ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2021) Red Cell Immunology and Genotyping
Gestational trophoblastic tumors (GTT): management and experience of the FEZ-Maroc oncology department
O.Zouiten*, K.Oualla, Y.Elouai, K.Darif, L.Amaadour, Z.Benbrahim, S.Arifi, N.Mellas
Department of Medical Oncology, Centre Hospitalier Universitaire Hassan, Hassan II, Morocco
- Corresponding Author:
- Dr. Othmane Zouiten
Department of Medical Oncology
Centre Hospitalier Universitaire Hassan Hassan II
Morocco
E-mail: drzouitenothmane@gmail.com
Accepted date: 04 November, 2021
The gestational trophoblastic diseases consist of a broad spectrum of tumors originating in the placental trophoblastic tissue after abnormal fertilization. Gestational trophoblastic tumors are represented by: invasive mole, placental choriocarcinoma, tumor of the implantation site. We conducted a retrospective and descriptive study to analyze the epidemiological profile, the gynecological and obstetrical profile, and the diagnostic and prognostic criteria used in the diagnosis of Gestational trophoblastic tumors, as well as a description of the therapeutic, monitoring and prognostic protocol of the patients followed for Gestational trophoblastic tumors with a review of literature.
Keywords
Gestational trophoblastic diseases, hydatidiform mole (HM), gestational trophoblastic tumors (GTT).
Introduction
Gestational trophoblastic diseases (GTD) are a group of distinct entities, and essentially include hydatidiform mole (HM) and gestational trophoblastic tumors (GTT). The last one includes all of the following oncological diseases: invasive mole, choriocarcinoma, placental insertion site disease, and epithelioid trophoblastic tumor.
TTGs are rare malignant tumors. They are characterized by a high metastatic potential and increased mortality in the absence of well-conducted treatment. This makes GTT a diagnostic and therapeutic oncological emergency [1-3].
Methods and Materials
Our study is a retrospective and descriptive study spanning 6 years between January 2010 and December 2016, including all patients aged ≥18 years, having been diagnosed with GTT on follow-up of hydatiform moles, or on an anatomopathological diagnosis of the aspiration or curettage product and/or on an anatomopathological examination of surgical parts for total hysterectomy, or referred from other hospital structures for specialized management of GTT within the Medical Oncology Department of CHU Hassan II of FEZ Morocco. The diagnostic criteria used to select the diagnosis of GTT are those proposed in 2000 by the FIGO Oncology Committee.
The objective of our study is the analysis of the epidemiological profile, the gynecological and obstetrical profile, and the diagnostic and prognostic criteria used in the diagnosis of GTT, as well as a description of the therapeutic, monitoring and prognostic protocol of the patients followed for GTT in the Medical Oncology Department of FEZ [4].
The data was collected from the hospitalization records. The data collected were analyzed using SPSS version 20, with 95% confidence interval (CI). A complete response (CR) was defined as the complete disappearance of all evidence of the disease. The median survival was measured from the date of diagnosis to the date of end or last follow-up, or to the date of death.
Results
During the period between January 2010 and December 2016, 29 patients followed for TTG were included in our study. The average age was 35 years. Women aged between 21-30 years and those over 40 years were the most presented in our series with a frequency of 38% and 35% respectively. 15 patients were from rural areas, and 13 patients were from urban areas, approximate frequencies for geographical origin. All of our patients were married and unemployed at the time of admission to the service. 76% of our patients were of low socio-economic status. The paternal age and blood type of our patients was not reported in our medical records.
In our series the gynecological-obstetrical profile was characterized by an average age of menarche at 14 years. A peak in frequency in paucigests with a frequency of 41% and an approximate 2nd peak in multigests (38% of our patients) was noted in the series. More than half of the patients were pauciparum, with a frequency of 65% of cases. We also noted the same number of cases for both primiparous and multiparous (7% of cases each). However, nulliparous women are not immune to this pathology, which constitutes 21% of our patients. In our series, 69% of our patients were diagnosed in the aftermath of complete mole, and 14% of cases in the aftermath of partial mole and 17% of cases in the aftermath of abortion. For contraception, only 4 patients were on oral contraception but with poor compliance before the occurrence of GTT. The majority of our patients wanted to become pregnant (25 patients).
For the diagnostic criteria, metrorrhagia was the most common clinical sign presented in our series, 86.2% of the cases. Pelvic pain and/or tenderness was reported in 17% of cases. 22 patients were diagnosed with TTG following mole, including 18 cases by the existence of an increase in the values of βHCG over at least 3 successive measurements over a period of 2 weeks (Day1, Day7, and Day14). 03 cases diagnosed by the existence of a plateau in the values of ΒHCG over at least 4 successive weekly dosages over a period of 3 weeks, and only one patient diagnosed by the persistence of ΒHCG detectable more than 6 months after evacuation.
The anatomopathological diagnosis was reported in 8 patients on the hemostasis hysterectomy specimen, thus objectivizing 5 cases of choriocarcinomas, 2 cases of invasive mole and one case of placental site trophoblastic tumor (PSTT)
Most of our patients were diagnosed with GTT in the aftermath of a molar pregnancy by monitoring values from ΒHCG (79.3% of cases).
All patients had received a pelvic Echo-doppler showing the appearance of an enlarged uterus with a heterogeneous intramyometrial vascularized Doppler image in 23 patients (79% of cases) and a retention image in 6 patients (21% of cases). Ultrasound also showed an absence of luteinic cysts in all patients.
All our patients were scored according to the FIGO 2000 risk score, having all undergone a lung X-ray, a cerebral and thoraco-abdominal CT scan, and a pelvic MRI as part of an extension assessment.
It was noted that 51.72% of cases had metastases, for 10 patients were pulmonary metastases, 03 patients had liver metastases, one patient with renal metastases, and one patient had brain metastases.
Concerning the delay between the causal pregnancy and the occurrence of GTT, for 12 patients the GTT occurred 1-2 months after the causal pregnancy (41% of cases), for 11 patients it was between 3-6 months (38% of cases), and after 6 months of the causal pregnancy for 6 patients (21% of cases). For the rate of ΒHCG before treatment, there was a predominance of patients with a rate between 103 and 104 equivalents to 48% of cases.
The prognostic scores of our patients were between 2 and 17. More than half of our patients were classified as high risk, and 48% of cases were classified as low risk.
In our series the therapeutic management of our patients was coded by the FIGO 2000 prognostic scores of our patients after pre-therapeutic clinical and biological evaluation and during therapeutic management.
Chemotherapy was proposed for 28 patients according to their prognostic scores. It is also noted that for 20 patients had received 1st line chemotherapy only, compared to 8 patients who had received 1st and 2nd line chemotherapy, whose indication was either resistance to chemotherapy 1st line or relapse during post-chemotherapy monitoring.
For low-risk patients, the 1st line monochemotherapy was indicated, consisting of methotrexate (MTX) alone administered at a dose of 40 mg/m2 weekly in IM or IV, until negativation of ΒHCG, followed by 2 courses of MTX after negativation. It was noted that 13 patients classified as low risk who had received MTX-based chemotherapy, one of whom had received 2 courses of MTX and had developed febrile neutropenia with grade IV mucositis hence the indication for Actinomycin D-based monochemotherapy administered at a dose of 5mg/day over 05 days. The average number of cures received was 6.5 cures, with a minimum of 4 cures and a maximum of 14 cures. The response rate to 1st line chemotherapy in the low-risk group was 61.53%, with 4 cases of MTX resistance and 1 case of ACT-D resistance.
In our series 14 patients received multidrug therapy. The protocols (Table 1) used in 7 of our patients was the EMA protocol CO.5 patients were put on the CEM protocol. The BEP protocol was used in 2 patients. The average number of cures was 3.28 cures [Extreme 1-5 cures], all chemotherapy protocols combined. And the response rate to 1st line multidrug therapy was 85.71%, with only one case of resistance and one case of death [5].
| Indication | protocol | Doses-Modalities | Number of patients |
|---|---|---|---|
| High-risk TTGs, on the front line | EMA-CO | D1+D2 :( EMA) | 7 |
| -Actinomycin D 0.5 mg IV J1, J2 | |||
| -Etoposide 100 mg/m² IV J1, J2 | |||
| -Methotrexate 100 mg/m² IV then 200 | |||
| mg/m² over 12 hrs, | |||
| -Folinic acid 15 mg IM x 2/d 12 hours after the end of MTX; 4 IVL injections 12 hours apart. | |||
| D8: (CO) | |||
| -Vincristine 1mg/m² IV. | |||
| -Cyclophosphamide 600 mg/m² IV. | |||
| Weekly until normalization of hCG then consolidation :2 cures | |||
| Cisplatin-etoposide-MTX | J1 : | 5 | |
| Cisplatin 70-80 mg/m² IV J1 | |||
| D1->D3 : | |||
| Etoposide 100 mg/m² IV | |||
| J1 : | |||
| Methotrexate 50 mg/m² IV. | |||
| Every 21 days until negativation of the | |||
| hCG then consolidation: 3cures | |||
| BEP | D1, D8, and D15: | 2 | |
| -Bleomycin 30mg IV, | |||
| J1 ->J5: | |||
| -Cisplatin 20 mg/m2 IV, | |||
| - Etoposide 100 mg/m2 IV. | |||
| Every 21 until negativation of the Bhcg then consolidation :2 cures. |
Table 1: Polychemotherapy protocols used in the treatment of patients with high-risk GTT at the medical oncology department of CHU HASSAN II in Fez between 2010 and 2016.
One patient put on EMA-CO, 3 patients put on MTX, and the patient who was put on Actinomycin D, showed a ascension of the ΒHCG either during treatment or after negativation (during monitoring), hence the indication to put them on 2nd line chemotherapy; BEP protocol for the patient previously treated with EMA-CO, and 2nd line EMA-CO chemotherapy for the 4 patients who were under monochemotherapy.
12 patients had experienced side effects from the administration of chemotherapy (41.37%), of which 2 patients developed haemato-toxicity following the BEP protocol, and one patient died from febrile pancytopenia, which developed after receiving 4 courses of BEP.
8 patients underwent hemostatic hysterectomy, classified as high risk, one of whom had negatively affected her ΒHCG levels after surgery, and therefore did not receive chemotherapy. An interanxial hysterectomy was performed, in a low-risk patient who had developed resistance to first-line chemotherapy, and then she received additional multidrug therapy. Embolisation was carried out in a single patient with hemostatic aims. No patient received radiotherapy.
All the patients were followed regularly in our training, the majority (26 patients) were declared cured, and so far without any abnormality. The remission period for this category was on average 12 weeks, all stages taken together, with a minimum of 3 weeks and a maximum of 32 weeks.
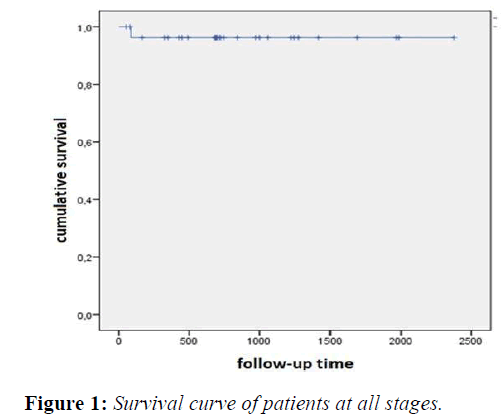
After a median follow-up of 68.3 months; 90% of our patients in all stages survived, with a survival of 76.4 ± 2.7 months. (Figure 1)
26 patients from our series were monitored in our training after negating their rates from ΒHCG. Estroprogestogenic contraception was systematically prescribed for all patients before the start of treatment and continued throughout the treatment period. Pregnancy is only permitted in our training after the end of monitoring, and all patients were routinely put on contraception.4 patients who became pregnant carried their pregnancies to term without maternal or fetal complications, of which 3 had met the recommended timeframes and 1 patient had not, and became pregnant 2 months and 2 weeks after the end of her weekly methotrexate (MTX) chemotherapy.
Discussion
Gestational trophoblastic diseases consist of a broad spectrum of tumors originating in the placental trophoblastic tissue after abnormal fertilization. They account for less than 1% of all gynecological malignancies. Some of these diseases are benign: complete or partial hydatidiform mole, these can evolve into malignancy (10-20%), we then speak of persistent trophoblastic diseases (PTD).
TTPs are represented by: invasive mole, placental choriocarcinoma, tumor of the implantation site. Thus, the incidence of malignant transformation towards a TTP is estimated at 10 to 20% in China, 20% in the USA, and in France the incidence is estimated at 16% of CDs and 0.5% of PMs.
Among the risk factors for AMD described in the literature: Maternal age, which seems to be an important and major factor in the occurrence of AMD. Numerous studies have shown that women >40 years of age are 14 times more likely to develop GTT and in particular choriocarcinoma, while women <20 years of age, specifying adolescents <16 years of age in some studies, also appear to be at increased risk compared to mothers aged 20-39 years. In our study, the average age was 35 years, and the age group (21-30 years) and the age above 40 years were most affected by the pathology with an incidence of 38% and 35% respectively.
Most studies have considered that paternal age does not influence the occurrence of GTT, while Parazzini et al found a high incidence in men over 45 years of age. This factor could not be determined in our study.
Living conditions are often considered as a risk factor although no real causal relationship has been demonstrated. Low socio- economic status associated with malnutrition was suspected. However, it is difficult to determine in the different published studies the respective impact of each of these two factors. In our study, 76% of our patients were of low socioeconomic status, which is consistent with the data in the literature, and 54% were from rural areas.
The patient's blood group was also reported to be an important factor with a higher risk for group A or AB patients compared to group B or O patients, but no pathophysiological mechanism is proposed to explain this observation. Blood grouping has not been mentioned in any of our patients, so we cannot make a comparison with other studies that have been carried out.
The increased risk of GTT with parity is almost constantly reported. Andria Altieri et al reported a notion of a significant increase in the risk of choriocarcinoma with parity and estimated that the risk is multiplied by 5.2 after the fourth parity. The same notion was reported in a study in Senegal where large multiparous women were three times more likely to develop choriocarcinoma than those with a parity of 4 or less.
However, more recent studies, notably the one conducted by TCHEGNIKIN M in 2011, have concluded that the gestation/ parity profile of patients with GTT has changed, with younger and nulliparous patients followed by pauciparum patients. In our series, 41% of the cases are paucigestes. The distribution according to parity shows a predominance among the paucipares with a frequency of 65% of the population studied. This is consistent with recent data in the literature.
The risk of hydatidiform mole is increased threefold by the history of abortion, but this factor is still under discussion. However, there seems to be a genetic predisposition since recurrences are not always consecutive and do not always occur with the same partner. In our study, In our study, 5 cases had at least one history of outside abortion. None of our patients had a hydatidiform mole TCDA outside of the causal pregnancy.
In the literature, 50% of GTTs occur after a mole pregnancy, 25% after abortion and 25% after full-term pregnancy. More precisely, invasive mole complicates 10 to 20% of complete hydatiform moles, and 0.5% of partial moles. In parallel with the literature, the results of our study show that more than half of our patients had experienced GTT after a complete mole, a frequency of 69% of cases, compared to 14% after a partial mole, and 17% after an abortion.
Oral contraception does not seem to have a role in the incidence of choriocarcinoma, however an American study concluded that the risk of this tumor increases from 2.2 to 6.4 in patients who have already taken contraception compared to those who have never taken it. A relationship between duration of oral contraceptive use and the occurrence of GTT was discussed by Palmer JR, Rosenberg L, in a study published in 1999, which showed that the longer the duration of oral contraceptive use before conception, the higher the risk of GTT. In our study, only 4 patients were on oral estrogen- progestin-only contraception. The duration of use was not specified.
The symptomatology of GTT varies according to the clinical form present. The patient most often presents spontaneous metrorrhagia, abdomino-pelvic pain, vaginal discharge, latero- uterine masses, anaemia, and respiratory signs in relation to the presence of pulmonary or hepatic metastases. In the context of paraneoplastic symptoms, hyperthyroidism is noted in 3-5% of cases, probably related to the thyreotropic effect of ΒHCG.
There are also cases where metastases inaugurate the clinical picture. Patients generally present with dyspnoea, and more rarely with intracranial hypertension. Our study is in line with the literature, in that 86.2% of cases presented with metrorrhagia as a primary, isolated clinical sign, and 17% of cases presented in consultation for associated pelvic pain and/or tenderness.
The diagnosis of GTT is suspected in the face of an abnormally high dosage of ΒHCG compared to the theoretical term of the pregnancy.
MH is diagnosed on ultrasound, which finds an abnormally large uterus at term containing blurred, diffuse, flaky images giving the snowstorm-like appearance characteristic of the pathology.
The diagnosis is confirmed by an anatomopathological examination of the aspiration or curettage product, or in the event of an abnormal evolution of the BHCG after evacuation of a CBM.
In our study, the majority of our patients, a frequency of 79.3% of cases, were diagnosed as having TTG in the aftermath of a partial or complete molar pregnancy, by biological monitoring of the values from ΒHCG, compared to 20.6% of cases that had been diagnosed on an anatomopathological proof of a hysterectomy piece of hemostasis hysterectomy. These data are similar to those found in North America and Europe, where the diagnosis is made at an early stage, based on the biological evolution disturbed during the monitoring in the aftermath of hydatidiform mole.
In Rabat, a 2012 study found that only 33% of cases were diagnosed as TTG, based on the biologically disturbed rates of ΒHCG. The anatomopathological examination is then not essential for the diagnosis and the implementation of chemotherapy.
In 79% of cases, the delay was less than 6 months, between the causal pregnancy and the occurrence of GTT, which is perfectly in line with the results of studies conducted worldwide (Table 2), with an average delay of 6 months. This justifies the need for intensive monitoring of patients during the first 12 months following evacuation of a mole, the risk being much lower after 12 months.
| Region | Average time from causal pregnancy to GTT occurrence |
| Senegal | 7 months |
| France | 6 months |
| Chine | 6 months |
| Norway | 4 months |
Table 2: Delay between causal pregnancy and diagnosis of GTT.
This surveillance is based essentially on repeated serum ΒHCG measurements and on the search for metrorrhagia during mole pregnancy .
The extension assessment consists of a standard lung X-ray to detect pulmonary metastases. An additional of the thoracoabdominopelvic CT scan stages is requested if ΒHCG elevated or the standard lung X-ray is pathological. An MRI is performed unless pulmonary metastasis or neurological signs are present.
All the cases included in our study benefited from a complete extension workup, consisting of a lung X-ray, cerebral and thoracoabdominal CT scan and a pelvic MRI.
The classification of AHT has evolved over the years, due to the incrimination of several morphological and biological stigmas, which have been recommended in the evaluation of the prognosis of AHT. Hence the diversity of classifications.
In 2000, the International Federation of Gynecology and Obstetrics (FIGO) took up and modified the classification in partnership with the Committee of the International Society for the Study of Trophoblastic Diseases (ISSTD) and the International Society of Gynecologic Cancers (IGCS), the scoring system established by the World Health Organization (WHO).
The diagnosis of our patients has been carried out according to the FIGO 2000 criteria. (Table 3). In our study, prognostic scores ranged from 2 to 17, with 52% of cases classified as high risk and 48% as low risk.
| SCORE | 0 | 1 | 2 | 4 |
|---|---|---|---|---|
| Age | < 40 years | > ou= 40 years | ||
| Initial pregnancy type | Môle | Abortion | Term Pregnancy | |
| Numbers of months since previous pregnancy | <4 | 44293 | 44390 | > ou= 13 |
| hCG plasma before ui/l treatment | < 10*3 | 10*3 _< 10*4 | 10*4 _<10*5 | >ou =10*5 |
| Larger tumor size | <3 cm | 3 - 5 cm | > ou = 5cm | |
| Metastatic sites | Lungs | Spleen-Kidneys | Digestive tract | Liver, Brain |
| Number of metastases | 0 | 44200 | 44324 | > ou= 8 |
| Pre-ablative chemotherapy | Mono-Chemotherapy | Poly-Chemotherapy |
Table 3: FIGO 2000 Staging.
In terms of treatment, GTTs are highly chemosensitive: survival has increased from 19% (when the treatment was only surgical) to over 90% since chemotherapy, with sometimes heavy protocols seeming necessary.
It is now possible to speak of the cure of almost all patients with GTT, thus constituting a specificity of this tumour pathology. The treatment is adapted to the severity of the disease as determined by the FIGO score.
Chemotherapy is the mainstay of treatment for GTT, which is one of the only cancers for which monotherapy is used.
The therapeutic decision for the choice of agent or protocol (mono- or multidrug therapy) depends on the risk score for each patient diagnosed with GTT, which must be established as soon as the diagnosis is confirmed.
Monochemotherapy is indicated from the outset to treat low- risk GTT (FIGO score<7), the first drugs recognised as active and effective were methotrexate (MTX) and actinomycin D (ACT-D) used as monotherapy.
Several studies have compared the efficacy of these 2 agents in the treatment of low-risk GTT, taking into account the treatment's effectiveness, side effects and toxicity on patients; without being able to assert the superiority of one agent over the other.
Etoposide (VP16) seems to give a complete remission exceeding MTX and ACT-D, reaching 100% according to some studies at a dose of 200 mg/m2, daily OV for 5 days; however, it is currently little used because of its increased risk of secondary tumours and its high toxicity on the bone marrow.
MTX is used according to different protocols (table 4). Actinomycin D is used as first-line therapy in cases of renal or hepatic insufficiency, which contraindicates MTX, and as second-line therapy when the patient develops resistance to MTX.
| Protocol | Administration mode | Side effects | Rate of remission in%. |
|---|---|---|---|
| Protocol Herts | 0.4mg/kg/d (max 25mg) per IV or IM route 1 injection per day for 5 days every 14 days up to 3 weeks after negativation of βHCG. |
Mucite, alopecia Poor hematological tolerance: Pancytopenia (71% according to Hammond et al.) | 82 93 |
| Protocol GOLDSTEIN | 1mg/Kg (IM track) J1, J3, J5, J7 in association with folinic acid 0.1mg /Kg at d2, D4, D6, D8 per os |
Mucite, alopecia, skin rash. | 73 89 |
| Protocol modified of Bagshawe | 1 mg/kg at d1, d3, d5 and d7 repeated every 14 days. | 74,2 77 |
|
| Protocol GOG*/ Homesley. | 30 to 50mg/m2 weekly with degression until 3 normal dosages are obtained from βHCG. | Good tolerance | 78 |
| Protocol | Bolus of 100mg/m2 then infusion of 200mg/m2 for 12h and folic acid 15mg/12h in IM or per os. | Good tolerance | 81.5 |
| New England Trophoblastic Disease Center group. |
Table 4: Frequently used methotrexate (MTX) protocols.
It has more side effects (nausea, alopecia) than MTX, and a risk of local tissue damage in case of extravasation during IV infusion.
The most effective protocols are ACT-D 10-12 mg / kg IV daily for five days every fortnight, or a single intravenous dose of 1.25 mg/m2 every fortnight.
In our series, the treatment regimen adopted for low-risk scoriated GTT was MTX monochemotherapy at a dose of 40 mg/m² weekly with monitoring of plasma BHCG at each course. Except for one patient; who was clinically intolerant to MTX and was subsequently put on ACT-D. Our study found a complete response rate in low-risk patients of 61.53% with 4 cases of MTX resistance and 1 case of ACT-D resistance; This is in line with litterature.
A FIGO score greater than or equal to 7 means, above all, prior resistance to monochemical therapy. Therefore, a protocol based on a combination of agents or polychemotherapy should be instituted. Several protocols have been developed which are MAC (Methotrexate, actinomycin D and cyclophosphamide), MAF (Methotrexate, folinic acid and actinomycin D), CHAMO-CA (Methotrexate, dactinomycin, cyclophosphamide, doxorubicin, melphalan, hydro- xycarbamide), and vincristine), EMA-CO (Etoposide, methotrexate and actinomycin D, cyclophosphamide and vincristine), EMA-EP (Etoposide, methotrexate and actinomycin, cisplatin), ACE (Actinomycin D, Cisplatin and Etoposide) and BEP (Bleomycin, Cisplatin and Etoposide).
In the literature, and since 1979, EMA-CO has been the reference treatment for high-risk GTT at Charing Cross Hospital. Gerulat et al. found a cure rate of 83% when this protocol was administered as first line treatment; in 2002, he listed the benefits of EMA-CO: better response rate, better long-term survival, minimum short and long-term toxicity. Thus several recent studies continue to report the usefulness of the EMA-CO protocol as a first-line treatment for high-risk GTT. Also, for the British centre, the EMA-CO is the mainstay of treatment of high-risk GTT with a 5-year survival rate reaching 70-90% of patients.
However, the side effects of multidrug therapy are not negligible. They must be taken into consideration when making therapeutic choices. Some deaths of patients are secondary not to the evolution of GTT itself but to the direct toxicity of chemotherapy. In our series, high-risk GTT patients have received multidrug therapy with EMA-CO or BEP. In parallel with the data in the literature, our study found, for the category of high-risk scoriated patients, a response rate to the polychemotherapy used was 85.71%, with only one case of death and only one case of resistance to 1st line polychemotherapy.
A controlled reascension of ΒHCG during treatment at eight days, or a stagnation ΒHCG (variation of less than 10%) during treatment over at least two cures, with or without new metastases developing, while the patient is undergoing treatment defines resistance to 1st line chemotherapy. On the other hand, a diagnosis of recurrence or relapse is made when there are two elevations in plasma BHCG concentrations in the absence of pregnancy after a normal ΒHCG result, called negative. Both circumstances are delicate in the case of GTT and therefore require remedial treatment. Resistance and relapse occur in about 3% of low-risk cases of FTT and in 7-10% or even 20% of high-risk cases of FTT.
The prognosis of patients with chemoresistance remains serious compared to those with relapse. Surgical treatment or even radiotherapy can be considered. As far as chemotherapy is concerned, many schemes have been developed for catch-up treatment such as the EMA-EP protocol (Etoposide, Methotrexate, actinomycin D and Etoposide, Cisplatin) or the TE / TP protocol (paclitaxel and Etoposide alternating weekly with paclitaxel and cisplatin) or others.
Results vary and response rates range from 20 to 75%. EMA- EP remains the most commonly used and has a cure rate of over 75%, but the reported toxicity remains significant.
For low-risk patients, as remedial treatment; Numerous studies have demonstrated the efficacy of EMA-CO or Actinomycin, administered to patients after an initial MTX-based protocol, with rates as high as 94-100% for some. Hence the need for good follow-up after negativation of ΒHCG.
For high-risk TTGs, EMA-EP appears to be the best choice for achieving complete remission in most patients initially treated with EMA-CO. In our study, 6 patients had developed resistance to 1st line chemotherapy, and their catch-up chemotherapy protocol depended on the chemotherapy previously received.
Cytotoxic drugs can have various adverse effects ranging from simple inflammation of the mucous membranes, stomatitis, gastrointestinal disorders to mortality. According to Lurain [5], 11% of GTT deaths are due to the direct toxicity of chemotherapy drugs, responsible among other things for septic and haemorrhagic accidents. The risk of the appearance of secondary cancers (especially acute myeloid leukaemia and thyroid cancer) would be without tangible proof, as already demonstrated by Rustin et al in 1983 and confirmed by several studies, the most recent in 2017 by Brown et al.
Hysterectomy was the cornerstone of GTT treatment prior to the advent of chemotherapy, which revolutionized treatment and remission rates. In 1960, Lurain reported a 5-year survival rate of 41% for patients with non-metastatic GTT and 19% for metastatic GTT. Despite the undeniable efficacy of chemotherapy, its toxicity and the chemoresistance of GTT are constraints which have led to the reconsideration of the place of surgery in distinct indications: it constitutes an effective treatment to reduce or resect the tumour mass. The importance of surgery in the management of GTT should not be underestimated. Surgery may involve the initial site and may involve hysterectomy or metastatic sites (pulmonary, cerebral and hepatic in particular). In our series, 9 patients underwent a hysterectomy. 8 for haemostatic indication, and one patient for chemotherapy resistance. No metastatic site surgery was performed.
Embolisation can be proposed for TTG patients who have a high risk of haemorrhage, due to the hyper-vascular nature of these tumours. Vaginal haemorrhage in particular can be a difficult circumstance to control in the management of these patients. For example, Carlini et al. reported the case of a patient who negated her ΒHCG levels after arterial embolisation alone without secondary chemotherapy. In our study, only one patient had undergone haemostatic embolisation.
Radiotherapy has a limited role in the management of GTT, especially as a palliative treatment for metastases. In our study, none of our patients had recourse to radiotherapy.
The post-therapy monitoring of patients is based on the clinic as well as on paraclinical examinations of which biology is the fundamental element, without forgetting the psychological support of patients facing mortality and morbidity linked to the cancer pathology as well as to the treatment undertaken.
For the CNGOF, the monitoring of a gestational trophoblastic tumour after chemotherapy treatment is based on repeated measurements of total serum BHCG, at the following rate. Once a week for the duration of chemotherapy and for the following 8 weeks and then every 15 days for the following 8 weeks followed by monthly dosing of ΒHCG up to 12 months in the case of low-risk GTT and up to 18 months in the case of high-risk GTT.
As regards contraception, and depending on the different centres, it should be maintained throughout the treatment period and for 1 year after completion of chemotherapy, preferably using oral contraceptives.
In our department we have adopted the same CNGO monitoring scheme. An imaging check-up of all the pathological sites of the initial check-up is carried out two weeks after the normalization of the CNGOs. This check-up can highlight the persistence of residual masses. These masses may regress spontaneously and do not need to be surgically removed, as the negativation of the BHCG is a sign of healing.
In our study, it was found that the majority of patients who were followed, had respected the monitoring rhythm, and 3 patients were lost to follow-up. And all patients were put, systematically, on oral contraception during chemotherapy and 1 year after the end of treatment.
The prognosis of GTT has been revolutionised by the introduction of chemotherapy in the management of GTT, making this tumour entity the most curable gynaecological cancer.
According to a study conducted by Hextan, the overall survival of patients reached a threshold of 90% if EMA-CO treatment was started and managed early. In our study, the remission rate was 90%. With only one reported case of death, following febrile pancytopenia, occurring after receiving 4 courses of BEP, a mortality rate of 3%.
The literature shows that 83% of patients maintain their fertility despite chemotherapy treatment, and most will be able to carry a pregnancy to term resulting in a live, deformity-free newborn. In our series, we found 4 patients who became pregnant, of which 3 patients had met the recommended time frame and only one patient who became pregnant just after the end of her chemotherapy.
Conclusion
GTTs represent a heterogeneous group of diseases with different prognoses, requiring a therapeutic approach adapted to each case, hence the importance of treatment by a competent specialist team so as not to compromise patients' chances of recovery due to errors in diagnosis or treatment, justifying the creation of reference centers for the treatment of these tumors.
References
- Soper JT, Mutch DG, Schink JC. Diagnosis and treatment of gestational trophoblastic disease: ACOG Practice Bulletin No. 53. Gynecol Oncol. 2004;16:93-99
- Golfier F, Raudrant D, Frappart L, et al. First epidemiological data from the French trophoblastic disease reference center. Am J Obstet Gynecol. 2007;196: 172–e1.
- Sebire NJ, Foskett M, Fisher RA, et al. Risk of partial and complete hydatidiform molar pregnancy in relation to maternal age. Int J Obstet Gynaecol. 2002;109:99–102.
- Altieri A, Franceschi S, Ferlay J, et al. Epidemiology and aetiology of gestational trophoblastic diseases. Lancet Oncol. 2003;4:670–676
- Parazzini F, Vecchia C, Pampallona S. Parental age and risk of complete and partial hydatidiform mole. BJOG Int J Obstet Gynaecol. 1986;93:582–585.