ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 21
Gastrodin has in vitro anticancer effects in human glioma U118 cells
1Department of Neurosurgery, the First Affiliated Hospital of Yangtze University, Jingzhou, Hubei, PR China
2Department of Neurosurgery, the Second People’s Hospital of Jingzhou, Jingzhou, Hubei, PR China
- *Corresponding Author:
- Hanjiang Cui
Department of Neurosurgery
The Second People’s Hospital of Jingzhou
Jingzhou, Hubei, PR China
Accepted date: August 24, 2017
Gastrodin is a biologically active substance extracted from Gastrodia elata Bl. which is a traditional Chinese medicine. The aim of this study was to determine the in vitro anticancer effects in human glioma U118 cells. Two concentrations of gastrodin, (0.5, 1.0 and 2.0 mg/mL) both had the strong growth inhibitory effects in U118 cells determined by MTT assay. Using flow cytometry assay, high concentration of gastrodin (2.0 mg/mL) treated U118 cells contained the most apoptosis cells (43.2%) and the low concentration of gastrodin (1.0 and 0.5 mg/mL) treated U118 cells also had the more apoptosis cells (23.3% and 9.8%) than control cells (2.1%). By qPCR assay, the experiments results showed that gastrodin could raise caspase-3, caspase-8, caspase-9, Bax, p53, p21, IκB-α, Fas, FasL, TIMP-1, TIMP-2 and reduce Bcl-2, Bcl-xL, NF-κB, EGF, EGFR, VEGF, Fit-1, MMP-2, MMP-9 mRNA expressions. From these results, gastrodin could be used as a medicine for cancer treatment.
Keywords
Gastrodin, Cancer, U118 cells, mRNA expression.
Introduction
Cancer is the second most serious disease that threatens human health. Therefore, it is a big dream of scientists to conquer cancer. Natural product is one of the most important way from which new drug and lead compound may possibly be discovered and from which actually many kinds of anticancer drugs come directly or indirectly. Paclitaxel is now the most outstanding natural anticancer drug among those discovered which is used widely in clinical practice in treatment of breast cancer, ovarian cancer and some of head and neck cancer and lung cancer [1,2].
Cancer inhibitors exist in various plants naturally, and have very good effects on human cancer prevention. These cancer inhibitors that occur naturally are safer as having low toxicity, as well as can reduce the pain of patients during the treatment [3]. But the activity of many cancer inhibitors existing in natural plants is lower than that of synthetic drugs, and these natural cancer inhibitors can substantially improve the treatment effect of cancer, so finding out a useful cancer inhibitor becomes the most important thing to improve and enhance the anti-cancer effects resources that occurs naturally.
Gastrodin is extracted from dried root of Gastrodia elata Blume in China using a medicine, gastrodin has a good sedative and hypnotic effects, and can relieve the symptoms of neurasthenia, insomnia and headache in traditional Chinese medicine. Gastrodin is used for blood pressure, antiplatelet effects, action on nerve cells and also showed the effects of anti-free radical, protects cell membrane, increases immune function, and treats acute lung injury [4-8].
Studies reported written by experts and scholars as well as clinical application showed that gastrodin has good curative effects on hepatic ascitic cancer and colonic adenocarcinoma [9,10]. Gastrodin had anticancer effects in H22 hepatic ascitic tumor cells through NF-κB signaling activation in CD4+ T cells [4]. Gastrodin also had anticancer effects in colonic adenocarcinoma Caco-2 cells using passive paracellular transport pathway. In this study, we study the anticancer effects of gastrodin in human glioma U118 cells, to induce the apoptosis of cancer cells, meanwhile observe the mechanism of these anticancer effects.
Materials and Methods
Cell line
Human glioma U118 cell line was purchased from ATCC (American Type Culture Collection, Manassas, VA, USA). The RPMI-1640 medium (Gibco Co., Birmingham, MI, USA) containing 10% foetal bovine serum (Gibco Co., Birmingham, MI, USA) and 1% penicillin-streptomycin (Gibco Co.) was used for Human glioma U118 cells culturing. The cancer cells were cultured in the RPMI-1640 medium at 37°C in a humidified atmosphere containing 5% CO2 (Forma, model 311 S/N29035; Waltham, MA, USA). The medium was changed once per 2-3 d.
MTT assay
Culture solution was added to adjust the concentration of cancer cells in logarithmic growth phase to 2 × 104/dish, which were added to the 96-well culture plate with 50 μL per well, and placed in incubator with 5% CO2 at 37°C for 24 h. Gastrodin were added into the 96-well plate with 50 μL per well, to adjust the concentration of cancer cells to 1 or 2 mg/mL. The 50 μL culture solution was added to the blank control group, which was cultured in CO2 incubator for 48 h. Followed by, the blank control group which was added with MTT solution after the supernatant was removed and then incubated for 4 h. The 100 μL DMSO was added to the blank control group after the supernatant was removed and shocked for 30 min, the enzyme standard instrument were used to detect at 492 nm [11].
Flow cytometry assay
Single cell suspension was centrifuged to remove stationary liquid and washed by 3 mL PBS twice, and then centrifuged for 5 min; added with 1 ml PI staining solution and incubated in refrigerator at 4°C for 30 min without explosion to sunshine; and then filtered by 500-well copper mesh; flow cytometry detection and argon ion laser with 15 mA excitation light source and 488 nm wavelength were used for testing, and 630 nm band-pass filter to receive the light. The 1 × 104 cells were collected by FSC/SSC scattered point diagram method, with gating technology used to exclude adhesive cells and cell debris, to analyze the percentage of apoptotic cells in PI fluorescence histogram [11].
qPCR assay
RNAzol reagent was used to extract the total RNA from cancer cells, and DNase RNase-free was adopted to digest total RNA at 37°C for 15 min, and then RNeasy kit to purify RNA to adjust its concentration to 1 μg/μL. RNA (2 μg) was used as the template to synthetize cDNA by reacting with reverse transcriptase at 37°C for 120 min, at 99°C for 4 min, and at 4°C for 3 min respectively. After that, reverse transcriptionpolymerase chain reaction method was adopted to amplify the DNA expressions (Table 1), to measure the transcription level of mRNA, and GAPDH was used as the housekeeping genes of internal control group [12].
| Gene Name | Sequence |
|---|---|
| Caspase-3 | Forward: 5′-CAA ACT TTT TCA GAG GGG ATC G-3′ |
| Reverse: 5′-GCA TAC TGT TTC AGC ATG GCA-3′ | |
| Caspase-8 | Forward: 5′-CCC CAC CCT CAC TTT GCT-3′ |
| Reverse: 5′-GGA GGA CCA GGC TCA CTT A-3′ | |
| Caspase-9 | Forward: 5′-GGC CCT TCC TCG CTT CAT CTC-3′ |
| Reverse: 5′-GGT CCT TGG GCC TTC CTG GTA T-3′ | |
| Bax | Forward: 5′-AAG CTG AGC GAG TGT CTC CGG CG-3′ |
| Reverse: 5′-CAG ATG CCG GTT CAG GTA CTC AGT C-3′ | |
| Bcl-2 | Forward: 5′-CTC GTC GCT ACC GTC GTG ACT TGG-3′ |
| Reverse: 5′-CAG ATG CCG GTT CAG GTA CTC AGT C-3′ | |
| Bcl-xL | Forward: 5′-CCC AGA AAG GAT ACA GCT GG-3′ |
| Reverse: 5′-GCG ATC CGA CTC ACC AAT AC-3′ | |
| p53 | Forward: 5'-GCT CTG ACT GTA CCA CCA TCC-3' |
| Reverse: 5'-CTC TCG GAA CAT CTC GAA GCG-3' | |
| p21 | Forward: 5'‑CTC AGA GGA GGC GCC ATG‑3' |
| Reverse: 5'‑GGG CGG ATT AGG GCT TCC‑3' | |
| NF-κB | Forward: 5′-CAC TTA TGG ACA ACT ATG AGG TCT CTG G-3′ |
| Reverse: 5′-CTG TCT TGT GGA CAA CGC AGT GGA ATT TTA GG-3′ | |
| IκB-α | Forward: 5'‑GCT GAA GAA GGA GCG GCT ACT‑3' |
| Reverse: 5'‑TCG TAC TCC TCG TCT TTC ATG GA‑3' | |
| Fas | Forward: 5′-GAA ATG AAA TCC AAA GCT-3′ |
| Reverse: 5′-TAA TTT AGA GGC AAA GTG GC-3′ | |
| FasL | Forward: 5′-GGA TTG GGC CTG GGG ATG TTT CA-3′ |
| Reverse: 5′-TTG TGG CTC AGG GGC AGG TTG TTG-3′ | |
| TIMP-1 | Forward: 5'-GTC AGT GAG AAG CAA GTC GA-3' |
| Reverse: 5′-ATG TTC TTC TCT GTG ACC CA-3′ | |
| TIMP-2 | Forward: 5′-TGG GGA CAC CAG AAG TCA AC-3′ |
| Reverse: 5′-TTT TCA GAG CCT TGG AGG AG-3′ | |
| MMP-2 | Reverse: 5′-CTT CTT CAA GGA CCG GTT CA-3′ |
| Forward: 5'‑GCT GGC TGA GTA CCA GTA‑3' | |
| MMP-9 | Reverse: 5'‑TGG GCT ACG TGA CCT ATG AC‑3' |
| Forward: 5′-GCC CAG CCC ACC TCC ACT CC-3′ | |
| EGF | Reverse: 5′-GCC AAG CTC AGA AGG CTA C-3′ |
| Forward: 5'‑CAG GCC AGC CTC GTC TCA T‑3' | |
| EGFR | Reverse: 5'‑TCG GTG CTG TGC GAT TTA‑3' |
| Forward: 5′-TTT CTG GCA GTT GCT CCT C-3′ | |
| VEGF | Reverse: 5′-GCA CCC ATG GCA GAA GGA GGA G-3′ |
| Forward: 5'-GTG CTG ACG CTA ACT GAC C-3' | |
| Fit-1 | Reverse: 5'‑CAA GTG GCCAGA GGC ATG GAG TT-3' |
| Forward: 5′-GAT GTA GTC TTTACC ATC CTG TTG-3′ | |
| GAPDH | Reverse: 5′-CGG AGT CAA CGG ATT TGG TC-3′ |
| Forward: 5'-AGC CTT CTC CAT GGT CGT GA-3' |
Table 1: Sequences of primers were used in this study.
Statistical analysis
The in vitro experiments were presented as mean ± standard deviation (SD). Differences between the mean values for individual groups were assessed with one-way analysis of variance (ANOVA) with Duncan’s multiple range test using SAS version 9.1 (SAS Institute Inc., Cary, NC, USA).
Results
Growth inhibitory effects of gastrodin in U118 cells
By the MTT assay, the untreated U118 cells showed the OD540 value at 0.479 (Table 2), after 0.5, 1.0 and 2.0 mg/mL gastrodin treatment, the OD540 values were reduced at 0.317, 0.197 and 0.063 respectively. The 0.5, 1.0 and 2.0 mg/mL gastrodin showed the inhibitory effects at 33.8%, 58.9% and 86.8%, respectively.
| Treatment | OD492 value | Inhibitory rate (%) | |
|---|---|---|---|
| Control | 0.479 ± 0.006a | / | |
| Gastrodin (mg/kg) | 0.5 | 0.317 ± 0.011b | 33.8 ± 2.9c |
| 1 | 0.197 ± 0.009c | 58.9 ± 2.5b | |
| 2 | 0.063 ± 0.005d | 86.8 ± 1.4a | |
Table 2: Growth inhibitory effects of human glioma U118 cells treated by gastrodin by MTT assay.
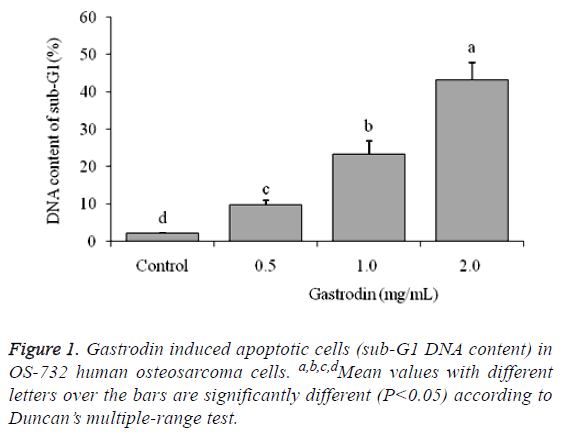
DNA content of sub-G1 U118 cells
The flow cytometry showed that control cells has only 2.1 ± 0.2% DNA content of sub-G1 of U118 cells (apoptotic cells), and as per the results in the other groups the cells have more apoptosis because of gastrodin treatment (Figure 1). The 2.0, 1.0 and 0.5 mg/mL gastrodin treated U118 cells had 43.2 ± 4.7%, 23.3 ± 3.6% and 9.8 ± 1.2% apoptosis cells, respectively.
mRNA expressions of caspases
By qRCR experiment, control cells showed the weakest caspase-3, caspase-8 and caspase-9 also the mRNA expressions were weakest (Table 3). Gastrodin treated cells showed remarkably stronger caspase-3, caspase-8 and caspase-9 expressions than control cells, and 2.0 mg/mL gastrodin treatment had the strongest caspase-3 (4.87 fold of control), caspase-8 (5.32 fold of control) and caspase-9 (4.33 fold of control) expressions.
| Group | Caspase-3 | Caspase-8 | Caspase-9 | |
|---|---|---|---|---|
| Control | 1.00 ± 0.21d | 1.00 ± 0.17d | 1.00 ± 0.11d | |
| Gastrodin (mg/kg) | 0.5 | 2.31 ± 0.29c | 2.12 ± 0.24c | 1.68 ± 0.23c |
| 1 | 2.92 ± 0.21b | 3.68 ± 0.38b | 3.06 ± 0.15b | |
| 2 | 4.87 ± 0.48a | 5.32 ± 0.52a | 4.33 ± 0.41a | |
Table 3: Quantitative analysis of mRNA expressions of caspase-3, caspase-8 and caspase-9 in U118 cells treated by gastrodin.
mRNA expressions of Bax, Bcl-2 and Bcl-xL
Gastrodin treated U118 cells had the high Bax mRNA expressions and low Bcl-2, Bcl-xL expressions (Table 4). Whereas the high concentration of 2.0 mg/mL treatment were showing the highest Bax (3.36 fold of control) expressions than other groups cells, but this concentration treatment showed the lowest Bcl-2 (0.22 fold of control), Bcl-xL (0.12 fold of control) expressions.
| Group | Bax | Bcl-2 | Bcl-xL | |
|---|---|---|---|---|
| Control | 1.00 ± 0.08d | 1.00 ± 0.06a | 1.00 ± 0.05a | |
| Gastrodin (mg/kg) | 0.5 | 1.86 ± 0.22c | 0.82 ± 0.08b | 0.77 ± 0.04b |
| 1 | 2.31 ± 0.19b | 0.53 ± 0.05c | 0.41 ± 0.05c | |
| 2 | 3.36 ± 0.33a | 0.22 ± 0.04d | 0.12 ± 0.03d | |
Table 4: Quantitative analysis of mRNA expressions of Bax, Bcl-2 and Bcl-xL in U118 cells treated by gastrodin.
mRNA expressions of Fas and FasL
The control cells had the lowest Fas and FasL mRNA expressions (Table 5), after gastrodin treatment, these expressions were elevated, raising concentration treated cells had the remarkably higher Fas (6.39 fold of control at 2.0 mg/kg gastrodin) and FasL (2.82 fold of control at 2.0 mg/kg gastrodin) expressions with respect to low concentration treated cells.
| Group | Fas | FasL | |
|---|---|---|---|
| Control | 1.00 ± 0.14d | 1.00 ± 0.09d | |
| Gastrodin (mg/kg) | 0.5 | 3.05 ± 0.38c | 1.45 ± 0.12c |
| 1 | 4.38 ± 0.42b | 2.32 ± 0.21b | |
| 2 | 6.39 ± 0.50a | 2.82 ± 0.36a | |
Table 5: Quantitative analysis of mRNA expressions of Fas and FasL in U118 cells treated by gastrodin.
mRNA expressions of p53 and p21
The p53 and p21 mRNA expressions of control cells were weakest (Table 6), gastrodin could increase these expressions, and high concentration of gastrodin had the stronger capability to increase p53 (5.31 fold of control at 2.0 mg/kg gastrodin) and p21 (4.88 fold of control at 2.0 mg/kg gastrodin) activities.
| Group | p53 | p21 | |
|---|---|---|---|
| Control | 1.00 ± 0.15d | 1.00 ± 0.14d | |
| Gastrodin (mg/kg) | 0.5 | 2.31 ± 0.30c | 2.11 ± 0.18c |
| 1 | 3.50 ± 0.45b | 3.09 ± 0.37b | |
| 2 | 5.13 ± 0.44a | 4.88 ± 0.46a | |
Table 6: Quantitative analysis of mRNA expressions of p53 and p21 in U118 cells treated by gastrodin.
mRNA expressions of NF-κB and IκB-α
Gastrodin (2.0 mg/mL) group cells had the lowest NF-κB (0.19 fold of control) mRNA expression and the highest IκB-α (3.81 fold of control) expression (Table 7). Control cells had the highest NF-κB expressions and the lowest IκB-α expression.
| Group | NF-κB | IκB-α | |
|---|---|---|---|
| Control | 1.00 ± 0.05a | 1.00 ± 0.06d | |
| Gastrodin (mg/kg) | 0.5 | 0.69 ± 0.06b | 1.49 ± 0.11c |
| 1 | 0.41 ± 0.04c | 2.41 ± 0.25b | |
| 2 | 0.19 ± 0.03d | 3.81 ± 0.33a | |
Table 7: Quantitative analysis of mRNA expressions of NF-κB and IκB-α in U118 cells treated by gastrodin.
mRNA expressions of TIMP-1, TIMP-2, MMP-2 and MMP-9
The TIMP-1, TIMP-2 mRNA expressions in control cells were lowest than other groups cells, but MMP-2, MMP-9 expressions were highest than other groups cells (Table 8). Gastrodin could raise TIMP-1, TIMP-2 expressions and reduce MMP-2, MMP-9 expressions as compared to the control cells, and after raising its concentration showed higher TIMP-1 (4.20 fold of control at 2.0 mg/kg gastrodin), TIMP-2 (3.80 fold of control at 2.0 mg/kg gastrodin) expressions and lower MMP-2 (0.14 fold of control at 2.0 mg/kg gastrodin), MMP-9 (0.22 fold of control at 2.0 mg/kg gastrodin) expressions than low concentration treated cells.
| Group | TIMP-1 | TIMP-2 | MMP-2 | MMP-9 | |
|---|---|---|---|---|---|
| Control | 1.00 ± 0.08d | 1.00 ± 0.09d | 1.00 ± 0.06a | 1.00 ± 0.09a | |
| Gastrodin (mg/kg) | 0.5 | 2.03 ± 0.28c | 1.75 ± 0.19c | 0.70 ± 0.08b | 0.68 ± 0.06b |
| 1 | 2.68 ± 0.24b | 2.59 ± 0.31b | 0.39 ± 0.05c | 0.41 ± 0.05c | |
| 2 | 4.20 ± 0.41a | 3.80 ± 0.39a | 0.14 ± 0.04d | 0.22 ± 0.04d | |
Table 8: Quantitative analysis of mRNA expressions of TIMP-1, TIMP-2, MMP-2 and MMP-9 in U118 cells treated by gastrodin.
mRNA expressions of EGF, EGFR, VEGF and Fit-1
Gastrodin treatment reduce EGF, EGFR, VEGF, Fit-1 mRNA expressions as compared to the control cells (Table 9), and high concentration gastrodin showed further reduction in expression of EGF (0.34 fold of control at 2.0 mg/kg gastrodin), EGFR (0.28 fold of control at 2.0 mg/kg gastrodin), VEGF (0.11 fold of control at 2.0 mg/kg gastrodin), Fit-1 (0.29 fold of control at 2.0 mg/kg gastrodin).
| Group | EGF | EGFR | VEGF | Fit-1 | |
|---|---|---|---|---|---|
| Control | 1.00 ± 0.11a | 1.00 ± 0.12a | 1.00 ± 0.08a | 1.00 ± 0.10a | |
| Gastrodin (mg/kg) | 0.5 | 0.83 ± 0.07b | 0.76 ± 0.08b | 0.64 ± 0.07b | 0.73 ± 0.09b |
| 1 | 0.62 ± 0.06c | 0.52 ± 0.05c | 0.35 ± 0.07c | 0.45 ± 0.06c | |
| 2 | 0.34 ± 0.06d | 0.28 ± 0.06d | 0.11 ± 0.05d | 0.29 ± 0.07d | |
Table 9: Quantitative analysis of mRNA expressions of EGF, EGFR, VEGF and Fit-1 in U118 cells treated by gastrodin.
Discussion
Apoptosis of cancer cell plays an important role in the occurrence and development of cancer, Wong found that a lot of receptor-mediated cell signal transduction and many different genes are involved in the activation of cancer cells apoptosis, and regulation of cancer cell apoptosis respectively [13]. As an upstream protein involved in exogenous apoptosis, caspase-8 shears and activates downstream apoptosis-inducing proteins such as caspase-3, caspase-6 and caspase-7, causing cell apoptosis [14]. Apaf-l can bond to the original structural domain of the precursor of caspase-9 through the complementary domain of caspase, leading to the selfactivation of caspase-9, which further activates downstream caspase-3, caspase-6 and caspase-7, and ultimately inducing endogenous apoptosis of cells [15]. Caspase-3 involves both exogenous and endogenous apoptosis, and many apoptotic factors work on downstream effector caspase-3 ultimately to induce cell apoptosis [16].
The inhibition of apoptosis has a vital significance to the incidence and development of cancer. Proteins in Bcl-2 family play the important roles in regulating the apoptosis of cancer cells. Bcl-2 family is made up of apoptosis inhibitory factor (Bcl-2 and Bcl-xL) and apoptosis-promoting factor (Bax); their ratio determines whether the cell is able to accept the apoptotic signal [17]. To a certain extent, apoptosis or apoptosis inhibition are regulated by the above two genes. The disturbance of apoptosis regulation is crucial in the development of tumor, and Bcl-2 family plays a major role in this process [18]. As the main members of Bcl-2 family, Bcl-2, Bax and Bcl-xL mainly regulate the apoptosis of cells by affecting mitochondrial pathway. When cells get death signals, the Bax which is bonded to Bcl-2 or Bcl-xL will be displaced, results increase in the permeability of the mitochondrial membrane and leading to the release of a series of substances, thus eventually causing the death of cells [19].
Fas, FasL and caspase-3 are the important proteins mediating the apoptosis of cells. At present, it has been found that FasL can be induced by certain stress responses, such as ultraviolet and DNA damage, and the interactions between FasL and Fas can induce programmed death of cells, which may be an important mechanism of the body to clear cells having mutation [20]. FasL can express on the surface of tumor cells, and tumor-specific antigen can induce tumor infiltrating T lymphocytes (TIL) to express Fas in large quantity, it enhances the sensibility of T cells to apoptosis. Tumor cells induce the apoptosis of T lymphocyte which cause the high expression of Fas by FasL, resulting in immunosuppression. Fas-mediated apoptosis is also related to many other factors, such as p53 gene mutation or the lack of co-stimulatory factor [21].
p53, the major protein regulating Bcl-2 family, regulates different proteins of Bcl-2 family in various ways, affecting the biological behaviours of pancreatic cancer. p53 can up-regulate Bax and down-regulate Bcl-2 or Bcl-xL, affecting the apoptosis of cancer cells, and changing the permeability of mitochondria, thus affecting the function of downstream proapoptotic genes [22]. As the clumping factor of CDK, low concentrations of tumor suppressor gene p21 positively regulates the function of CDK, facilitating the development of cells and promoting the transition from G1 stage to S stage, but highly expressed p21 protein and cyclin bind to CDK competitively to inhibit the activity of CDK, causing the cell development stagnating in G1 stage, thus inhibiting cell proliferation or inducing cell apoptosis [23]. p73 and p53 protein have homology in target gene binding, but their functions have great differences. As p73 can arrest cell cycle and induce cell apoptosis, it can inhibit tumor to certain extent [24].
NF-κB system is composed of NF-κB family and its inhibitor IκB-α. NF-κB is an extremely important transcriptional activator, and IκB-α is the inhibitory protein of NF-κB [25]. NF-κB is important to inflammation process, and also serves as regulatory protein in the development of cancer. It plays an important role in information transmission in relation to tumor growth, closely related to the incidence and development of tumor [26]. Studies have found that NF-κB highly expresses in many types of tumors, and activated NF-κB promotes the expression of a variety of genes which involve the development of cancer [27,28]. Wu et al. found that Hp infection activated NF-κB and the expression of COX-2 play important roles in the incidence and development of cancer [29].
Malignant tumors are characterized by local invasion and distant metastasis, which are the main reasons that malignant tumor threaten patients’ health and life. MMPs play an important role in the invasion and metastasis of tumor, it not only mediates tumor cells’ degradation of extracellular matrix including the basement membrane, but also controls the process of angiogenesis, that affects the function of cell adhesion molecules and regulates the growth of tumor cells [30]. Study has shown that the expression of MMP-2 and MMP-9 is closely related to cancer angiogenesis; tumor cells which can secrete MMP-2 and MMP-9 have high invasion and metastases ability, drugs can also be used to inhibit the growth of tumor cells through lowering the activity of MMP-2 and MMP-9 [31]. In addition, ECM play a key role in local invasion and distant metastasis of cancer cells, the degradation of ECM is complex, as it involved a lot of factors, and MMPs and inhibitors play important functions, MMPs can degrade ECM, while TIMPs can inhibit the degradation of ECM through lowering the activity of MMPs, to protect normal cells [32]. The formation of intravascular cavity depends on the balance of MMPs and TIMPs, introducing exogenous inhibitors may break the balance of MMPs and TIMPs, inhibiting the process of angiogenesis, as well as the invasion and metastasis of tumor cells. Therefore TIMPs can inhibit tumor invasion and metastasis, and has a remarkable use in the research of tumor treatment [33].
EGF is a kind of growth factor which can affect many reactions by combining with EGFR. Study has shown that EGF and other growth factors can promote the proliferation of human cells. EGFR is a member of ErbB receptor family located on the surface of cells, involved in cells proliferation, growth, migration and infiltration [34]. Study has found that EGFR can be adjusted by EGF-meditated cancer cell proliferation through sialylation [35].
VEGF can promote the growth of tumor and angiogenesis, and provides a foundation for tumor metastasis, affecting the prognosis of patients with tumor. VEGF is the strongest vascular endothelial cell growth factor which can directly work on blood vessels, and specifically promote the division, proliferation and migration of endothelial tumor cells, playing an important role in the formation of tumor blood vessel, and it is also one of the key factors of promoting angiogenesis [36]. Fit-1 is the receptor of VEGF; it can bind to VEGF in high affinity. Fit-1 receptor deficient mice are mainly characterized by vascular endothelial cell damage; the expression of Fit-1 is mainly related to the early-stage angiogenesis and wound healing of mouse embryos [37].
Conclusion
In this study, MTT assay, flow cytometry and qPCR assays were used to determine the anticancer effects of gastrodin in human glioma U118 cells. Gastrodin had a good anticancer effect on U118 cells, and from these results, we can conclude that gastrodin could be used as a cancer cell inhibitor for glioma treatment, and this inhibitor might be used in clinical application to save human life in future.
Conflict of Interest
There is no conflict of interest.
References
- Davidson M, Smyth EC, Cunningham D. Clinical role of ramucirumab alone or in combination with paclitaxel for gastric and gastro-esophageal junction adenocarcinoma. Onco Targets Ther 2016; 9: 4539-4548.
- Yu B, Tan L, Zheng R, Tan H, Zheng L. Targeted delivery and controlled release of Paclitaxel for the treatment of lung cancer using single-walled carbon nanotubes. Mater Sci Eng C Mater Biol Appl 2016; 68: 579-584.
- O'Connor SE. Plant Biochemistry: Fighting cancer while saving the Mayapple. Science 2015; 349: 1167-1168.
- Fang H, Zhang JC, Yang M, Li HF, Zhang JP, Zhang FX, Wang QY, Wang RR, Liu J. Perfusion of gastrodin in abdominal aorta for alleviating spinal cord ischemia reperfusion injury. Asian Pac J Trop Med 2016; 9: 688-693.
- Li Y, Zhang Z. Gastrodin improves cognitive dysfunction and decreases oxidative stress in vascular dementia rats induced by chronic ischemia. Int J Clin Exp Pathol 2015; 8: 14099-14109.
- Liu B, Li F, Shi J, Yang D, Deng Y, Gong Q. Gastrodin ameliorates sub-acute phase cerebral ischemia-reperfusion injury by inhibiting inflammation and apoptosis in rats. Mol Med Rep 2016; 14: 4144-4152.
- Du F, Wang X, Shang B, Fang J, Xi Y, Li A, Diao Y. Gastrodin ameliorates spinal cord injury via antioxidant and anti-inflammatory effects. Acta Biochim Pol 2016; 63: 589-593.
- Zhang Z, Zhou J, Song D, Sun Y, Liao C, Jiang X. Gastrodin protects against LPS-induced acute lung injury by activating Nrf2 signaling pathway. Oncotarget 2017; 8: 32147-32156.
- Shu G, Yang T, Wang C, Su H, Xiang M. Gastrodin stimulates anticancer immune response and represses transplanted H22 hepatic ascitic tumor cell growth: Involvement of NF-κB signaling activation in CD4+ T cells. Toxicol Appl Pharmacol 2013; 269: 270-279.
- Wang XD, Zeng S. The transport of gastrodin in Caco-2 cells and uptake in Bcap37 and Bcap37/MDR1 cells. Yao Xue Xue Bao 2010; 45: 1497-1502.
- Zhao X, Kim SY, Park KY. Bamboo salt has in vitro anticancer activity in HCT-116 cells and exerts anti-metastatic effects in vivo. J Med Food 2013; 16: 9-19.
- Zhao X, Wang Q, Li GJ, Chen F, Qian Y, Wang R. In vitro antioxidant, anti-mutagenic, anti-cancer and anti-angiogenic effects of Chinese Bowl tea. J Funct Food 2014; 7: 590-598.
- Wong RS. Apoptosis in cancer: from pathogenesis to treatment. J Exp Clin Cancer Res 2011; 30: 1096-1104.
- Chen G, Cheng X, Zhao M, Lin S, Lu J, Kang J, Yu X. RIP1-dependent Bid cleavage mediates TNFα-induced but Caspase-3-independent cell death in L929 fibroblastoma cells. Apoptosis 2015; 20: 92-109.
- Guerrero AD, Chen M, Wang J. Delineation of the caspase-9 signaling cascade. Apoptosis 2008; 13: 177-186.
- Agostini-Dreyer A, Jetzt AE, Stires H, Cohick WS. Endogenous IGFBP-3 mediates intrinsic apoptosis through modulation of Nur77 phosphorylation and nuclear export. Endocrinol 2015; 156: 4141-4151.
- Nakazawa M, Matsubara H, Matsushita Y, Watanabe M, Vo N, Yoshida H, Yamaguchi M, Kataoka T. The human Bcl-2 family Member Bcl-rambo localizes to mitochondria and induces apoptosis and morphological aberrations in drosophila. PLoS One 2016; 11: e0157823.
- Tiwari P, Khan MJ. Molecular and computational studies on apoptotic pathway regulator, Bcl-2 gene from breast cancer cell line MCF-7. Indian J Pharm Sci 2016; 78: 87-93.
- O'Neill KL, Huang K, Zhang J, Chen Y, Luo X. Inactivation of pro-survival Bcl-2 proteins activates Bax/Bak through the outer mitochondrial membrane. Genes Dev 2016; 30: 973-988.
- Chen SQ, Lin JP, Zheng QK, Chen SJ, Li M, Lin XZ, Wang SZ. Protective effects of paeoniflorin against FasL-induced apoptosis of intervertebral disc annulus fibrosus cells via Fas-FasL signalling pathway. Exp Ther Med 2015; 10: 2351-2355.
- Shin EM, Kim S, Merfort I, Kim YS. Glycyrol induces apoptosis in human Jurkat T cell lymphocytes via the Fas-FasL/caspase-8 pathway. Planta Med 2011; 77: 242-247.
- Zhang J, Huang K, O'Neill KL, Pang X, Luo X. Bax/Bak activation in the absence of Bid, Bim, Puma, and p53. Cell Death Dis 2016; 7: e2266.
- Gongpan P, Lu Y, Wang F, Xu Y, Xiong W. AS160 controls eukaryotic cell cycle and proliferation by regulating the CDK inhibitor p21. Cell Cycle 2016; 15: 1733-1741.
- Wang Y, Wang X, Flores ER, Yu J, Chang S. Dysfunctional telomeres induce p53-dependent and independent apoptosis to compromise cellular proliferation and inhibit tumor formation. Aging Cell 2016; 15: 646-660.
- Huang C, Wang J1, Lu X1, Hu W1, Wu F1, Jiang B, Ling Y, Yang R, Zhang W. Z-guggulsterone negatively controls microglia-mediated neuro-inflammation via blocking IκB-α-NF-κB signals. Neurosci Lett 2016; 619: 34-42.
- He G, Li LI, Guan E, Chen J, Qin YI, Xie Y. Fentanyl inhibits the progression of human gastric carcinoma MGC-803 cells by modulating NF-κB-dependent gene expression in vivo. Oncol Lett 2016; 12: 563-571.
- Lu YX, Ju HQ, Wang F, Chen LZ, Wu QN, Sheng H. Inhibition of the NF-κB pathway by nafamostat mesilate suppresses colorectal cancer growth and metastasis. Cancer Lett 2016; 380: 87-97.
- McLoed AG, Sherrill TP, Cheng DS, Han W, Saxon JA, Gleaves LA. Neutrophil-derived IL-1β impairs the efficacy of NF-κB inhibitors against lung cancer. Cell Rep 2016; 16: 120-132.
- Wu CY, Wang CJ, Tseng CC, Chen HP, Wu MS, Lin JT, Inoue H, Chen GH. Helicobacter pylori promote gastric cancer cells invasion through a NF-kB and COX-2-mediated pathway. World J Gastroenterol 2005; 11: 3197-3203.
- Köhrmann A, Kammerer U, Kapp M, Dietl J, Anacker J. Expression of matrix metalloproteinases (MMPs) in primary human breast cancer and breast cancer cell lines: New findings and review of the literature. BMC Cancer 2009; 9: 188.
- Roomi MW, Monterrey JC, Kalinovsky T, Rath M, Niedzwiecki A. Patterns of MMP-2 and MMP-9 expression in human cancer cell lines. Oncol Rep 2009; 21: 1323-1333.
- Bourboulia D, Stetler-Stevenson WG. Matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs): positive and negative regulators intumor cell adhesion. Semin Cancer Biol 2010; 20: 161-168.
- Kousidou OC, Roussidis AE, Theocharis AD, Karamanos NK. Expression of MMPs and TIMPs genes in human breast cancer epithelial cells depends on cell culture conditions and is associated with their invasive potential. Anticancer Res 2004; 24: 4025-4030.
- Appert-Collin A, Hubert P, Crémel G, Bennasroune A. Role of ErbB receptors in cancer cell migration and invasion. Front Pharmacol 2015; 6: 283.
- Yen HY, Liu YC, Chen NY, Tsai CF, Wang YT, Chen YJ, Hsu TL, Yang PC, Wong CH. Effect of sialylation on EGFR phosphorylation and resistance to tyrosine kinase inhibition. Proc Natl Acad Sci USA 2015; 112: 6955-6960.
- Carmeliet P. VEGF as a key mediator of angiogenesis in cancer. Oncol 2005; 69: 4-10.
- Hoar FJ, Lip GY, Belgore F, Stonelake PS. Circulating levels of VEGF-A, VEGF-D and soluble VEGF-A receptor (sFIt-1) in human breast cancer. Int J Biol Markers 2004; 19: 229-235.