ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 6
Gd3+-DTPA-bis (N-methylamine)-Dendrimer significantly alters Bax/Bcl2 gene expression levels in MCF-7 cancer cells
Bahar Javani1, Sara Fattah1, Mostafa Saffari1 and Mehdi Shafiee Ardestani2*
1Islamic Azad University Pharmaceutical Sciences Branch (IAUPS), No 99, Yakhchal, Gholhak, Shariati, Tehran, Iran
2Department of Radiopharmacy, Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran
- *Corresponding Author:
- Mehdi Shafiee Ardestani
Department of Radiopharmacy
Faculty of Pharmacy
Tehran University of Medical Sciences, Iran
Accepted on October 24, 2016
Nanoparticles and their engineering are widely used in diagnosis, drug delivery and therapeutic agents, and have provided a new perspective to clinical diagnostic methods, advanced imaging and treatment of cancer. In recent years, investigating pharmacogenetics and corona protein effects of nanoparticles in biological applications has been seriously considered. Since apoptosis is a major mechanism of tumor suppression in the body so investigating the effects of drugs such as nanoparticles through inducing apoptosis pathway can be a proper therapeutic strategy for a variety of cancers. In this study, the mechanism of apoptosis induced by Gd3+-DTPA-bis (N-methylamine)-Dendrimer was investigated compared to Omniscan as a contrast agent on epithelial cell line of MCF-7 through investigating the expression of Bcl-2, Bax, caspase 3 and 9, and GAPDH in mitochondrial pathway. In order to demonstrate the effect of drugs on the genome, a quantitative method was used to estimate PCR products called Real-time PCR. Also the interaction of plasma proteins was investigated by Gd3+-DTPAbis (N-methylamine)-Dendrimer compared with Omniscan using SDS-PAGE technique to determine the fate of nanoparticles. Conducted study showed acceptable therapeutic effect of Dendrimer Gd3+-DTPAbis (N-methylamine)-compared with Omniscan in vitro of model of breast cancer compared to normal cells. Therefore, the use of the nanoparticles can be effective on increasing expression of some suppressing metastasis genes and pro-apoptotic. No significant result of the effect on caspases was observed. But a significant effect was observed on Bax and Bcl-2. (p<0/05) Also in this study, corona protein was investigated and the protein with weight less than 70 kDa was observed around A nanoparticle that seems albumin protein with a molecular weight of 67 kDa.
Keywords
Gd3+-DTPA-bis (N-methylamine), Dendrimer, Real-time PCR, Bax and Bcl-2, MCF-7 cell line, Apoptosis, Corona protein.
Introduction
Cancer is a group of diseases, which are characterized by their unregulated cell growth, invasion and spread of cells from the primary site or the original position to other parts of the body. Several points are emphasized in this definition, Firstly, cancer is considered as a group of diseases, and more than 100 types of cancer have been recognized so far. Each tissue gives the origin, and specific and distinct characteristics to the cancers arisen from that region. Merely 85 percent of cancers occur in epithelial cells and are known as Carcinomas. Malignancies with mesodermal origin (e.g. bone and muscle) are called sarcoma and malignancies of glandular tissue (such as breast) are called adenocarcinomas. Most cancers have 6 obvious symptoms. Apoptosis is a Greek word that means falling off, dropping of leaves from a tree or petals from a flower, which was first used by Kerr, Wyllie and Currie in 1972 to describe the distinct forms of cell death. Apoptosis normally occurs as a mechanism to preserve the stability of cell populations in tissues as well as a defense mechanism in immune responses or when the cells are destroyed by pathogens or disease [1-5]. Radiations or drugs used in cancer chemotherapy used in cancer chemotherapy can lead to DNA damage in somatic cells, which in turn leads to the initiation of apoptosis pathway through the activation of tumor suppressor protein p53. Apoptosis plays an important role in cellular growth, differentiation, homeostasis, and the regulation of immune function and removal of damaged cells. So, dysfunction and dysregulation of apoptosis results in pathological damages. Defects in apoptosis can give rise to cancer, autoimmune diseases and spread of viral infections, whereas increased apoptosis may lead to neuronal degenerative diseases, AIDS and anemia. Due to the importance of apoptosis in these biological processes, programmed cell death is an important phenomenon in all types of organisms from Metazoa, including mammals, insects and nematodes. Cells that are undergoing apoptotic death show several morphological changes, including: cell shrinkage, chromatin condensation, budding plasma membrane and ultimately nuclear fragmentation and formation of apoptotic bodies that are ingested by phagocytic cells of the immune system [6].
One of the most important changes that occurs during apoptosis, is in the biochemistry of plasma membrane so that phospholipid phosphatidylinositol serine (PS), a phospholipid with a negative net charge and normally found on the inner leaflet surface of the plasma membrane, is transferred to the cell surface i.e. the outer leaflet of cell membrane the, in apoptotic cells. Translocation of PS to the outer leaflet of the membrane is a surface marker to identify apoptotic cells. This marker is also as a signal for macrophages to phagocytosis of apoptotic cells. In addition to signaling phagocytic cells, the presence of PS on the cell surface also acts as an agent to prevent inflammation. Since PS-mediated surrounding of apoptotic cells prevents production of inflammatory proteins by phagocytic cells.
In general pathways involved in apoptosis are divided into two categories:
1. Extrinsic pathway (Or the death receptor pathway) which initiates by ligand binding to TNF receptor family leading to the activation of caspase-8 and subsequently caspase-3. "Caspases" are proteases that are synthesized as inactive Zymogens and are proteolytically cleaved during apoptosis to produce the active enzyme. After the proteolytic cleavage, two subunits, one large and other being small, are produced that together form heterodimers which are the active forms of the enzyme [7].
2. The intrinsic (or mitochondrial pathway): various intracellular changes such as DNA damage, a mutation in a gene involved in apoptosis and oxidative stress can act as internal signals. In the presence of these signals, through the particular pathways and by the interaction of specific proteins which are members of Bcl-2 family, cytochrome C is released from the space between the two membranes of mitochondria and enters the cytosol and activates the caspase cascade by the formation of a complex called “apoptosome”, directing the cell to the programmed cell death.
In the intrinsic pathway of apoptosis Bak and Bax proteins are oligomerized in the outer membrane of mitochondria and produce some temporary permeable pores in the outer membrane of mitochondria. This opens a route to release proteins located in the space between the mitochondrial membranes into the cytosol. Cytochrome C can be noted as the prototype of these proteins. Bax and Bak are also present at the surface of the endoplasmic reticulum and the nucleus which their activation leads to the release of Ca2+ in the cytosol. This helps increase the concentration of Ca2+ in the process of apoptosis. Anti-apoptotic Bcl2 and Bax proteins prevent polymerization of Bax and Bak proapoptotic proteins by sliding into the surface of the endoplasmic reticulum, mitochondria and nucleus [8-22].
The “BH3 only” proteins are a large subfamily of proteins from the Bcl-2 family (Figures 1 and 2). These proteins are the stimulators of intrinsic pathway, although each protein is activated by a certain stimuli. For example, Bim protein is activated through the withdrawal of survival signals of JNK. P53 is activated by DNA damage and upregulates the expression of Noxa and Puma proteins. In the extrinsic pathway of apotosis, caspase-8 activates Bid protein and activated Bid increase the apoptotic ability of extrinsic pathway by stimulating the intrinsic pathway as well [9,22-43]. One of the new approaches in early diagnosis of cancer is molecular imaging that could be used to diagnose different types of cancer.In modern clinical treatment of cancer, questions such as, where is the cancer located? What size is it? Whether it has spread in the body or not? And many of these questions could be answered through new methods of imaging. With the advent of proteomic and genomic techniques, hope for the clinical treatment of cancer in the future has been raised. In this regard the imaging techniques, play a central role because they are able to integrate molecular and physiological data [10].
It is hoped that the technique could one day reach the following objectives:
1. Detection of molecular and physiological changes could function as a signal to detect cancer in its early and curable stages.
2. The ability to evaluate and adjust treatment protocols at the right time.
3. The ability to simplify the process of developing drugs that are useful in the treatment of cancer.
In fact, molecular imaging is a radiological technique and allows us to recognize the progress of cancer in its early stages of establishment as well as determining the location of tumor. This makes the treatment of cancer take place at an earlier time and be more effective [11]. MRI is one of the most widely used techniques of molecular imaging for diagnostic purposes. MRI or magnetic resonance imaging is a non-invasive medical imaging technique that allows us for differential imaging and diagnosis of the structures inside the body. This technique is one of the most powerful diagnostic methods that gives crosssectional images, showing each part separately [19]. The ability to image molecular changes during disease which leads to early diagnosis and treatment, made MRI a popular and widely accepted technique.
MRI provides good contrast of soft tissue and work on this device based on initial exposure to radio waves into the body. The device works based on initial exposure of radio waves into the body then detects the reflections from the water spins after discontinuation of the radiation. The image is formed in the device based on the differences in absorption rates resulting from the relaxation and the presence or absence of water and fat in tissue tract an. What distinguishes this technique with PET and CT techniques it that MRI do not produce radioactive ions, however its sensitivity is lower than PET. The contrast in MR images depends on many factors that it is necessary to understand the physical mechanism to explain its action. Protons in the body has a dipole moment (μ) arising from their spin movement (permanent rotation on its axis) which is an inherent feature for them. If a number of these protons spin together and a uniform direction a magnetic field will be created. However, since the axis of protons is normally determined by random in the body, the resultant mean quantity of the magnetic field in the absence of an external magnetic field is zero.
Usually, the improvement in image quality is measured with resolution scale in MRI. The higher the resolution is, the greater will be the image quality. The main disadvantage of MRI device is the lack of sufficient resolution due to the lack of contrast in desired tissue compared to tissues around it. To fix the problem, chemicals called Contrast media are administered orally or injected before performing MRI imaging. The contrast media, as the name implies, increases the contrast in the image, resulting in a greater clarity of the MRI images [20]. Today, lots of contrast media are used for medical purposes and efforts are being made to change their characteristics so that they would give the lowest risk with the highest efficacy. Although the current contrast media are not ideal, the following criteria should be taken into account for developing contrast media:
• To be non-toxic.
• To be biodegradable.
• To be effective at low concentrations.
• To be targeted. (Could be used to increase the contrast of the image in a particular target tissue).
Materials are divided into four categories based on their magnetic behavior:
1. Diamagnetic materials: materials that their electron do not have non-paired orbitals. When these materials are exposed to external fields, induce a weak magnetic field, against the external field and thus reduce the effective magnetic field. These materials are totally considered non-magnetic. The majority of body tissues exhibit such characteristics.
2. Paramagnetic materials: These materials have unpaired electrons in orbitals, and when placed in an external magnetic field, induce a field in the same direction to increase the effective magnetic field. These materials shorten T1. Rare earth elements have the largest number of unpaired electrons in the periodic table of elements. Such as gadolinium, which has seven unpaired electrons.
3. Superparamagnetic materials: are materials that their magnetic susceptibility is 100 to 1,000 times stronger than that of paramagnetic materials, like hemosiderin with 1,000 unpaired electrons.
4. Ferromagnetic materials: are materials that are strongly absorbed by the external magnetic field and could be permanently magnetized and remain magnetized even after the external field is removed. Three types of ferromagnetic materials have been known so far: iron (Fe), cobalt (Co) nickel (Ni).
Various metals are used in the manufacture of Contrast agents which usually have paramagnetic and superparamagnetic properties. These ions when subjected to strong external magnetic field show strong paramagnetic properties and this changes the signal intensity in the tissues of the body [21]. Now, the question that arises is that how the presence of contrast agents containing metal ions such as gadolinium (Gd3+), manganese (Mn2+) and iron (Fe3+, Fe2+) could change the received signal intensity. Contrast media alter the T1 and T2 relaxation times through changing protons relaxation time or through affecting tissue proton density. Ability of a contrast agent to change the relaxation time depends both on the agent concentration in the tissue and the inherent tissue relaxation time. The presence of a contrast agent causes the protons exert the extra energy they have received from RF pulsed and it affects both spin–network interactions and spin-spin interactions and also reduces T1 and T2 relaxation times.
Contrast agents can be classified in different ways. Some of these classifications are based on:
1. The presence and nature of the central metal in contrast media. (Such as gadolinium, or iron)
2. The extent at which contrast media affect the image. (Weak, medium and strong)
3. The chemical structure of the ligands in contrast agent. (Such as DTPA, DOTA)
4. Contrast media with the specific purpose (such as biointelligent contrast agents)
5. Organ-specific contrast agents (e.g. for bile system or liver)
6. The distribution of the contrast media in body (6 modes of distribution have been described)
7. The type of the drug (oral or IV)
The most used type of contrast media are administered by injections and enters the extracellular space, 90% of which are excreted from the body via glomerular filtration within 24 hours. This material does not pass the blood-brain barrier due to their high hydrophilic nature. Normal injection dosage of this category of contrast media is 0.1 – 0.3 mM per kg of body weight [22,27,44-55].
Gadolinium (Gd3+)
Gadolinium is one of the rare-earth elements and since these category of metals has metal-like properties similar to lanthanum they are called lanthanide. This metal is solid, with silvery white color and can be seen in nature only in the form of salt. It is more known as a heavy metal and paramagnetic. Cadmium metal ions with free electrons tend to accumulate in tissues that have a natural affinity to metals. Its Metal ions with free electrons tend to accumulate in tissues that have a natural affinity to metals. Gadolinium atoms lead to the emission of higher signals through a rapid decline in relaxation time of the atoms in the nuclei of surrounding tissues, which produces the desired contrast in the image.
Gadolinium atomic number is 64, with its standard atomic weight being 157 g/mol and its density 90.7 gr/cm3. Owning to its paramagnetic properties and 7 unpaired electron, this metal is widely used in MRI imaging. Since the metal is highly toxic, it is administered to the body in the form of complex. Compounds that bind with gadolinium to produce non-toxic contrast media complex are called chelates. Contrast agents that function more specialized and more effectively are being gradually employed. There are several strategies to create effective contrast agents that some of them will be mentioned in the following:
Due to the fact that some molecules such as sialic acid are present on the surface of cancer cells more than other cells, which is due to its overexpression in cancer cells, they are known as tumor markers. It is possible to produce contrast agents that are designed to bind to cancer cell markers. In this case, to recognize cancer cells ,the contrast agents should selectively bind to sialic acids instead of binding to blood sugars like fructose and glucose. Some compounds that are nominated for such receptor purposes, are made of urea basic units and are able to strongly interact with the carboxylate anions (present in the sialic acid) and thus migrate toward cancer cells to establish a greater contrast from the tumor site. So far, several Gadolinium-containing contrast agents have been introduced for detection of many tumor types. In a researches conducted by Min-Ying Su and colleagues in 1998, three Gd containing complexes were utilized to study the tumor in three different mouse models of cancer. Positive results have been reported from this study [27,53-68].
These materials are physic-chemical factors to which paramagnetic system is sensitive. Contrast material could be devised from these category that are sensitive to changes in pH, temperature, oxygen pressure, enzymatic activity, redox potential and the concentration of a particular metal ion. For example, contrast agents sensitive to pH changes can be used in cancer detection studies. Because the cancer cell surface pH is lower than normal tissue by 0.4 units. It is possible to devise contrast agent series with different bodily distribution and pH sensitive by adding carboxylate or phosphate to the arms in contrast agents. Temperature-sensitive contrast agents are those encapsulation of gadolinium chelates into the liposome. In this case, it is said that transition of membrane from a gel to a liquid happens at a special temperature. Thus, membrane permeability changes thus alter relaxation time through stimulating the water molecules. Many studies have also been carried out about intelligent metal contrast agents [28].
The most important factor in making intelligent metal contrast agents is the "chemical selection" of anions in the presence of multiple target anions, small organic molecules and macromolecules found in the body. A wide range of metal ions like sodium, potassium and calcium, are often present at high concentrations in biological systems so that contrast agents should only be specific for one of these ions and do not emit the signal of others. This specificity is achieved by selecting the appropriate architecture of metal-sensitive element in contrast media. Characteristics such as the type of donor (electrically charged or neutral), the number of donors, the size of the cavity and its geometry should be appropriate for binding to the intended metal ion. The binding strength should be compatible with biological systems in designing sensors.
Dendrimers are a class of macromolecules that are constructed for targeted and programmed delivery of the drugs. Dendrimers are highly branched spherical units that Polymeric branches are of different size and have a symmetrical spherical shape. There is a lot of spaces inside their structure that provides a suitable space for embedding drugs in it. Each branch of the dendrimer is called a Dendron. Ideally a dendrimer has three parts: the core, the inner shell and the outer shell. Synthetic dendrimers have various capabilities in each of these parts for controlling the properties such as solubility, thermal stability and number of branches to design dendrimers with a particular application. The core could be made of protein or polymer (linear, branched or spherical).
Dendrimers are divided according to their generation which refers to the number of duplicate branches. For example, if the dendrimer is produced by convergent synthesis its branches are twice connected to the core of a dendrimer the second generation will be formed. Dendrimers with lower generation, have asymmetric shapes and more open structures compared to higher generation dendrimers. Dendrimers have a wide range of applications including: Targeting, Radio ligand, drug delivery, gene transfer and manufacture of sensors. Studies also have shown that the use of nanoparticles in the manufacturing of contrast media improves their influence.
The objectives of this research which is going to happen for the first time, are as the following:
• To Study the Effects of DTPA-bis (N-methylamine) - Dendrimer Gd3+ in comparison with Omniscan, on bcl2, caspase 3, GAPDH genes in MCF-7 the cell line
• To study the effects of plasma protein aggregation or Binding on Gd3+ DTPA-bis (N-methylamine) -Dendrimer by Corona protein technique
• To study Effects of DTPA-bis (N-methylamine) -Dendrimer Gd3+ on caspase-3 gene in comparison with Omniscan.
• To study Effects of DTPA-bis (N-methylamine) – Dendrimer on GAPDH genes compared with Omniscan.
• The effects of DTPA-bis (N-methylamine) -Dendrimer on Bcl2 and Bax genes compared with Omniscan.
• To investigate the interactions of plasma proteins with Gd3+ DTPA-bis (N-methylamine)-Dendrimer compared with Omniscan, which determines the fate of the nanoparticle. This assessment was performed qualitatively using SDSPAGE techniques.
Materials and Methods
Synthesis of dendrimer
Synthesis method of Omniscan nanoparticles: At first, 2.5 -3 cc liquid polyethylene glycol weighing 600 was added in the container, and then 4 ml DMSO solvent was added to the reaction (DMSO makes a matrix). In reactions that a nucleophile binds by nucleophilic substitution, the best solvents are solvents that help to create a matrix for reactions. The best solvents are DMF and DMSO that functionally are more appropriate. One g of DCC (dicyclohexane Hexyl carbo di imide) was added to the solution under stir. In the first place, Citric acid dendrimers were synthesized with polyethylene glycol core. Then the end of these dendrimer were functionalized with imidazole functional groups in the presence of DCC and thus the carboxyl groups were enabled and made ready to bind to the carboxyl groups of citric acid. 5 minutes later one gram of citric acid was added. (The reaction was performed at room temperature). Before all that, at the time when polyethylene glycol and DMSO were mixing, two pieces of calcium chloride was added. Calcium chloride absorbs excess water and increases efficiency. After adding one gram acid citric, the reaction stirred for hours and then 2 gr DCC was added for 2 hours, then two grams of citric was added. The reaction stirred for a week.
Magnetic stirrer device
Magnetic Stirrer is a Laboratory device that uses a rotating magnetic field to rotate the little tube without having physical contact with it. Rotation of the magnet causes the content of beaker to be stirred. As indicated in Figure 3, the device has a metal plate which influence the magnet leading to rotation, and warms up the fluid as well and facilitate the reaction. The device is used in biology and chemistry, and in experiments with a volumes less than 4 L and is usually preferred to motor rib stirrers. Because they produce less noise and do not need to replace external parts, because they do not have any external part [47].
Purification
Purification is the separation of synthesized dendrimer from other impurities including extra citric acid and unreacted DCC. DCC is converted to DCU (D cyclohexane Hexyl urea) during the reaction. Since it is not possible to synthesize absolutely pure dendrimer in vitro, a Dendrimer with the purity of more than 90% was produced. Although DCC and DCU are insoluble especially in aqueous system, their dendrimers are soluble. 5 ml water is added, the dendrimer dissolved and DCU and the DCU precipitate. Then the solution passed through a simple filter paper and funnel and comes out. Then for further purification and expelling excess citric acid Sephadex advanced column was used which is called "gel filtration". Tiny molecules attach the column and large molecular pass and are eluted. Thus, impurities are stuck in and the dendrimer is eluted. The best strategy is to pass a solution through the column and no solvent is added to perform a natural washing so that the impurities stuck in the column and the dendrimer is purified.
Adding the dendrimers to Omniscan
Half the volume of the vial was drawn by a syringe and the same volume of dendrimer was added. Sonication was performed for 10 minutes. Then the solution was put in the fridge.
At this point Gadolinium concentration is very important and the amount of dendrimer does not matter.
• Concentration: Base
• Net Omniscan concentration: 287 (mg / 5cc)
• Thus the basal concentration of dendrimer-drug was 5143 (mg / 5cc)
• Sonicator device
Eventually, to increase the contact surface, extreme mechanical shaking was applied and Kst7703 sonicator model was used. The device uses ultrasound waves with frequencies higher than 20,000 Hz to create shakes in materials, thus increases the contact surface of particles. This increase in surface area is applied in chemical reactions to facilitate the reaction and in nano-technology to uniformly disperse nanoparticles in liquid [48].
Cell culture preparation
The foundation of all culture media are the same and all contain essential amino acid, vitamins, minerals and color indicator And their differences relies in their type or amount of available amino acids. Given the type of cells, each cell has its own culture medium. RPMI 1640 Medium, was first introduced in 1966 by Moore et al. at Roswell Park Memorial Institute. At first, the media was designed for maintaining Lymphoblastoma cells in liquid medium, but today is used as the basic culture medium to feed a large group of adherent cells. RPMI1640 is an economic environment for most cell types 60 to 70 percent of available cell lines are compatible with this medium.
For preparation of this medium, initially, 1000 ml of double distilled water was added in a glass container with lid and autoclaved. then 10.4 gr of RPMI1640 was dissolved in 1000 ml of double distilled autoclaved water, then a Magnet which was disinfected with 70% alcohol put into it and stirred for four hours on a magnetic stirrer to completely dissolve and be no visible particles in it. About 15 to 30 minutes before filtering medium, other two compounds added to the culture medium.
1. Two grams of sodium bicarbonate in 1000 ml. All culture media require bicarbonate because according to culture conditions at 37°C and 5% CO2, presence of a water container within the incubator is necessary to prevent evaporation of culture medium water. Therefore, in these conditions, CO2 which is a very soluble gas, dissolves in the medium and makes it acidic which is stressful to cells. Therefore, to control the pH alteration, bicarbonate buffer of HCO3 and H tampons or 7.5% solution of sodium bicarbonate is used.
2. 99% HEPES: 2.5 gr HEPES buffer is added to 1000 ml. Hepes is a buffer that prevents acidification or alkalization of culture and provides the optimum which for cell growth which is 7.5. When HEPES is used, there is no need to use pH meters. Since pH meters are prone to produce contamination it is better not to be used.
Filters can be used to sterilize the medium. The basis of filtration is pressure and vacuum. Here, we used syringe type filters with the pore diameter of 0.22 and total diameter of 30 millimeters. This filter has fine pores and does not allow the passage of Mycoplasma and fungi. These two organisms are the main causes of contaminants of culture media. To Filtration, the prepared culture medium was drawn by a 20 ml syringe, and the syringe containing the medium was installed on the filter, then the sterile filtered medium was discharged into a sterile lidded glass container with a proper size. Vacuum pump creates suction that causes pressure on the filtered solution, followed by a quick exit of solution through the filter into a sterile container. The filtered medium is kept overnight at a temperature of 4°C (in the refrigerator). In this case, if bacteria are present, they will grow and make the medium turbid.
Cell culture and cell passage
Materials and equipment needed for cell culture and cell passage:
• Fetal bovine serum
• Trypsin
• PBS
• Pipette and pipette tips 100-1000
• T25 Flasks with filter caps
• Special capped centrifuge tubes (Falcon), 15 and 50 ml
Cell culture method
Studied cells were cultured in RPMI 1640 medium containing 10 to 20% fetal calf serum and incubated at 37°C, with 5% CO2 gas. Since antibiotic could affect gene expression of the cells, it is to carefully implement aseptic techniques rather than using antibiotics for reducing contamination risk of infection. Bacterial contamination detaches the cells from the bottom and generates turbidity in culture medium, while fungal contamination produces hyphae, which is visible as colony-like fragments. Fungal contamination is very dangerous because samples containing the fungus produce spores which spreads and contaminates all media in the incubator altogether. So as soon as fungal contamination is seen, the flask should be removed from the incubator.
The cells produce acidic metabolites which leads to the gradual color change of culture medium from purple to yellow, so usually every 48-24 h the cell culture medium is replaced. To evacuate the previous medium Pasteur pipettes and pipette can be used. Because the cells usually release some signals into the culture medium, the complete evacuation of medium places great stress and pressure onto cells and gene expression pattern might be changed, thus, to exchange the medium 10 to 20 percent of previous media is kept in the flask. Then fresh medium with the same temperature as the previous, is slowly added to the flask to the direction that does not hurt adhered cells on the bottom side and thus media replacement is carried out in this way.
Cell passage method
The basis of the method: during the passages, cells are drawn from one position to another position with the aim of increasing the number of cells. In an in vitro condition, and when the optimum culture conditions (temperature 37°C, 5% CO2, in the presence of a suitable culture medium and absence of contamination) is available, cells will proliferate in the flask. Then the cells should be transferred to other culture dishes when the entire surface of the bedding is covered by the cells or cell density exceeds over the bed capacity. This process is called cell passage. Depending on the type of the cell, being suspended or adherent, the passage method is different. Suspended cells are poured from the flask and into Falcon tubes, centrifuged, and then the supernatant is discarded and cell pellets are transferred into new culture dishes with media. But in the case of adherent cells, to separate the cells from the bottom, the flask is treated with trypsin. It should be noted that when using trypsin, temperature should be 37°C. For this reason, after removal from the refrigerator, it is placed in a bain-marie.
For cell passaging, the medium is completely discharged from the flask with the help of Pasteur pipette or pipettors, then add 2-3 ml 0.25% trypsin is added and kept for 3-5 minutes in this state until the cells are completely detached from the bottom of the flask. Trypsin breaks cellular junctions and separates the cells from the bottom of the flask and make them floating. Care should be taken in the use of trypsin, since if trypsin is removed out earlier than the right time, the number of cells remained attached to the bottom of the flask and this reduces efficiency. If trypsin is left more than right time, it could deform cell surface proteins as a result cells can not adhere to the flask.
After a suitable period of time and to neutralize trypsin, serum containing medium was added to the flask and slowly was pipetted. The resulting suspension was transferred to Falcon tubes and centrifuged at 1,000 rpm, for 7 minutes. After centrifugation, the supernatant was discarded and the cell pellet was transferred into new flasks with fresh medium. It should be noted that the adherent cells were suspended after detachment from the bottom of the flask and are easily transferable. Depending on cell count, now at least two and maximum four new cell culture flasks could be obtained.
Determining cell viability
Trypan blue preparation Method: For preparing 4.0% Trypan blue, 400 mg of Trypan blue powder is weighed and the volume brought to 100 ml with distilled water, then it is filtered with filter paper, percolates and to prevent mold growth, a few drops of sodium azide was added. Then the dye color is ready for use. For performing the experiment, 100 ml of cellular suspension was transferred to a test tube in sterile condition, then the same volume of Trypan blue was added. And after a few minutes and pipetting, a drop of the mixture is removed and the number of cells was counted using a Neubauer slide and cover slip, cells were counted only in squares specific for WBC counting. Trypan blue does not permeates the living cells due to the presence of membrane pumps. Thus, it only stains dead cells, so the cell viability is determined by counting dead cells and calculated using the following formula:
Cell viability % = No. of Viable Cells Counted / Total Cells Counted (viable and dead) × 100
To investigate the effect of drugs on cells, it is necessary that at least 90% of cells be viable viability before the experiment begins. Because the viability lower than 90%, reduces the accuracy of the test.
Safety precautions
The following safety tips should be followed at all stages of the cell culture:
1. Always get gloves and use safety goggles when working with the materials. When freezing and thawing, special attention should be paid and the aforementioned considerations must be taken into account.
2. thoroughly wash Hands after each time od working
3. Never pipette by mouth
4. Never smoke and never eat or drink at laboratory environment
5. Products with human origin have potential biological risk, even for registered samples that are negative for HIV and hepatitis C, B. Appropriate care and necessary precautions should be taken to prevent inadvertent contact.
MTT assay technique
Materials and equipment needed to perform MTT:
• MTT Powder
• Trypan blue dye
• Neubauer slide
• Flat-bottomed 96 well cell culture plates
Several methods exist for indirectly measuring the proliferation and survival of the cell. Most of these methods, measure the activity of a cellular enzyme in living cells. One of the most well-known of these methods is the MTT test. MTT test, is a quantitative laboratory test to measure cell proliferation on exposure with various factors and determine the toxicity of these agents on cells when they are due. In fact, this technique will help to determine the toxicity of a particular drug. This technique was first used in 1983 by the Monsoon. The method is based on the ability of mitochondrial reductase enzyme in living cells to reduce yellow tetrazolium rings of MTT to the purple insoluble crystals of Formazan that are able to cross the cell membrane (Figures 1-3). These crystals are then dissolved by detergent. The most used detergent is usually dimethyl sulfoxide (DMSO) and sometimes sodium dodecyl sulfate diluted in hydrochloric acid. Isopropanol is also an excellent solvent for Formazan but due to its high expenses, is less commonly used. During an MTT test, a reaction takes place in living cells, as shown in Figures 1-3.
Optical density of the resultant solution could be obtained by spectrophotometry at a wavelength of 570 nm. Regarding to the fact that the above-mentioned reduction reaction occurs only in living cells having active reductase enzyme, the numerical value obtained by this technique is proportional to the number of living cells. In this measurement, the amount of formazan produced by the cells treated with a special agent, is compared with the formazan produced by control cells that did not receive the treatment. Then through this comparison the effects of certain factors on cell death or growth inhibition is determine. For this purpose, lower absorption read-outs compared to the control corresponds to a lower number of living cells and more growth inhibition.
MTT test was performed for 24 hours as follows
First day: In MTT test cells are seeded in 96-well culture plates. Given that the wells of culture plate is too small and it is not possible to passage the seeded cells while testing, to prevent cell over growth, the proportion of medium FBS, which is normally 10%, is cut to %5 or even 2.5% . In the first step, the cells are detached from the flasks using trypsin (the process is similar to cell passaging), but in the last step, instead of using the medium supplemented with 10% FBS, one ml of cell culture medium containing %5 FBS was added to cell pellets and then pipetted. 10 microliters of this cell suspension is drawn and 10 microliters of trypan blue (the dye enters dead cells but not living cells) is added. Then one drop put on Neubauer slide and cells were counted. Cell counting is crucial on the first day, because it must be done in such a way that an equal number of cells are seeded in each well. Then, the cell suspension is diluted so that each well contains 10,000 cells in 100 ml medium supplemented with 5% FBS.
The second day: In the second day, different concentrations of Omniscan nanoparticles (1.25, 3.0, 6.25, 12.5, 25, 50, and 100 mM) were prepared. Then the medium was emptied and replaced with 100 ml of prepared concentrations.
Third day: MTT dye was added on the third day. In order to prepare 5 mg/ml MTT solution, 50 mg MTT powder was dissolved in 10 ml PBS and when using, it was diluted 10 times with PBS to obtain a 5 mg/ml MTT solutions. MTT powder can be stored up to 2 years in -20° C but not later than one month as the solution in the refrigerator. Then 100 ml of the prepared solution is added to each well. Culture plates are left in an incubator for three hours. Then removed out of the incubator, the medium is evacuated, then to dissolve the produced formazan, 100 ml of DMSO was added to each well and placed at room temperature for thirty minutes for formazan to be dissolved. In The next step absorption was read by Elisa Reader and was measured at 570 nm. To investigate the effects of gadodiamide nanoparticles for 24, 48 and 72 hours, the process was repeated in the fourth and fifth days.
Survival rate of breast cancer cells, and normal cells that were treated with nanoparticles, Omnisacn drug and nanoparticles loaded with Omniscan treatment was calculated using 1 and 2 formulas:
% Cytotoxicity=1-(mean absorbance of toxicant treated cells)/ (mean absorbance of negative control) ×100 → (1)
% Viability=100-% cytotoxicity → (2)
Determination of IC50 for gadodiamide nanoparticles in MCF-7cell lines
To determine lethal dose 50% of toxic substances on three classes of cancer and normal cells mentioned above, all obtained information (toxicity rate) of cases and controls was evaluated by using the Pharm-PCS statistical software package and the exact IC50 was determined.
RNA extraction
Materials and equipment needed:
• RNase-free Microtube
• RNX solution (Cinnagen)
• Chloroform
• Isopropanol (Sigma)
• Ethanol
• DEPC Water (Cinnagen)
Test method: Firstly, 2 × 106 cells were transferred to T-25 flasks and after a night, were treated with different concentrations of gadodiamide nanoparticles and then RNA was extracted after 24, 48 and 72 hours. One of the most important factors in the production of cDNA with good quality, is the extraction of pure integrated total RNA. RNase is one of the most stable proteins that are not denatured even with boiling, so when working with RNA it must not be supposed that something could be free of RNase (especially our hands). In all the process of working with DNA including RNA extraction and preparation of the solution required for the purification, disposable gloves must be used. In addition, utensils and equipment used must be free of this enzyme. For this purpose, containers must be autoclaved twice or be treated with DEPC water. The work surfaces should be washed using NaOH 50 mM. In the case that a sufficient amount of RNA is obtained, it is recommended to check the RNA by electrophoresis on agarose gel containing ethidium bromide. When a non-broken RNA is run on a gel, two distinct bands of 28 S and 18S ribosomal RNAs with the approximate 5.4 and 9.1 kb can be seen, respectively. The ratio of these two bands (28S/18S) should be about 1.5- 2.5 to 1, and if these two bands were observed, materials and equipment which were used for RNA extraction RNA purification must be controlled.
RNA extraction consists of five steps
1. Homogenization
2. Phase Separation
3. RNA precipitation
4. Washing RNA
5. Re-dissolving the RNA
Step 1: The first step differs depending on whether RNA is to be extracted from tissue, monolayer adherent cells or suspension cells. Since the cells are attached to the bottom of the flask as a monolayer, the culture medium or the drug are emptied. To detach the cells from the bottom of the flask, the cells are separated by trypsin, and centrifuged at 1100 rpm and then washed twice with PBS. Then for lysing the cells, one ml of cold RNA extraction solution was added and homogenized by Vortex. It should be noted that if RNX solution is not enough, extracted RNA will have DNA contamination. Then the content of each falcon was transferred to RNase-free microtubes.
Step 2: Homogeneous samples are incubated for five minutes at room temperature. For The nucleoprotein complexes to be completely break down. 200 ml of chloroform was added. Microtubes are vigorously shaken by hand for 15 seconds, and are placed for 5 minutes at room temperature. Samples were centrifuged for 15 minutes at rpm12000 at C4. After centrifugation three phases are formed:
1. The bottom phase colored red
2. The middle phase
3. The upper aqueous phase containing the RNA which is colorless.
Step 3: The aqueous phase is transferred to a microtube.
Because RNA precipitates, aqueous phase is mixed with isopropyl alcohol (equal volume of aqueous phase). Samples are placed on ice for 15 minutes. Samples centrifuged for 15 minutes at 4°C with 12000 rpm. Precipitated RNA is not visible after centrifugation, only a gel-like pellet is collected at the bottom of microtube.
Step 4: The supernatant is emptied and RNA sediment is washed once with 75% ethanol. At least 1 ml of 75% ethanol per 1 ml of RNX should be used. The sample was mixed with upside down movements and centrifuged at 7500 rpm for eight minutes at 4°C.
Step 5: RNA sediment was air-dried for five to 10 minutes. RNA should not be dried by centrifugation under vacuum. It should be noted that the RNA sediment must be completely dry because its insolubility is low. DNA precipitate was dissolved in DEPC Water. RNA was stored in a freezer at -70°C. Quality and quantity control of extracted RNA. All experimental procedures were performed under the laminar hood as much as possible.
Assessing the quantity and quality of extracted RNA: At this stage, the quantity and purity of extracted RNA using was determined photonanometer (IMPLEN GmbH, Germany). The ratio of RNA absorption at two different wavelengths was measured: 260 nm to 280 nm and 260 nm to 230 nm. Examples that their 280/260 nm ratio was between 2. 2 to 8.1 and the 260/230 ratio of 1.7 to 1.9, were used for cDNA the synthesis.
Synthesis of complementary DNA (cDNA): cDNA molecules were synthesized by Revert AidTM First Strand cDNA Synthesis kit (Fermentas, USA). The reaction mixture contained one microgram RNA, 5 uL of 5x reaction buffer, 0.5 uL primer Oligo (dT), 0.5 uL random hexamer primer, 2 uL dNTPs (10 mM), 1 uL RNase inhibitor (20 units /uL), 1 uL MMulV reverse transcriptase and DEPC water (to a final volume of 20 uL) . The temperature-time program was as the following: C25 for 5 minutes (primer annealing), C42 for 60 minutes (synthesis of cDNA), C70 for 5 minutes (inactivation of reverse transcriptase) and C4 for 5 minutes. PCR reaction was performed using iCycler Thermal Cycler (Bio-Rad, Hercules, CA, USA).
Primer design
Primers are oligonucleotides with a length of 25-17 bases that bind to specific parts of the template DNA and allow the enzyme to function and synthesize the DNA fragment located between two primers. The following points should be noted when designing primers:
• Primers should be of appropriate length (25-17 nucleotides).
• Primers should be rich in terms of GC content (60-45%).
• Sequences with duplicates or regions containing repetition of one nucleotide should be avoided, since they may lead to mispriming.
• Possibility of secondary structures due to the presence of internal complementarity sequences should be avoided.
Sequences at the 3' end of primers should not have binding tendency to each other or to any primer co-existing in PCR reaction. Otherwise the primers may form primer dimer. GCs with three or more repeats at the 3' end should be avoided because it may lead to incorrect binding at GC-rich regions. Tandem repeats of poly-Pyrimidine and poly-purine should be avoided. The primers should be designed so that they are able to function at temperatures appropriate for binding. For primer design, the cDNA sequences of genes were obtained from GenBank and then the best primer sequences were determined by Gene Runner and Primer express software v.3.0 (Applied Biosystems, USA). The best and confident method For primer design is to select the forward or reverse primer at exon junction regions or set the forward primer in one exon and reverse primer at the other exon, thus by such a method, DNA replication in contaminated samples is prevented. To ensure the accuracy of primer sequence and not binding to non-specific sequences in other parts of the genome, primers were BLAST (http://www.ncbi.nlm.nih.gov/blast) (Table 1).
| Gene of interest | Primer sequence |
|---|---|
| Bcl-2 | Forward: 5'-TGTGGATGACTGAGTACCTGAACC-3 |
| Revers: 5'-CAGCCAGGAGAAATCAAACAGAG-3' | |
| Bax | Forward: 5'-TTGCTTCAGGGTTTCATCCAG-3' |
| Revers: 5'-AGCTTCTTGGTGGACGCATC-3' | |
| GAPDH | Forward: 5'-CGTCTGCCCTATCAACTTTCG-3' |
| Revers: 5'-CGTTTCTCAGGCTCCCTCT-3' |
Table 1. Primers used in real time PCR reactions.
Real-time RT- PCR
Real-Time PCR method is based on fluorescence activity. The most widely used fluorescence dye is SYBER-Green, which emits a fluorescent light in case of reacting with small grooves of double-stranded DNA. As a result, by measuring the fluorescence generated at each stage of the reaction progress of the reaction can be measured. The disadvantage of this method is the non-specific binding of SYBER Green to primer-dimers in the reaction vessel which leads to false-positive results. In order to address this problem, Melting Curve analysis is applied. So that after completion of the reaction, the temperature is gradually increased from 50°C to 95°C according to a program which has already been given to the device. Since the whole DNA is double-stranded at low temperature, the fluorescent emission is maximum. But with the time and temperature increases, the amount of doublestranded DNA decreases due to denaturation thus the fluorescence will be reduced and eventually reaches zero. Regarding this, and considering the fact that each PCR product has its own specific sequence length, each product melts at a distinct and specific temperatures and this melting produces a specific peak. As a result, different and distinct peaks will be seen in melting curve, if any primer-dimer or any possible contamination exists. This confirms the validity of the reaction and the resulting products.
In this study, ABI 7300 model Real Time PCR machine (Applied Biosystems, Foster City, CA, USA) was used. The device thermal cycling was performed in three steps. The first step leads to denaturation of cDNA molecules and activates the polymerase enzyme was done at 95°C for 10 minutes, the second phase was done at 95°C for 15 seconds and 60°C for 1 minute for 40 consecutive cycles, and the final stage to draw melting curve at 95°C for 15 seconds, 60°C for 30 seconds, and 95°C for 15 seconds. Real-time PCR reactions were performed in a final volume of 20 ml in triplets. Each reaction contained 10 microliter SYBR-Green PCR Master Mix (Applied Biosystems, Warrington, UK), one microliter of 400 nM forward and reverse primers specific for each gene, 5 microliter of cDNA (300 ng) and the remaining was double- Distilled water to the final volume of 20 ml. To draw the standard curve, serial dilutions of standard cDNA for concentrations of 75, 150, 300, 600 and 1200 nanograms per microliter was used. A standard curve was drawn based on the logarithm of the concentration of cDNA (horizontal axis) and threshold cycle (Ct) for each gene plotted as the vertical axis. The optimum concentration range of cDNA template and PCR efficiency for each gene was calculated. For each gene, Serial dilutions were carried out for primers and standard DNA along with reactions with no template.
Efficiency of replication= [10 (-1 / SLOPE) -1]
Then, proliferation curves were plotted for each PCR reaction. Data analysis was performed by comparing the threshold cycle. In this study, the difference in threshold cycles obtained from samples (cells treated with medication) and controls (untreated cells) was estimated and, the ratio of target gene to the reference gene (GAPDH) was calculated using the ΔΔCt formula. The calculation formula is as follows:
ΔCt= Ct target-Ct reference
ΔΔCt=ΔCt test sample - ΔCt control sample
Relative expression: 2 –ΔΔCt
Statistical analysis of data: For data analysis calculation of T1 and T2, Dicomwork, Matlab and Excel software were used. MicroDicom software was also applied for observing MR images. To compare quantitative data, paired sample t-test (Post hock Tukey) was performed using SPSS software. And if the data were compared in groups, one way ANOVA was used.
Results
Cytotoxicity test
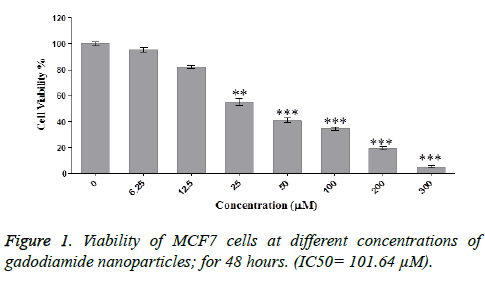
To evaluate the viability of MCF-7 cells exposed to different concentrations of Omniscan nanoparticles, the MTT assay was used. Results are presented as survival rate compared with control samples and are reported as mean ± SD (*: p<0.05, **: p<0.01, ***: p<0.001). In the control group, 100% of the cells were viable and gradually with dose increasing, cell viability. As shown in figure significant toxicity was observed at different concentrations of gadodiamide nanoparticles. For example, significant toxicity was seen at the concentration of 25 (p<0/01) and about 57% of the cells were viable. And at concentrations above 50 still significant toxicity (p<0/001) was observed.
Results are reported as the viability in comparison with control samples and as mean ± SD (*: p<0.05, **: p<0.01, ***: p<0.001), but gadodiamide alone did not cause significant toxicity. As the IC50 implies. Appropriate Concentration for our work 64.101. The impact of Omniscan nanoparticles on apoptosis gene expression. Nanodendrimers exert toxicity on to cancer cells through the mechanism of apoptosis. In Figure 3, effect of nanodendrimer on different apoptosis genes is compared and evaluated. IC50 concentration of 40% was used. (Concentration: 656.40).
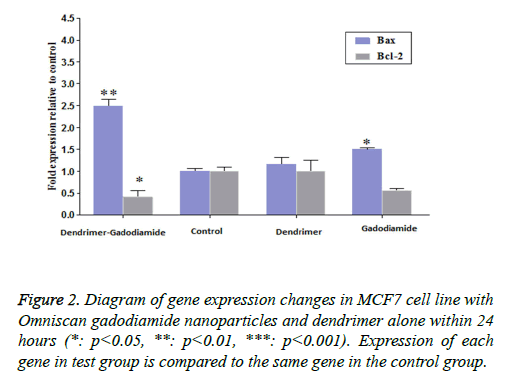
The impact of Omniscan nanoparticles compared with pure Omniscan and dendrimers alone, on pro-apoptotic genes Bax, Bcl2, caspase-9 and 6, and also GAPDH was assessed. No significant effect was observed on caspases, but significant effect was seen on Bax and Bcl2.
• Effect of dendrimer on Bax and Bcl 2 genes: noise is produced but was not significant, only the expression has been slightly increased especially in the Bcl2 gene. (All of these, was tested at constant concentrations and occurrence of significant changes in different concentrations is possible).
• Effect of Omniscan Bax and Bcl2 genes: raised Bax gene expression, but not only did not increase Bcl-2 but also decreased it.
The impact of omniscan nanoparticles (omniscan and dendrimer) on Bax and Bcl2 genes: Its effect on Bax is the sum of dendrimer and omniscan altogether and it quite significantly affects Bax and increases apoptosis. The effect on Bcl2 is much higher compared with the effect of pure omniscan and is almost equivalent to the impact of dendrimer alone. In fact, compared to the control group, a significant increase was observed in Bax but not any considerable effect was found on BCL2.
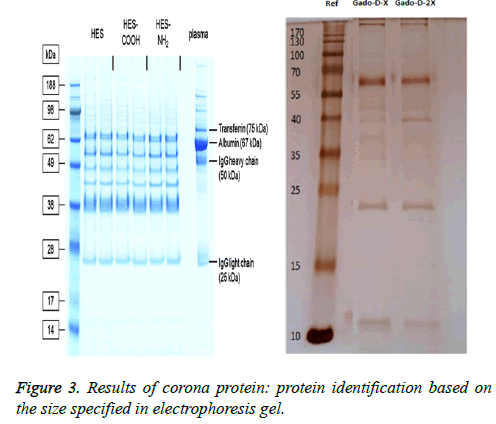
The results of electrophoresis and corona protein determination: In electrophoresis of Plasma serum, one protein weighing less than 70 kDa, and another protein weighting 22 kDa were found which were attached to the nanoparticle. As shown in Figure 3, it seems that the protein attached to omniscan nanoparticle, weighting less than 70 kDa, is albumin protein with a molecular weight of 67 kDa is.
Characterization of corona protein in nano-particles: After intravenous injection of nanoparticles, the blood is the first biological environment in which nanoparticles enter. Blood plasma contains many proteins and fat. Thus, after nanoparticles enter the biological environments, a competition arises among molecules bind to the particle, as a result, a crown of the proteins surround the nano-particles. Proteins that are directly absorbed to the nano-particles with high affinity are called hard crown and are not easily separated from the nano particles. Proteins binding with low affinity to nanoparticles form the soft crown that includes proteins with weak binding [49,50].
• SDS-PAGE gel electrophoresis: SDS-PAGE is a low-cost, rapid, reproducible method for protein studies which is typically used for the evaluation of purification steps, determine the relative amount and calculate and molecular weight of proteins and peptides.
• The role of sodium dodecyl sulphate: Sodium dodecyl sulfate is an Anionic detergent that normally binds to hydrophobic regions of proteins.
• The effect of polyacrylamide gel: Separation of proteins in SDS-PAGE depends on the specific characteristics of polyacrylamide gels for screening proteins with different molecular size.
Discussion
Given that many genetic changes are needed to form a cancer, and regarding the fact that most cancers have hereditary aspect so that the tumor relapses after the initial treatment and surgery, therefore, the utilization of substances that can inhibit the metastatic process is highly concerned. This means that although we cannot prevent tumor formation, we can stop the process of metastasis which is the main cause of mortality in cancer patients. In addition to metastasis, the phenomenon of apoptosis or programmed cell death, is the main form of cell suicide. In this process, the damaged, unwanted and dangerous cells are eliminated without any damage to the surrounding cells or tissue. Cancer cells escape apoptosis, which one reason is the change in the expression of genes that are involved in the regulation of this process. Most anti-cancer agents exert their therapeutic effects in part by inducing programmed cell death. Induction of apoptosis is one of the most important methods in destroying cancer cells without complication [51]. In this study, using the MTT assay, it was shown that Omniscan and gadodiamide nanoparticles have a killing effect on MCF-7 cancer cells that is depended on time and concentration. In addition, the study showed that gadodiamide nanoparticles compared with Omniscan alone, more inhibited the expression of Bcl-2 anti-apoptotic gene mRNA and further increased gene expression of Bax, caspase 3 and 9, and GAPDH during the specified time.
Today, the focus of cancer research is to find anticancer agents with high safety factor, functionality and more acceptance to patients. The targeted treatments with molecular targets that intervene in tumor growth or progression have a high specificity to tumor cells and a wide therapeutic window by reduced toxicity. In addition they can be used in combination with cytotoxic chemotherapy or radiation therapy for synergistic anti-cancer activity or as an adjuvant. Thus, targeted-therapy is considered as a new and promising approach for cancer treatment. To reduce cancer mortality, it is essential to develop new methods of treatment and prevention. So, today different types of standard treatment methods for patients with cancers have emerged. Some of these methods are surgery, chemotherapy, radiation therapy, biological therapy and hormone therapy [52]. That has not made any significant improvement in cancer metastasis, survival rate and complications in patients with peripheral hardware it [53].
Despite significant advances in medical technologies for diagnosis and treatment, cancer is still widely regarded as a threat of death. During recent decades, the structure and morphology of nanomaterials is of utmost importance. Because this material has the properties like low density and high specific surface area. So in order to optimize the test conditions and obtaining the smallest size of the nanoparticles in the research, study design was performed with Taguchi analysis. Several reports of anti-bacterial, anti-fungal and anti-cancer and other properties of nanoparticles was published several years ago and that is why synthesis of nanoparticles is very i