ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
- Biomedical Research (2016) Volume 27, Issue 2
Gentiopicroside inhibits cancer cell growth in OVCAR-3 ovary cancer cells through the mediation of apoptosis, loss of mitochondrial transmembrane potential and NF-kB signalling pathway.
| He Tian, Li Liu, Tong-Ju Yang, Yu-Qiu Wang* Department of Pharmacy, People’s Hospital of Zoucheng City, Shandong Province 273500, PR. China |
| Corresponding Author: Yu-Qiu Wang Department of Pharmacy People’s Hospital of Zoucheng City, PR. China |
| Accepted: January 30, 2016 |
The purpose of the current study was to demonstrate the antiproliferative and apoptotic effects of gentiopicroside in OVCAR-3 human ovary cancer cells. The effects on mitochondrial membrane potential loss and expression levels of various apoptosis-related proteins were also studied. The antiproliferative effect of gentiopicroside in OVCAR-3 cells was evaluated by MTT cell viability assay. Phase contrast and fluorescence microscopy techniques were employed to study the effect of the compound on cellular morphology and apoptosis. Flow cytometry using rhodamine-123 dye was used to measure changes in mitochondrial membrane potential (Δψm). Western blot analysis detected the changes in the expression levels of various proteins. The results indicated that gentiopicroside induces potent cytotoxic effects in OVACR-3 cells in a time-as well as dose-dependent manner. Fluorescence microscopy using acridine orange and propidium iodide double staining revealed that gentiopicroside induces moderate and extensive apoptosis in OVCAR-3 cells at lower and higher concentrations respectively. Gentiopicroside also induced potent depolarizing effects on the mitochondrial transmembrane potential with the percentage of depolarized mitochondria increasing from 5.2% in untreated cells to 16.7%, 44.5% and 67.3% in cells treated with 20, 40 and 100 μM dose of gentiopicroside respectively. Gentiopicroside treatment led to up-regulation of cleaved PARP-1, cytochrome c, caspase-3 and caspase-9 and down-regulation of NF-kB and Bcl-2 in a dose dependent manner. The current results indicate that gentiopicroside exerts potent anticancer and apoptotic effects in OVCAR-3 ovary cancer cells via disruption of mitochondrial membrane potential and altering the expression levels of several apoptosis-related proteins.
Keywords |
||||||||||||||
| Ovary cancer, Apoptosis, Gentiopicroside, Flow cytometry, Cytotoxic activity. | ||||||||||||||
Introduction |
||||||||||||||
| Cancer is one of the most deadly gynecological malignancies throughout the world and its incidence gets increased with age. Most of the ovarian tumors are already malignant on diagnosis due to the limited diagnostic tools and tissue anatomy. Almost 65-70% of the ovarian cancer patients have reached to late stages of the disease resulting in a low 5-year survival rate [1-3]. In China, the rate of ovary cancer was 8.0/100,000 and the age-adjusted rate was 5.35/100,000 overall during period 1999-2010. Surgery is the preliminary treatment of choice for ovarian cancer, provided patients are medically fit. Patients who are not fit for surgery may be given adjuvant chemotherapy and considered for surgery later. Chemotherapy involves use of chemical compounds of natural and synthetic origin to target and kill tumor cells [4]. Chemotherapeutic agents include carboplatin and paclitaxel among others. Despite of these agents, chemotherapy treatment protocol is often ineffective due to serious side-effects coupled with multidrug resistance by cancer cells which eventually leads to recurrence of the disease [5]. Therefore, there is a pressing need for novel, effective and relatively safer anticancer chemotherapeutic agents especially from plant origin. The objective of the current research work was to investigate the anticancer and apoptotic effects of gentiopicroside (chemically known as ((3S,4R)-4-Ethenyl-3-[(2S,3R,4S,5S,6R)-3,4,5- trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-4,6- dihydro-3Hpyrano[3,4-c]pyran-8-one) in OVCAR-3 ovary cancer cells. The effects of this compound on ROS generation and NF-kB signalling pathway were also elaborated. To the best of our knowledge, the current study constitutes the first such report on this molecule. | ||||||||||||||
Materials and Methods |
||||||||||||||
Chemicals and other reagents |
||||||||||||||
| Gentiopicroside (98% purity) was purchased from Sigma Chemical Company (St. Louis. Co), and 50 mg/ml solution dissolved in DMSO was stored at -20°C before use. 3-[4,5- dimeth-yl-2-thiazolyl]-2,5-diphenyl tetrazolium bromide (MTT) was purchased from Molecular Probes (USA). Dulbecco’s Modified Eagle’s Medium (DMEM), Fetal Bovine Serum (FBS), Penicillin-Streptomycin, Rhodamine 123 were obtained from Sigma-Aldrich (Sigma Aldrich, Shanghai, China). Primary antibodies against caspase-3, caspase-9, NF- κB, Bax, Bcl-2, PARP-1, cytochrome c, β-actin, and secondary antibodies (goat-anti-rabbit or goat-anti-mouse) were purchased from Millipore Pvt Ltd. | ||||||||||||||
Cell line and culture conditions |
||||||||||||||
| OVCAR-3 human ovary cancer cell line was purchased from the Shanghai Institute of Cell Resource Center of Life Science (Shanghai, China). The cells were cultured in DMEM medium supplemented with 10% FBS, 100 μg/ml Streptomycin, and 100 U/ml Penicillin maintained at 37°C in a humidified atmosphere with 5% CO2. | ||||||||||||||
Cell proliferation assay for cell viability |
||||||||||||||
| The cytotoxic effects of gentiopicroside were evaluated by MTT assay. Briefly, cells were placed in 96-well culture plates (2 × 105 cells/well). After 24 hours, the cells were treated with 0, 2.5, 5, 10, 20, 40 and 100 μM gentiopicroside or 0.1% DMSO respectively for 24 h. After that MTT (10 mg/ml) was added to each well. The cells were incubated for another 4 h, and 250 μL DMSO was added to each well. Absorbance was measured on a microplate reader (ELX 800; Bio-tek Instruments, Inc., Winooski, VT, USA) at a wavelength of 490 nm and the growth inhibition ratio was calculated. The halfmaximal Inhibitory Concentration values (IC50) were obtained from the MTT viability curves. | ||||||||||||||
Fluorescence microscopy using acridine orange and propidium iodide double-staining |
||||||||||||||
| In this assay, the apoptotic cell death induced by gentiopicroside was evaluated by fluorescence microscopy using propidium iodide and acridine orange double staining according to already reported methods [6]. OVCAR-3 cells were plated at a density of 2 × 105 cells/ml, and then treated with 0, 20, 40 and 100 μM concentration of gentiopicroside for 48 hours. The cells were centrifuged at 15, 000 g for 15 min, after which cells were washed three times with PBS. After that, 10 μL each of Acridine Orange (AO) and Propidium Iodide (PI) were added to the cell suspension. The stained cell suspension was put onto a glass slide and these slides were then monitored using fluorescence microscope for 30 minutes. Around 500 cells were chosen for determining the percentages of apoptotic, necrotic and living cells. | ||||||||||||||
Phase contrast microscopic evaluation of OVCAR-3 cancer cells after treatment |
||||||||||||||
| Phase contrast microscopic evaluation of OVCAR-3 cancer cells after drug treatment was done using inverted light microscope (Olympus, PA, USA). In brief, OVCAR-3 cells were seeded in 6-well plate at a density of 2 × 105 cells/well The cells were treated with 0, 20, 40 and 100 μM of gentiopicroside for 48 hours. The morphological changes were monitored and the same spot of cells was photographed. The images were captured at a magnification of 400X. | ||||||||||||||
Measurement of mitochondrial membrane potential (ΛΨm) loss |
||||||||||||||
| The effect of gentiopicroside on the loss of mitochondrial transmembrane potential was measured by flow cytometry using rhodamine-123 fluorescent probe. The OVCAR-3 cells (2 × 105 cells/well) were treated with 0, 20, 40 and 100 μM of gentiopicroside for 48 h. Rhodamine-123 dye was added 2 h before termination of the experiment. The cells were harvested, washed twice with PBS and then incubated in PI for 10 min. The decrease in fluorescence intensity, due to loss of ΛΨm, was analyzed using flow cytometry (FACSCalibur™; BD Biosciences). The mean fluorescence intensity was detected using the FL1 channel of the BD FACSCalibur™. | ||||||||||||||
Western blot analysis |
||||||||||||||
| OVCAR-3 cells were treated with 0, 20, 40 and 100 μM dose of gentiopicroside and then incubated for 48 h. The adherent and floating cells were harvested and then washed three times with PBS and then lysed in RIPA buffer and protease inhibitor for 10 min. After centrifugation, the protein content was determined by for Western blotting analysis. The protein lysates (20 μg/lane) were separated by 10% SDS-PAGE and blotted onto nitrocellulose membranes (Millipore, Bedford, MA, USA). Each membrane was blocked with 6% skim milk, and then incubated with the designated primary antibodies against caspase-9, caspase-3, Bcl-2, NF-κB, PARP-1, cytochrome c, and β-actin overnight at 4°C. Subsequently, the membrane was incubated with the secondary antibodies (HRPconjugated goat anti-rabbit or goat anti-mouse IgG) for 1 h at room temperature and the formed immunocomplex was visualized by western blotting detection reagents (Trans Gene, Beijing, China). | ||||||||||||||
Statistical analysis |
||||||||||||||
| Data are presented as the mean ± SEM. All experiments were repeated at least three times. The differences between groups were analyzed by one-way ANOVA, significance of difference was indicated as *P<0.05, **P<0.01. | ||||||||||||||
Results |
||||||||||||||
Cytotoxic activity of gentiopicroside in OVCAR-3 cancer cells |
||||||||||||||
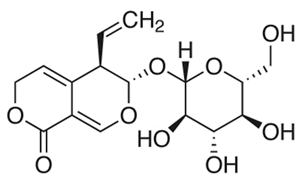
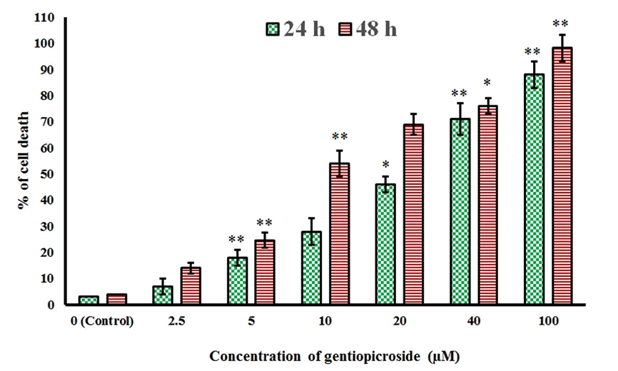
| The chemical structure of gentiopicroside is shown in Figure 1. The results of the current study indicated that gentiopicroside exerted potent cytotoxic effects in a time-dependent as well as dose-dependent manner. The potency of a chemical compound is expressed in terms of IC50 (half-maximal Inhibitory Concentration) value which is the concentration which causes 50% growth inhibition. The IC50 values at 24 and 48 h time intervals was found to be 22.4 and 8.2 μM respectively (Figure 2). | ||||||||||||||
Apoptosis quantification using Acridine Orange (AO) and propidium iodide staining |
||||||||||||||
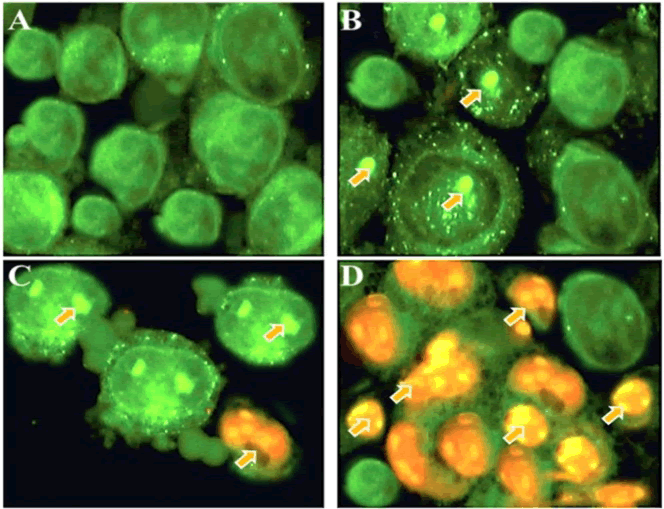
| Fluorescence microscopy can be used to examine the apoptotic morphological changes in cancer cells after drug treatment. The present study indicated that gentiopicroside induced potent apoptotic morphological changes in a dose-dependent manner (Figure 3). | ||||||||||||||
| As can be seen from Figure 3, untreated control cells (Figure 3A) revealed green colour indicating undamaged nuclear structure. However, after treatment with 20 and 40 μM dose of gentiopicroside, signs of mild apoptosis could be seen from the induced morphological changes including nuclear fragmentation and membrane blebbing (Figures 3B and 3C). However, at higher dose (100 μM) dose of gentiopicroside, extensive apoptosis could be observed characterized by reddish-orange fluorescence which arises due to the binding of AO dye to the fragmented DNA (Figure 3D). Around 500 cells were chosen for quantification of apoptosis. | ||||||||||||||
Phase contrast microscopic evaluation of the gentiopicroside-induced cell cytotoxicity |
||||||||||||||
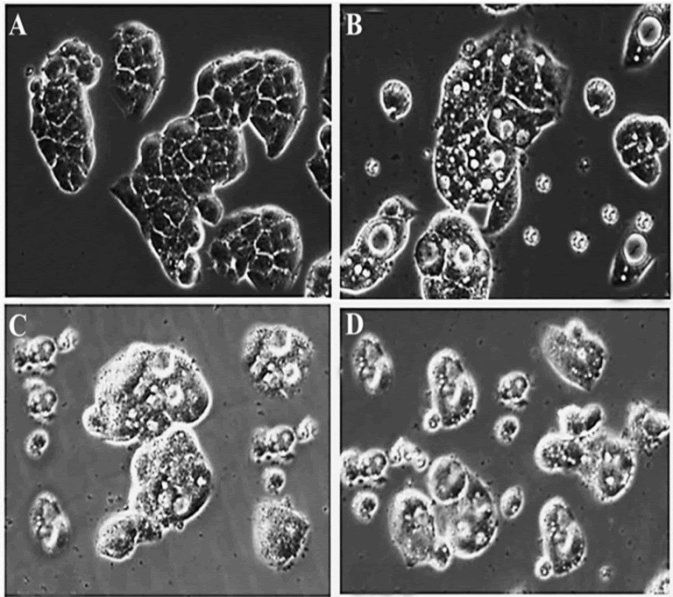
| Exposure of OVCAR-3 cells to 0, 20, 40 and 100 μM of gentiopicroside for 48 h resulted in a substantial decrease in cell count and, furthermore, induced morphological changes that were characteristic of cytotoxicity in OVCAR-3 cells under phase-contrasted microscopy following exposure to the drug (Figure 4). The results indicated that the number of cells with a vacuolated cytoplasm was markedly higher in the gentiopicroside-treated group compared with the control group. The appearance of vacuoles in the cytoplasm is an indication of early apoptosis. | ||||||||||||||
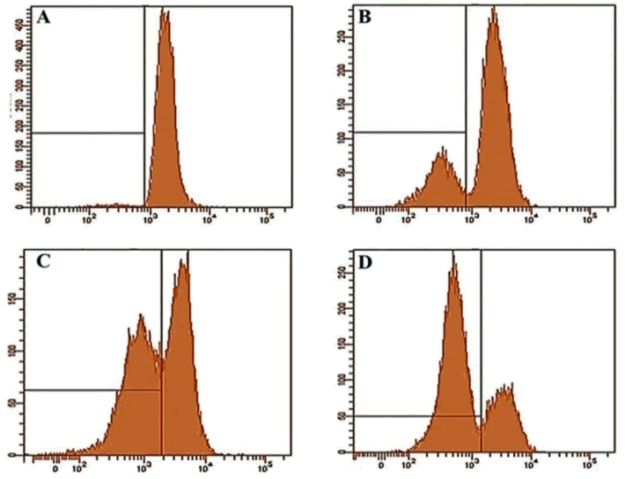
Gentiopicroside induced mitochondrial membrane potential loss (Δψm) in OVCAR-3 cancer cells |
||||||||||||||
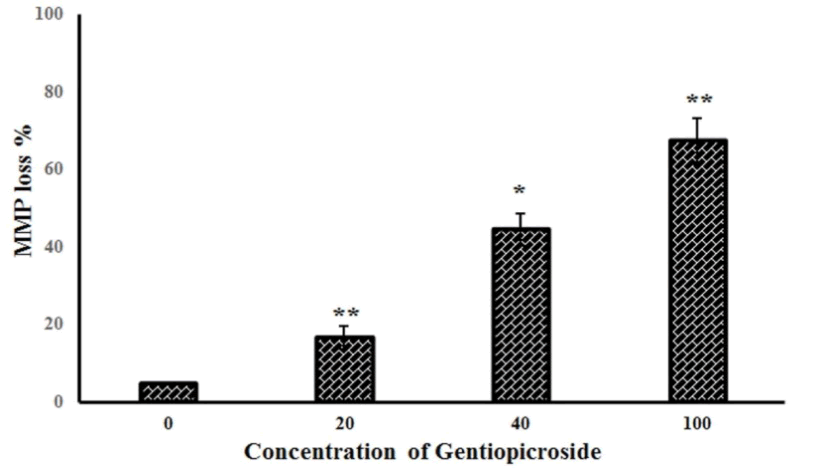
| Further experiments studied the effect of gentiopicroside on the loss of mitochondrial membrane potential in OVACR-3 cells using flow cytometry using Rh-123 as a fluorescent probe. Figure 5 A-D show the effect of the compound on Δψm indicating that gentiopicroside exerts potent depolarizing effects on the mitochondrial transmembrane potential. The percentage of OVACR-3 cells with depolarized mitochondria increased from 5.2% in untreated cells to 16.7%, 44.5% and 67.3% in cells treated with 20, 40 and 100 μM dose of gentiopicroside respectively (Figure 6). | ||||||||||||||
Effect of gentiopicroside on the apoptosis-related protein expressions including NF-kB, Bcl-2, caspase-3, caspase-9 and PARP |
||||||||||||||
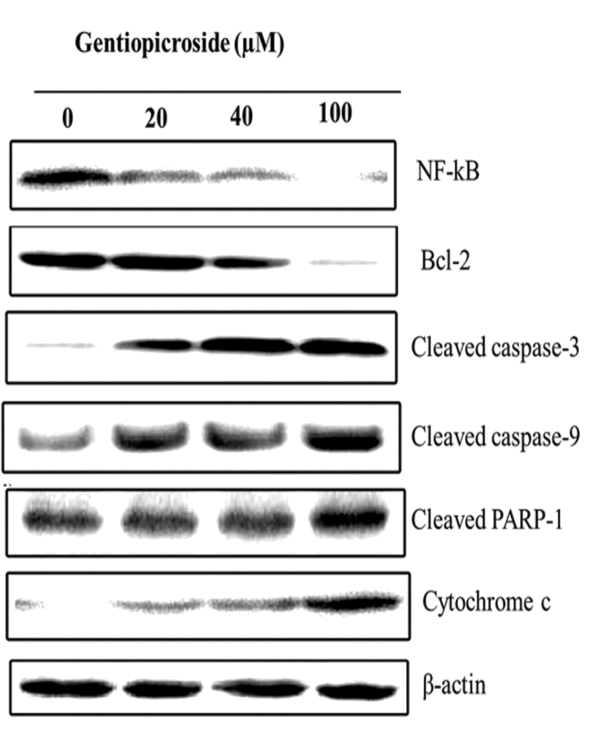
| Western blot analysis was employed to investigate the effect of gentiopicroside on the expression levels of various apoptosisrelated proteins and β-actin was used as a loading control. Gentiopicroside treatment at various doses (0, 20, 40 and 100 μM) for 48 h led to up-regulation of cleaved PARP-1, cytochrome c, caspase-3 and caspase-9 while as it was found that the gentiopicroside treatment resulted in down-regulation of NF-kB and Bcl-2 in a dose dependent manner. All these proteins play key roles in the induction of apoptosis in cells (Figure 7). | ||||||||||||||
Discussion |
||||||||||||||
| Apoptosis, which involves programmed cell death, is a highly organized biochemical process essential for the development of most of the organisms. Two main signalling pathways have been identified for the process of apoptosis, one is the extrinsic pathway and the other is the intrinsic pathway. Any deregulation of the apoptotic process leads to serious disorders including cancer [7,8]. Apoptosis process is characterized by several morphological changes in the cells including membrane blebbing, cell shrinkage, nuclear and DNA fragmentation and formation of apoptotic bodies. Currently, there is an increasing demand for searching chemotherapeutic agents which can act as apoptotic stimuli for the induction of apoptosis in cancer cells [9,10]. | ||||||||||||||
| Mitochondria has also been reported to play crucial roles in many biochemical processes including the apoptosis process. Mitochondria is involved in many key biochemical processes including release of caspase activators, loss of mitochondrial membrane potential and involvement of pro- and antiapoptotic proteins [11]. NF-kB, which is a pro-survival transcription factor, plays crucial role in cell proliferation and cell survival and it makes cells less prone to the process of apoptosis. This transcription factor is involved in inhibiting the apoptosis process and as such its down-regulation leads to induction of apoptosis [12]. NF-κB activation has also been witnessed in many solid tumors. NF-κB activation results from primary inflammation or the consequence of formation of an inflammatory microenvironment during cancer development. | ||||||||||||||
| NF-κB activation in cancer may be the result of either exposure to proinflammatory stimuli in the tumor microenvironment or mutational activation of upstream components in IKK–NF-κB signaling pathways [13]. Alterations in mitochondrial membrane potential has been shown to trigger induction of apoptosis and has even been suggested to be essential to the apoptotic pathway. Opening of the mitochondrial permeability transition pore has been shown to induce depolarization of the transmembrane potential, release of apoptogenic factors and loss of oxidative phosphorylation [14]. | ||||||||||||||
| Gentiopicroside, also known as gentiopicrin, is a plant secoiridoid constituent of many plant species of Gentiana genus including Gentiana macrophylla, Gentiana gelida, Gentiana lutea etc. It has been reported to exhibit smooth muscle relaxing activity in guinea pig ileum [15]. Gentiopicroside has also been reported to suppress the chemically and immunologically induced liver injuries in mice [16]. Gentiopicroside has been reported to exhibit antitumor activity in mice bearing the experimental tumor leukemia P 388. It has also shown activity in human hepatoma Hep3B cells [17,18]. In this study, we evaluated the effect of gentiopicroside on the proliferation and apoptosis in OVACR-3 ovary cancer cells. | ||||||||||||||
| The results indicated that gentiopicroside is a potent cytotoxic agent inhibiting the proliferation of OVCAR-3 cells in a dose and time-dependent manners. Fluorescence and phase contrast microscopic results indicated that gentiopicroside induces characteristic morphological features of apoptosis marked by reddish-orange fluorescence in treated cells. Gentiopicroside also led to the loss of mitochondrial membrane potential in OVCAR-3 cells. The percentage of cells with depolarized mitochondria increased from 5.2% in untreated cells to 16.7%, 44.5% and 67.3% in cells treated with 20, 40 and 100 μM dose of gentiopicroside respectively. | ||||||||||||||
| The current study also examined the effect of gentiopicroside on the expression levels of various apoptosis-related proteins. It was observed that 0, 20, 40 and 100 μM dose of gentiopicroside treatment for 48 h led to up-regulation of cleaved PARP-1, cytochrome c, caspase-3 and caspase-9 while as this treatment resulted in down-regulation of NF-kB and Bcl-2 in a dose dependent manner. | ||||||||||||||
Conclusion |
||||||||||||||
| The current findings reveal that gentiopicroside induces antiproliferative and apoptotic effects in OVCAR-3 ovary cancer cells through the induction of mitochondrial membrane potential loss and by altering the expression levels of various apoptosis-related proteins including NF-kB, Bcl-2, cleaved PARP-1, cytochrome c, caspase-3 and caspase-9. | ||||||||||||||
Figures at a glance |
||||||||||||||
|
||||||||||||||
References |
||||||||||||||
|