes were cultured alone [Figure 2A]. In contrast, the insulin-dependent glucose uptake showed a significant decrease (p<0.05) when the differentiated adipocytes were co-cultured with macrophages [Figure 2B].
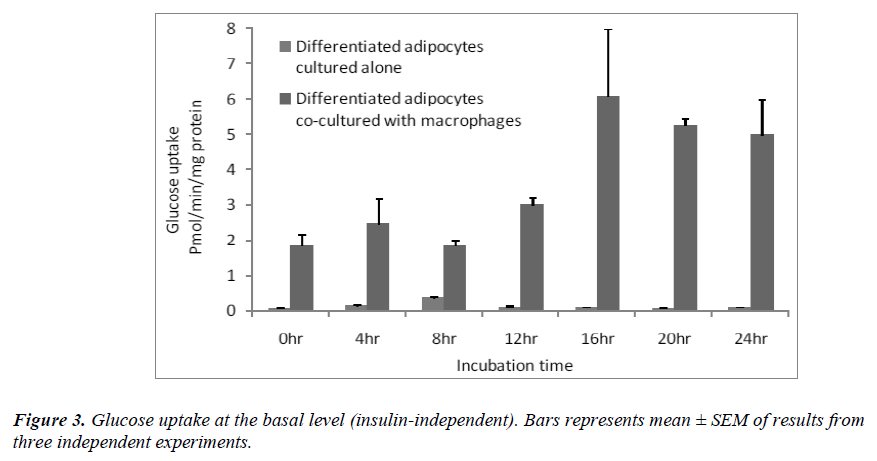
Effect of macrophages on insulin-independent glucose transport
Since the basal glucose uptake was significantly higher in the co-cultured adipocytes [Figure 2A], we determined basal glucose uptake over a period of 24 hrs. The results showed that at each time point, glucose uptake was significantly higher (p< 0.05 ) when the differentiated adipocytes were co-cultured with macrophages than when the differentiated adipocytes were cultured alone [Figure 3]. The increase in basal glucose uptake was timedependent in the co-cultured adipocytes, reaching a peak after 16 hrs of incubation.
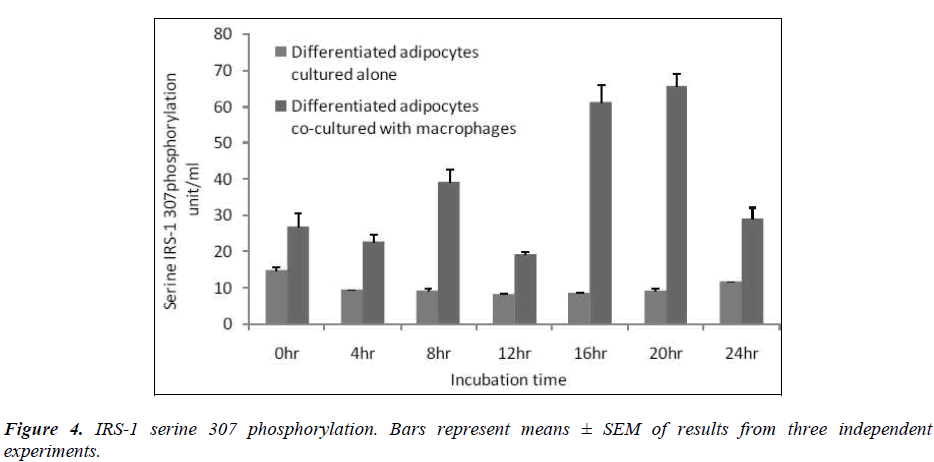
Effect of macrophages on IRS-1 serine-307 phosphorylation
In this study, we determined serine-307 phosphorylation in differentiated adipocytes co-cultured with macrophages and differentiated adipocytes cultured alone. The results showed that IRS-1 serine 307 phosphorylation was significantly higher (p< 0.05) when differentiated adipocytes were co-cultured with macrophages than when the differentiated adipocytes were cultured alone [Figure 4].
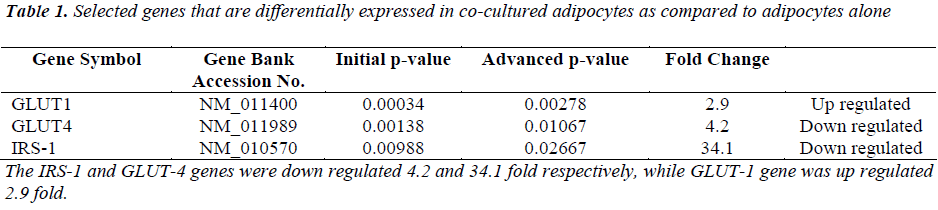
In order to verify the results obtained on the basal glucose uptake and insulin dependent glucose uptake using the radiometric assay and on serine phosphorylation using the ELIZA kit, we did a gene expression analysis on adipocytes that were cultured alone and on adipocytes cultured with macrophages using a quantitative real time PCR assay (RT2qPCR Primer assay). Table1 shows the expression of relevant genes involved in glucose uptake and serine phosphorylation with the fold changes.
Discussion
The main focus of our work was on the action of insulin on glucose metabolism in adipocytes co-cultured with macrophages. In tissues such as the skeletal muscle, insulin promotes glucose uptake by translocating GLUT-4 to the plasma membrane while impaired insulin signalling results in decreased insulin-dependent glucose uptake [18]. In recent years, the view of adipose tissue has transformed from that of a fuel store to that of an endocrine organ producing hormones like adipokines and proinflammatory cytokines [19]. Although whole-body glucose uptake takes place in muscle proper, glucose transport in adipocytes is required for normal glucose regulation [9]. Since adipocytes are infiltrated with macrophages in obese subjects, it is important to study the interaction between the two cell types. A study by Lehrke and Lazar [20] suggest that macrophages and adipocytes have many commonalities. They not only express overlapping sets of genes and have similar functions, but also comingle in the same part of the body: the adipose tissue.
A study by Lumeng [9] on indirect and direct co-culture demonstrated the importance of cellular contact as well as secreted factors in influencing the interaction between macrophages and adipocytes in adipose tissue. Therefore, it is important to study the function of macrophages and adipocytes where they are in direct physical contact, as in the adipose tissue of obese subjects. They influence one another by differential modification of the genes and the differential expression of the inflammatory proteins.
In culture, cells of preadipocytes are morphologically similar to fibroblasts. At confluence, induction of differentiation by appropriate treatment leads to drastic changes in the shape of the cells. Preadipocytes convert to a spherical shape, accumulate lipid droplets and progressively acquire the morphological and biochemical characteristics of matured adipocytes. The early indicator of adipocyte differentiation is the expression of lipoprotein lipase, which plays an important role in controlling lipid accumulation. Both a high level of lipogenesis and an increase in insulin sensitivity are observed in the last stage of differentiation [21].
In agreement with what has been previously documented, differentiated 3T3-L1 adipocytes showed lipid droplets [Figure 1]. Macrophages infiltrate adipose tissue, interact with the adipocytes and take on an elongated phenotype with long cellular processes. These processes interact with the surface of adipocytes, suggesting that adipocytes may modify macrophage properties and accumulate intracellular lipid. These observations suggest that macrophages co-cultured with adipocytes undergo morphological alteration similar to those in adipose tissue macrophages (ATM). ATMs from obese mice have an F4/80, + CD11B+CD68+CD14+ phenotype and form long cellular extension in culture [22]. Peritoneal macrophages take on the same morphology when cocultured with adipocytes. This suggests that direct contact-mediated factors derived from macrophages influence insulin sensitivity in adipocytes. As documented by Lumeng [24], our results show that macrophages in co-culture take on a long elongated phenotype with long cellular processes [Figure 2]. Although the adipose tissue in obese subjects is infiltrated with macrophages, the mechanisms involved in the interaction of the two cells resulting in insulin resistance is not well established.
Xie and coworkers [23] reported that C2D macrophages had a different impact on GLUT-1, GLUT-4 and adiponectin transcript levels as 3T3-L1 cells underwent differentiation, from the level of preadipocytes to adipocytes. Their data also revealed that adipocytes alter the C2D macrophage phenotype differently from preadipocytes and that the different phenotypic changes of the macrophage was important for the secretion of the different types as well as the quantity of proinflammatory cytokines. They also showed that the C2D Macrophages not only downregulated the number of GLUT-4 transcripts but also inhibited the differentiation of adipocytes .
It is now recognised that the stromal-vascular compartment of adipose tissue contains various precursors and stem cells that populate different cell lineages in vivo and in vitro [24]. Therefore, not only adipogenesis inhibited by the presence of macrophages, but macrophage activation/differentiation was also inhibited or activated depending on whether macrophages were interacting with preadipocytes or adipocytes.
Insulin uses phosphorylation and the resulting protein– protein interactions as essential tools to transmit and compartmentalise its signal [25].These intracellular protein–protein interactions are pivotal in transmitting the signal from the receptor to elicit final cellular effects, including glucose uptake. The transport of glucose across the cell membrane of the adipocyte is mediated by a set of stereo-specific transporter proteins such as glucose transporter-1 (GLUT-1), and glucose transporter-4 (GLUT-4). The transmission of the signal from the receptor results in the translocation of the vesicles containing GLUT-4 from the intracellular pool to the plasma membrane, activation of glycogen synthesis and initiation of gene transcription. At the basal level, the majority of glucose uptake is mediated by GLUT-1, whereas in the presence of insulin, GLUT-4 becomes the major glucose transporter resulting in a 10–20-fold increase in glucose uptake. Macrophages co-cultured with differentiated adipocytes have the ability to increase the expression of GLUT-1, and reduce the expression of GLUT-4 which results in an increase in glucose uptake at the basal level [26]. Our results using RTPCR showed a significant increase in GLUT-1 and a significant reduction in GLUT-4 [Table 1]. In support of this, our results show that co-cultured adipocytes increase the basal glucose uptake and reduce the insulin-dependent glucose uptake in a significant manner [Figures 2A and 2B]. The results of the basal glucose uptake in a time-dependent manner was determined in co-cultured adipocytes and adipocytes cultured alone [Figure 3]. Results showed a peak after 16 hours. A serine residue located near the phosphotyrosine-binding (PTB) domain in IRS-1 (serine- 307 in rat IRS-1and serine 312 in human IRS-1) is phosphorylated through several mechanisms. These findings suggest that inhibition of PTB domain function in IRS-1 by serine 307 (serine 312 in human IRS-1) phosphorylation might be a general mechanism regulating insulin signaling, resulting in the impairment of the insulin signalling pathway. In a 24-hour culture in our study, there was a significant increase in serine-307 phosphorylation in differentiated adipocytes cultured with macrophages compared to differentiated adipocytes cultured alone with maximal phosphorylation at 16 and 20 hours [Figure 4]. These results possibly indicate that in our invitro culture, the cells become insulin resistant after 16 hours.
Although several proteins serve as substrates for the IR, IRS-1 and IRS-2 are the most specific and the two key substrates involved in insulin resistance in obesity and type 2 diabetes. Decreased cellular levels of IRS-1 and IRS-2 have been shown to be associated with insulin resistance in animal and human subjects with type 2 diabetes [27]. In agreement with the above results, our study showed a significant reduction in the expression of GLUT-4 and IRS-1 and an increase in GLUT-1 [Table 1].
Conclusion
The onset of insulin resistance presents an interesting challenge because of the multifactorial impact of numerous cytokines and diverse differentiation stages of cells in the adipose tissue in the adipocyte–macrophage interaction. Our results demonstrate that macrophages, when in direct contact with adipocytes, increase serine- 307 phosphorylation and insulin-independent glucose uptake while reducing insulin-dependent glucose uptake, playing a crucial role in the development of obesity and type 2 diabetes. This is supported by the fact that the expression of GLUT-4 and IRS-1 were significantly downregulated while the expression of GLUT-1 was upregulated. Macrophages that accumulate in the adipose tissue of obese subjects may be one of the risk factors for the development of insulin resistance and type 2 diabetes.
Acknowledgements
Authors are grateful for the assistance provided by Shamila Vellapasamy and Arokiam Vincent Rayapan. This study was funded by e-Science grant number: 12-02- 03-2044
References
- Misra A, Khurana L. Obesity–related non communicable diseases: South Asians and Caucasians. Intl J Obesity 2011; 35: 167-187.
- Goossens GH, Bizzari A, Cajlakovic M, Blaak EE. Adipose tissue hypoxia and insulin resistance: role for adipose tissue blood flow. Obesity reviews T1:OS4.2 Abstract Book. 11th International Congress on Obesity Stockholm Sweden. Diabetese 2007:56;901-911 [details-year of publication and name of publisher be given].
- Kido Y, Nakae J, Accili D. The insulin receptor and its cellular targets. J. Clin Endocr Metab 2001; 86:972 – 979 final page ?
- Ward CW, Lawrence MC. Ligand-induced activation of the insulin receptor : a multi-step process involving structural changes in both the ligand and the receptor. Bioessays 2009; 31: 422-34.
- Aquirre, Vincent; Werner Eric D, Giraud Jodel, Lee Yong Hee, Shoelson Steve E, White Morris F. “ Phosphorylation of Ser307 in insulin receptor substrate -1 blocks interaction with the insulin receptor and inhibits insulin action”. J Biol Chem (USA) 2002; 277: 5980-3
- Greene MW, Sakaue H, Wang L , Alessi DR, Roth.RA. Modulation of Insulin stimulated degradation of Human Insulin Receptor Substrate-1 by serine 312 phosphorylation. J Biol Chem 2003; 278:8199- 211.
- Hribal ML, Oriente F and Accili D. Mouse models of insulin resistance. Am J Physiol Endocrinol Metab 2002; 282:E977- E981
- Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 2003; 112:1796-808.
- Lumeng CN, Deyoung SM, Saltiel AR. Macrophages block insulin action in adipocytes by altering expression of signalling and glucose transport proteins. Am J Physiol Endocr Metab 2007; 292:E166-174.
- Permana PA, Menge C, Reaven PD. Macrophage secreted factors induce adipocyte inflammation and insulin resistance. Biochem Biophys Res Com 2006; 341:50714.
- Miyazawa-Hoshimoto S, Takahashi K, Bujo H and Hashimoto N. Roles of degree on VEGF expression in adipocytes. Am J Physiol Endocrinol Metab 2005; 288:E1128-36.
- Yuan H, Zhao C. 3T3-L1 cell line revealing partially white adipogenesis of mice. Asian J Anim Vet Adv 2010; 6:482-87.
- Suganami T, Nishida J, Ogawa Y. A () paracrine loop between adipocytes and Macrophages aggravates inflammatory changes: role of free fatty acids and tumor necrosis factor alpha. Arterioscler Thromb Vasc Biol 2005; 25:2062-68.
- Kozma L, Baltensperger K, Klarlund J, Porras A, Santos E, Czech MP The Ras signaling pathway mimics insulin action on glucose transporter translocation. Proc Natl Acad Sci 1993; 90: 4460-64.
- Lakshmanan J , Elmendorf JS, Özcan S. Analysis of Insulin stimulated glucose uptake in differentiated 3T3-L1 adipocytes. In:ozcan S (eds ) Methods in Molecular Medicine. Diabetes Mellitus: Methods and Protocols Human press
- Totowa, NJ 2002; pp 97-103.
- SA Biosciences User Manual. Real Time Reverse Transcription Profile PCR Array System. Real Time PCR-Based Pathway Focused Gene Expression Profiling 2009.
- Khan BB, Rosen AS, Bak JF, Andersen PH, Damso P, Lund S, Pederson O. Expression of Glut1, and Glut4 glucose transporters in skeletal muscle of humans with insulin-dependent diabetes mellitus: regulatory effects of metabolic factors. J Clin Endocrinol Metab 1992; 74:1101-9.
- Ahima RS, Flier JS. Adipose tissue as an endocrine organ. Trends Endocrinol Metab 2000; 11:327-32.
- Lehrke M, and Lazar MA. Inflamed about obesity. Nature Med 2004; 10:126-27.
- Gregoire FM, Smas CM, Sul HS. Understanding adipocyte differentiation. Physiol Rev 1998; 78:783- 809.
- Lumeng CN , Bodzin JL and Saltiel AR . Obesity induces a phenotypic switch In adipose tissue macrophage polarization. J Clin Invest 2007; 11:175- 84.
- Linglin Xie, M.Teresa Ortega, Sylvia Mora and Stephen K.Chapes. Clin.Vaccine Immunol 2010;17:651.DOI:10.1128/CVI.00494-09.
- Ailhaud,G.Adipose tissue as a secretory organ: from adipogenesis to the metabolic syndrome. C R Boil 2006; 329:570-577.
- Virkamaki A, Ueki K, Kahn CR. Protein–protein interaction in insulin signaling and the molecular mechanisms of insulin resistance. J Clin Invest 1999; 103:931-43.
- Ebeling P, Koistinen HA , Koivisto VA. Insulin independent glucose transport regulates insulin sensitivity. FEBS Letters 1998; 43, 3:301-03.
- White MF. IRS proteins and the common path to diabetes Am J Physiol Endocrinol Metab 2002; 83:E413-22.