ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Advances in Health Science and Biotechnology Application
Experimental study on effect of calcitonin on osteoclast functions
Shunyu Wang1 and Xiaoying Yan2*
1Department of Gerontology, Second Affiliated Hospital of Dalian Medical University, Dalian, Liaoning Province 116027, PR China
2Department of General Practice, Second Affiliated Hospital of Dalian Medical University, Dalian, Liaoning Province 116027, PR China
- *Corresponding Author:
- Xiaoying Yan
Department of General Practice
Second Affiliated Hospital of Dalian Medical University, PR China
Accepted date: August 03, 2017
Objective: It's aimed to study the effect of calcitonins on osteoclast functions.
Method: Osteoclasts were acquired from 10 neogenic SD rats by mechanical separation method and then cultured in vitro by bone marrow derivation method; the calcitonins with different consistencies were added into the culture media, the numbers and forms of calcitonins were observed after tartrateresistant acid phosphatase staining, the changes in numbers and forms of bone resorption lacunas were analyzed with an image software of Image Pro Plus after 48h culturing and finally the apoptosis rate was measured by a flow cytometer.
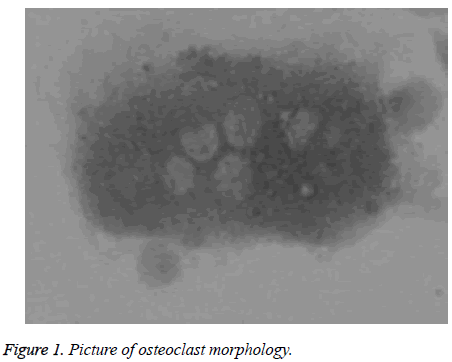
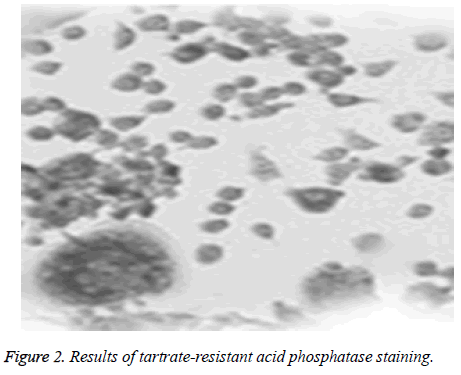
Result: In general, osteoclast was irregular and took the shape of strip, omelette, funnel or sausage and contains several to scores of nucleuses. The active element of tartrate-resistant acid phosphatase was granular and claret-red. The number of tartrate-resistant acid phosphatase-positive cells in the culture medium without calcitonin was (25.1 ± 3.4)/tablet; the number of tartrate-resistant acid phosphatasepositive cells in the calcitonin medium at concentration of 10-8 mol/L and 10-9 mol/L was (9.0 ± 2.6)/ tablet and (12.5 ± 3.8)/tablet, respectively, which were significantly less than those in the control group (P<0.01); 10-10 mol/(16.9 ± 4.1)/tablet, and there was significant difference between the two groups (P<0.01). The number of tartrate-resistant acid phosphatase positive cells in the calcitonin culture medium at the concentration of 10-10 mol/L was (16.9 ± 4.1)/tablet, significantly higher than that in the control group (P<0.05). The apoptosis rate of osteoclast rose as a function of the increase of calcitonin consistency.
Conclusion: Calcitonin was capable to inhibit proliferation of osteoclast, promote the apoptosis of osteoclast and further inhibit absorption function of osteoclast and was in positively correlated with the dose.
Keywords
Calcitonin, Osteoclast, Cell culture, Bone resorption, Apoptosis
Introduction
As calcitonin is capable to distinctly inhibit proliferation of osteoclasts and has definite pain-relief action-relieving pain caused by microfracture, it has been widely used in treating osteoporosis diseases and especially has maximal potential in treating post-menopausal osteoporosis and secondary osteoporosis [1]. Meanwhile, due to its safety, it may be possibly used in prevent osteoporosis. Calcitonin is produced mainly by parafollicular cells in parotids of lower animals and thyroids of mammals and slightly by cerebrospinal fluid, hypophysis, hypothyroid, thymus, lung, liver, intestine and bladder [2]. In recent years, more and more studies found that the apoptosis of osteoclast played an important role in occurrence of osteoporosis: the earlier and more the apoptosis of osteoclast was, the less and shallower the bone resorption became; the later and less the apoptosis of osteoclast was, the more and deeper the bone resorption became. Moreover, calcitonin was able to inhibit proliferation of osteoclast and promote apoptosis of osteoclast. This study showed that, calcitonin could inhibit the proliferation of osteoclasts, promote osteoclast apoptosis, and thus inhibit the absorption of osteoclasts, which was positively correlated to the dose, worthy of clinical applications and promotion.
Material and Method
Experimental instruments and reagents: The experimental animal was clean-grade SD neonatal rat, which came into the world for 1 day (ID No.: 00310) and the experimental materials included new-born calf serum provided by Nanjing Jian Cheng Bioengineering Institute; 1, 25 (OH) 2 Vit D3 provided by Sigma and a-MEM culture medium provided by GIBCO; tartrate resistant acid phosphatase; D-Hank’s solution of 100 U/ml penicillin and 100 U/ml streptomycin; ethanol solutions with volume fractions of 85% and 74%; fresh calf femur; low-speed sawing slicer; a-MEM culture solution; eel calcitonin injection produced by Asahi Chemical Industry Co., Ltd.
Experimental rats: 10 SD rats, male and female, provided by the Fourth Military Medical University Experimental Animal Center, weighed 80 ± 5 g, each. The treatment of experimental animals conformed to the relevant provisions of animal ethics.
Method
Preparation of bone-ground section and cover slip: Transected cortical bone of fresh calf femur into 100 m-thick segments with the low-speed sawing slicer, ground them to 10 m thick with energy stone and then cut them into 8 mm × 8 mm thin sections; cleaned the bone sections for 3 times with an ultrasonic cleaner, then steeped them in D-Hank’s solution containing 1000 U/ml penicillin and 1000 U/ml streptomycin and replaced the solution every 30 min for totally 3 times; put the prepared cover slips into the D-Hank’s solution containing 1000 U/ml penicillin and 1000 U/ml streptomycin for replacement of 2 times (20 min for each time). Dipped the bone sections and cover slips treated as above into ethanol (volume fraction 85%), took them out after 24 h, placed them under an ultraviolet light overnight and then put them into the a-MEM culture solution.
Separation of osteoclast: Osteoclasts mechanically separated from limb bones of neonatal rat were collected onto boneground sections and 1.8 cm × 1.8 cm cover slips for wall culture (volume fractures of carbon dioxide and air were 5% and 95%, respectively, and temperature was 37°C) for 30-60 min, cells not sticking to walls were washed off with prewarmed MRM culture solution for 2-3 times, then they were cultured under the conditions of carbon dioxide (volume fracture 5%) and 37°C saturated humidity and then transferred into a-MRM culture solution containing 10-8 mmol/L1, 25 (OH) 2 D3 after 24 h (1 ml per well), and the culture solution was changed once every 3 d [3,4].
Preparation of calcitonin liquor: The eel calcitonin injection produced by Asahi Chemical Industry Co., Ltd. was prepared into different liquors with consistency range of 10-8 mol/ L-10-12 mol/L. Meanwhile, a control group free of serum was prepared for comparison.
Appraisal of cultured osteoclast: the samples were taken out after 48 h of tartrate-resistant acid phosphatase staining of cover slips and toluidine blue staining of bone-ground sections and then an inverted phase contrast microscope was used to observe live cellular morphology of osteoclasts and formation of lacunas after culturing.
Positive cell count of tartrate-resistant acid phosphatase: Cover slips were taken out after 7 days of culturing, immobilized in glutaraldehyde solution (consistency 25 g/L) for 10 min, incubated in the incubation solution of tartrateresistant acid phosphatase under 37°C for 50 min and flushed with distilled water, and finally the positive count of tartrateresistant acid phosphatase was observed under an optical microscope.
Determination of apoptosis rate of osteoclast: Propidium iodide was inserted to DNA for quantitative staining; cells were normally digested and collected after 24 h; cells were washed with PBS solution for 2 times; cells were immobilized with ethanol (volume fraction 75%) under -20°C. The cells were centrifuged at 1200 rpm for 3 min to remove immobile liquid. The cells were washed with PBS for 2 times; 0.5 ml PBS was added to prepare cell suspension specimens; the specimens were put into a solution (volume fraction 1:50) containing 0.1% Triton-X-100, 5 g propidium iodide (50 mg/L) and 2 ml LRNAase and lightly shaken; then they were placed under -40°C for photophobic staining for 60 min and screened with a 400-eye sieve; the cell consistency was adjusted; finally the specimens were tested on the instrument. 5000-10000 cells were tested (under the argon ion laser with 488 nm wave length) for each specimen and the multicycle DNA analysis software was used to measure the apoptosis rate of cells. The observation indices were mainly the apoptosis rate of osteoclast and the positive cell count of tartrate-resistant acid phosphatase [5].
Statistical method
The statistical treatment was done for experimental data acquired in this study with SPSS13.0 statistical software by a specially assigned person. The normality test and homogeneity test for variance were conducted firstly, various sets of means passing the above tests were compared through the single factor analysis of variance and the LSD test was carried out to carry out pairwise comparison. The result was expressed in ͞x ± s and P<0.05 indicated statistical significance of the difference.
Result
Morphological observation
After the bone marrow cell suspension inoculated onto cover slips was cultivated for 40 min, it was found that the osteoclasts had attached to walls and the cytoplasm extended gradually and took the form of large volume, clear profile and more nucleuses with different sizes and forms. The shape was irregular but changed gradually and the convex-concave stretching motion of pseudopods could be seen. In the beginning, it could be seen that the interstitial cells of bone marrow or other cells were mingled in it, but were washed off later, leaving the osteoclasts, which were more tightly attached to walls, were large in volume, contained more nucleuses (several to scores), were irregular in forms (taking the shape of strip, omelette, funnel or sausage) and had bubbles of unequal sizes in cytoplasm and higher purity (Figure 1).
Result of tartrate-resistant acid phosphatase staining
The active element of tartrate-resistant acid phosphatase was uniformly distributed in cytoplasm, is granular and claretred and has vivid pseudopods and clear negative nucleoli (Figure 2).
Effect of calcitonin on positive cell count of tartrateresistant acid phosphatase
After 48 h cultivation in the calcitonin culture solutions with different consistencies, positive cell count of tartrate-resistant acid phosphatase was carried out and the results indicated: the positive cell count of tartrate-resistant acid phosphatase was 25.1 ± 3.4 pcs/slice in the calcitonin-free culture solution; the counts were 9.0 ± 2.6 pcs/slice and 12.5 ± 3.8 pcs/slice in the 10-8 mol/L and 10-9 mol/L calcitonin culture solutions, respectively, which were substantially less than that of the control group, and the difference was of extreme significance (P<0.01); the count was 16.9 ± 4.1 pcs/slice in the 10-10 mol/L calcitonin culture solution, of which the difference whole compared with the control group was of substantial significance (P<0.05).
Effect of calcitonin on apoptosis rate of osteoclast
It was found in the inspection with a flow cytometer that the osteoclast cultured in this study had a certain basic apoptosis rate, the calcitonin could increase the apoptosis rate of osteoclast in the positive dose correlation with its consistency; when the consistency was ≥ 10-10 mol/L, the effect of the calcitonin was of great significance while compared with the control group (P<0.05) (Table 1).
| C (CT)/(mol/L) | Apoptotic rate of osteoclasts (%) |
|---|---|
| 0 | 2.66 ± 0.49 |
| 10-12 | 5.83 ± 0.24 |
| 10-10 | 9.32 ± 0.34∆ |
| 10-9 | 13.11 ± 0.51* |
| 10-8 | 18.02 ± 0.75# |
Table 1. Effect of calcitonins with various consistencies on apoptosis rate of osteoclast.
Discussion
Calcitonin is an endogenous hormone, can inhibit bone resorption by inhibiting formation and function of osteoclasts and is a kind of anti-bone-resorption drugs. The osteoclast has special calcitonin receptors, which, if activated, can inhibit formation and function. The calcitonin can inhibit escaped phenomenon in bone resorption, can lift up the deceased bone resorption rate again and relief bone ache of osteoporosis diseases [6-8]. Studies indicate that the occurrence of the phenomenon is related to decrease of calcitonin receptor on cell membrane and the calcitonin receptor recovers slowly after the calcitonin is removed [9,10]. In this study, after the calcitonins with different consistencies were added into the osteoclasts cultured in vitro, their number and dose decreased in positive correlation and the possible reason was that the osteoclasts were split into mononuclear cells under the action of calcitonin and their lives were shortened, or the amalgamation of bone marrow mononuclear cells was arrested by the calcitonin so that the formation rate of osteoclast was lowered down [11]. This study counted the osteoclasts, which had been cultured in the culture solution containing calcitonins of different consistencies after 48 h, and found that the positive cell count of tartrate-resistant acid phosphatase significantly decreased in case of calcitonin consistency ≥ 10-10 mol/L, which indicated that the calcitonin could substantially inhibit the osteoclast.
Main cells for activity of bone resorption were osteoclasts, which play a critical role in fracture, osteoporosis and other metabolic bone diseases. So, researchers have paid more and more attention to them [12-14] and gradually started to investigate their in-vitro culturing and purification methods. However, there have been no reports on sufficient quantity of acquired osteoclasts so far and it is insufficient to carry out biochemical studies. Therefore, relevant report is rare. At present, the existing osteoclast separating methods include: the method of using a compound of Type I collagen gel and phosphorite collagen is adopted for separation; non-adhesive bone marrow cells are excited with RANKL and PGE2V and then put in the culture medium containing M-CSF megacell colony stimulating factor for 24 h to acquire the osteoclasts. Although the osteoclasts acquired by these methods are higher in purity, their absorption functions still need to be further researched.
In this study, 1, 25 (OH) 2 D3 was used to induce differentiation of bone marrow stroma cells to obtain the osteoclasts, which were large in volume, contained more nucleuses and had bubbles of unequal sizes in cytoplasm and microvilli on surfaces. The most characteristic feature of the osteoclasts was: after tartrate-resistant acid phosphatase staining of the cytoplasm, the red insoluble deposits were generated in active element s and bone resorption lacunas were formed simultaneously. In this study, osteoclasts, which were positive in tartrate-resistant acid phosphatase staining, contained more nucleuses and were in pseudopod motion, were separated from limb bones of SD neonatal rat and then planted onto bone sections to form absorption lacunas, which met the standard of appraising the cell and this separation method was feasible accordingly.
Normal and orderly apoptosis of cells is a necessary condition for homoiostasis, growth and development and the programmed cell death under gene control. So, abnormal apoptosis may lead to various diseases. In the occurrence of osteoporosis disease, the apoptosis of osteoclast is of far reaching important: the earlier and more the apoptosis of osteoclast is, the less and shallower the bone resorption become; the later and less the apoptosis of osteoclast is, the more and deeper the bone resorption become, and osteoporosis, bone damage and so on may come into being accordingly [15]. This study indicates that calcitonin can act on osteoclast in the same form of other cell factors and drugs to adjust Fas and their ligands on surfaces and promote the apoptosis of osteoclast. Moreover, it has been verified that osteoclast has a basic apoptosis rate and the calcitonin added into the culture solution can promote the apoptosis of osteoclast, which has time dependence and dosage correlating effect. However, osteoclasts acquired from long bone of neonatal rat is used in this experiment and whether corresponding change may occur in human osteoclast under the action of calcitonin still needs to be verified by further research.
References
- Wang D. The progress of cathepsin K in bone resorption. Prog Vet Med 2014; 35: 114-116.
- Hao Y, Gao K, Wang Y. Effect of calcitonin on the proliferation and apoptosis of osteoclasts. J Clin Rehab Tissue Eng Res 2008; 12: 5410-5413.
- Zhang Y, Niu EL, Li Y. Effects of aspirin on differentiation and maturation and bone resorption of osteoclasts in rats. Chinese J Osteoporosis 2013; 19: 17-22.
- Leung P, Pickarski M, Zhuo Y. The effects of the cathepsin K inhibitor odanacatib on osteoclastic bone resorption and vesicular trafficking. Bone 2011; 49: 623-635.
- Simon LS. Osteoporosis. Rheum Dis Clin North Am 2007; 33: 149-176.
- Guo J, Watterson SH, Spergel SH. Identification and syn-thesis of potent and selective pyridyl-isoxazole based agonists of sphingosine-1-phosphate1(S1P1). Bioorg Med Chem Lett 2016; 26: 2470 -2474.
- Xiong M, Zhu JP, Li L. Effect of adoptive transfer FTY720 - DC on embryonic loss rate in spontaneous abortion model of pregnant rats. J Shanghai Jiaotong University Med Sci 2016; 36: 793-798.
- Li S, Xiang F, Zhang T. Effect of calcitonin on analgesia and expression of calcitonin receptor in perlaqueductal gray. Chinese J Endocrinol Metabol 2011; 27: 113-117.
- Yu H, Herbert BA, Valerio M. FTY720 inhibited proin-flammatory cytokine release and osteoclastogenesis induced by Ag-gregatibacter actinomycetemcomitans. Lipids Health Dis 2015; 14: 66.
- Zhang D, Huang Y, Huang Z. FTY-720P suppresses oste-oclast formation by regulating expression of interleukin-6 (IL-6),interleukin-4 (IL-4),and matrix metalloproteinase 2( MMP-2). Med Sci Monit 2016; 22: 2187-2194.
- Lotinun S, Kiviranta R, Matsubara T. Osteoclast-specific cathepsin K deletion stimulates S1P-dependentbone formation. Cfini Invest 2013; 123: 666-681.
- Xiao Q, Xiong L, Yang Q. Effect and mechanism of calcitonin on osteoporoic fracture healing in rats. Shandong Med J 2010; 50: 25-26.
- Chen B, Chen G, Gong S. Application of calcitonin for the treatment of unstable intertrochanteric fractures in elderly patients. Chinese J Orthopaedics 2014; 34: 24-28.
- You X, Yang H. Changes of cytokines and calcitonin in the pathogenesis of knee osteoarthritis. Guangdong Med J 2012; 33: 306-309.
- Boyce BF, Zuscik MJ, Xing L. Biology of bone and cartilage//Thakker RV, Whyte MP, Eisman J. Genetics of bone biology and skeletal disease. Elsevier Inc, New York, 2013.