ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 10
Experimental study on curcumin inhibiting proliferation and invasion of human osteosarcoma cells
Wang Jun, Chen Peng, Jiang Wen and Gong Mingzhi*
The Second Hospital of Shandong University, Shandong University, PR China
- *Corresponding Author:
- Gong Mingzhi
The Second Hospital of Shandong University
Shandong University, PR China
Accepted date: February 22, 2017
The natural antioxidant curcumin has been proposed for cancer chemoprevention, as it reduces proliferation of several types of cancer. In this study, we investigated anticancer efficacy of curcumin in two human osteosarcoma cell lines, U2OS and MG-63. The results demonstrated that the proliferation of the osteosarcoma cells was significantly inhibited in a time- and concentration-dependent manner when incubated with curcumin. Furthermore, after being treated with curcumin, the level of apoptosis significantly increased, and the expression level of apoptosis-related proteins increased. The invasion properties of the cell lines decreased gradually. MG-63 cells, which have a P53 gene mutation, were more sensitive to curcumin than U2OS cells. Therefore, curcumin has an obvious inhibition effect on the human osteosarcoma cell lines, and the P53 gene may play an important role in this process. Curcumin is a promising antitumor drug for human osteosarcoma treatment.
Keywords
Osteosarcoma, U2OS, MG-63, Curcumin, Apoptosis, Bcl-2, p53.
Introduction
As one of the most common malignant bone tumors, osteosarcoma can be found in approximately 30% of the patients with malignant bone tumors. It is of especially high prevalence in children. Osteosarcoma first peaks in the adolescence stage of human, less prevails among the age group that begins with 65. About 400 patients are diagnosed with osteosarcoma each year in the United States. Osteosarcoma originates in mesenchymal cells, and presents mainly at the tip of long bones, such as the knee, hip and shoulder. The ten-year survival rate of osteosarcoma patients is approximately 60% to 75%. At present, osteosarcoma is usually treated by preoperative chemotherapy, amputation or limb salvage tumor resection and postoperative chemotherapy. Chemotherapy drugs have an obvious lethal effect on both osteosarcoma cells and normal human cells, which may further reduce the survival of osteosarcoma patients due to its side effects [1]. To this end, a new chemotherapy drug is needed that kills more osteosarcoma cells but does less damage to non-cancerous tissues.
Curcumin is a natural product extracted from plants. It is used in China as a traditional medicine to treat a variety of diseases. Curcumin can exert anti-tumor effects by acting on the multiple targets, such as transcription factors, apoptosis genes, and cell signaling molecules [2,3]. Studies have shown that curcumin had clear anti-cancer effect on liver cancer, stomach cancer, pancreatic cancer and other cancers [4,5]. Curcumin has a wide range of pharmacological effects, low toxicity and is well tolerated in humans [6].
This study explores the inhibition effect of different concentrations of curcumin on osteosarcoma U2OS and MG-63 cell lines, and analyses the effect of curcumin on cell proliferation, apoptosis and invasion of U2OS and MG-63 cells, to summarize the anti-cancer effect of curcumin on osteosarcoma.
Materials and Methods
Drugs
Curcumin (Sigma-Aldrich, Inc., St. Louis, MO, USA) was dissolved in DMSO at 20 mM as a stock solution. Dilutions of all reagents were freshly prepared before each experiment.
Cell culture and cell lines
Human osteosarcoma cell lines U2OS and MG-63 were purchased from the cell bank of the Shanghai Institute of Cell Biology, Chinese Academy of Sciences. Cells were cultured in DMEM medium (GIBCO/BRL, Grand Island, NY, USA) supplemented with 100 U/ml penicillin, 100 mg/ml streptomycin and 10% fetal bovine serum. All cells were incubated at 37°C in an atmosphere containing 5% CO2.
U2OS cells were divided into four groups. Group A, the control group, was treated with Phosphate Buffered Saline (PBS); and groups B, C and D were treated with PBS containing 5, 10 and 20 μmol/L curcumin, respectively.
The same method was used to for the study groups of MG-63 cell lines.
Proliferation assay
Human osteosarcoma cell proliferation was evaluated by 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay. All cellular growth assays were performed in 96 well plates (1 × 104 cells/well). MG-63 and U2OS cells were cultured with increasing concentrations of curcumin for 48 and 72 h. Cells were treated with 10 μl MTT (5 mg/ml)/well and incubated for 4 h at 37°C. The formazan dye generated by cellular dehydrogenase activity was dissolved in DMSO and quantified by measurement of absorbance at 490 nm.
Flow cytometric analysis of apoptosis
MG-63 and U2OS cells seeded in 12-well plates (1.5 × 105 cells/well) were treated with increasing concentrations of curcumin for 24 h before harvest. Cells were assessed for apoptosis using an Apoptosis Detection kit (eBioscience, San Diego, CA, USA) according to manufacturer’s protocol. Briefly, cells suspended in binding buffer were incubated with Annexin V-FITC and propidium iodide (Becton Dickinson, Mountain View, CA, USA) for 15 min at room temperature in the dark. Cells were then analyzed by flow cytometry performed on a FACScalibur cell analyser using CellQuest software (Becton Dickinson, Mountain View, CA, USA). Cells undergoing apoptosis were defined as those positive for Annexin V.
Western blotting assay
MG-63 cells were treated with 0-20 μmol/L curcumin for 12 h, and then subjected to western blotting using antibodies against Bcl-2, Bax, cleaved poly (ADP-ribose) polymerase (PARP), caspase-3, and β-actin. At the indicated time, the treated cells were harvested and washed twice with ice-cold PBS (pH 7.4). Cells were then lysed with ice-cold buffer for half an hour on ice, and the lysates were centrifuged at 12000X g at 4°C for 15 min. Protein concentration was determined, and sodium dodecyl sulfate polyacrylamide gel electrophoresis was used to separate equal amounts of protein. The separated proteins were then transferred to polyvinylidene difluoride membrane. The indicated antibodies were used to probe the proteins, followed by appropriate secondary antibodies (Rockland Immunochemicals, Gilbertsville, PA). Detection was carried out with an odyssey infra-red imaging system (LI-COR, Lincoln, NE). Equal loading was confirmed using primary antibodies against β-actin.
Invasion assay
Matrigel (Cat. 356234, BD Biosciences, USA) was diluted 1:19 with serum-free DMEM and 50 ml was used to coat each invasion chamber (Transwell; Corning 8 mm pore size; Corning, NY, U.S.A.). MG-63 and U2OS cells (1 × 105) were treated with increasing concentrations of curcumin for 48 h in the upper chamber, while the lower compartment was filled with 10% fetal bovine serum in DMEM. After 48 h of incubation, the Matrigel on the upper side of the filter was removed. The cells that had migrated through the membrane between the chambers were fixed with methanol and stained with 1 mg/ml crystal violet dye. Images of cells that invaded the lower chamber were obtained under a microscope (IX81, Olympus, Japan).
Measurement of caspase-3 activity
Caspase-3 activity was measured with a caspase-3 colorimetric assay kit (KeyGEN Biotech, Nanjing, China) according to the manufacturer’s instructions. Briefly, cells were harvested and lysed after treatment, then centrifuged at 12000X g for 15 min at 4°C. The supernatant was collected, and a bicinchoninic acid protein assay kit (Pierce, Rockford, IL, USA) was used to measure protein concentration. Supernatant containing 50 μg of total protein was incubated with caspase substrate at 37°C for 4 h in the dark. Absorbance at 405 nm was assessed using a microplate reader. Caspase-3 activity was calculated as the Optical Density (OD) in curcumin-treated cells as a percentage of the OD in control cells.
Statistical analyses
Results
Curcumin suppresses MG-63 and U2OS cell proliferation
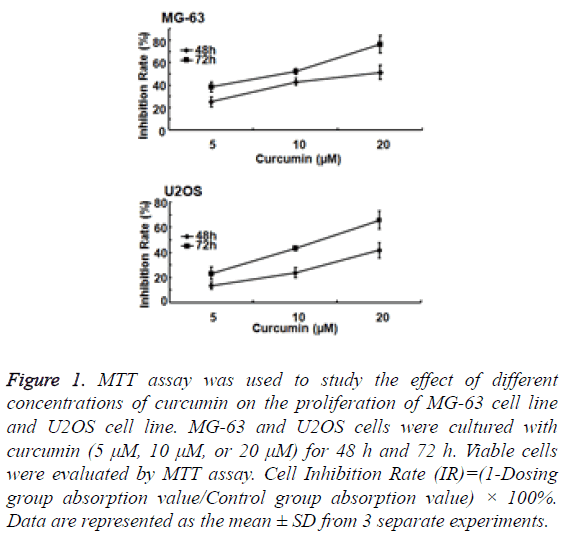
To study the effect of curcumin on osteosarcoma cell proliferation, MG-63 and U2OS cells were cultured with curcumin (5 μM, 10 μM, or 20 μM) for 48 h and 72 h. MG-63 and U2OS cell proliferation was significantly inhibited by curcumin. The degree of inhibition was concentration dependent (Figure 1).
Figure 1: MTT assay was used to study the effect of different concentrations of curcumin on the proliferation of MG-63 cell line and U2OS cell line. MG-63 and U2OS cells were cultured with curcumin (5 μM, 10 μM, or 20 μM) for 48 h and 72 h. Viable cells were evaluated by MTT assay. Cell Inhibition Rate (IR)=(1-Dosing group absorption value/Control group absorption value) × 100%. Data are represented as the mean ± SD from 3 separate experiments.
MG-63 cell line was more sensitive to curcumin than U2OS cell line. With the same action time and under the same concentration of curcumin, MG-63 cell line was more inhibited than U2OS cells, and the difference was statistically significant (P<0.05). Treated with 20 μM curcumin for 72 h, the inhibitory rate of MG-63 cell line could reach to 80%.
The degree of inhibition of MG-63 and U2OS cell proliferation also significantly correlated with the duration of curcumin treatment. The inhibitory effect of curcumin in the 72 h group was significantly greater than that in the 48 h group (P<0.01).
Curcumin induces MG-63 and U2OS cell apoptosis
Many studies have shown that curcumin not only inhibits the proliferation of tumor cells, but also promotes tumor cell apoptosis. To verify the promoting effect of curcumin on apoptosis of human osteosarcoma cell lines, flow cytometry was used to evaluate apoptosis of MG-63 and U2OS cells.
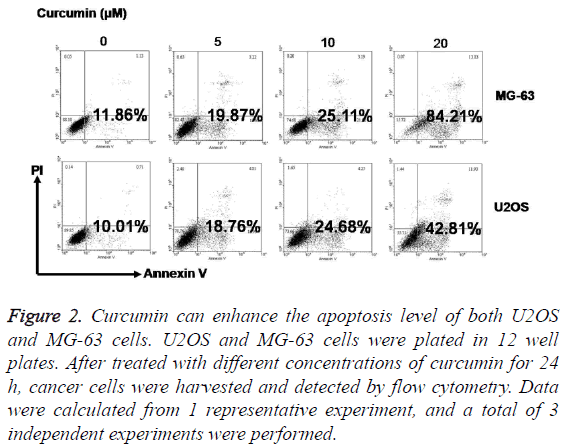
After treatment with 5 μM, 10 μM, and 20 μM curcumin for 24 h, the rate of apoptosis in MG-63 and U2OS cells increased significantly. Furthermore, the increase in apoptosis was positively correlated with the curcumin concentration (Figure 2).
Figure 2: Curcumin can enhance the apoptosis level of both U2OS and MG-63 cells. U2OS and MG-63 cells were plated in 12 well plates. After treated with different concentrations of curcumin for 24 h, cancer cells were harvested and detected by flow cytometry. Data were calculated from 1 representative experiment, and a total of 3 independent experiments were performed.
The differences in apoptosis rates between MG-63 and U2OS cells were significant. After treatment with 20 μM curcumin for 24 h, the MG-63 cell apoptosis rate was approximately 84.21%, compared to 42.81% in the U2OS cell group (P<0.01).
Effect of curcumin on caspase-3, Bcl2, and Bax protein expression levels
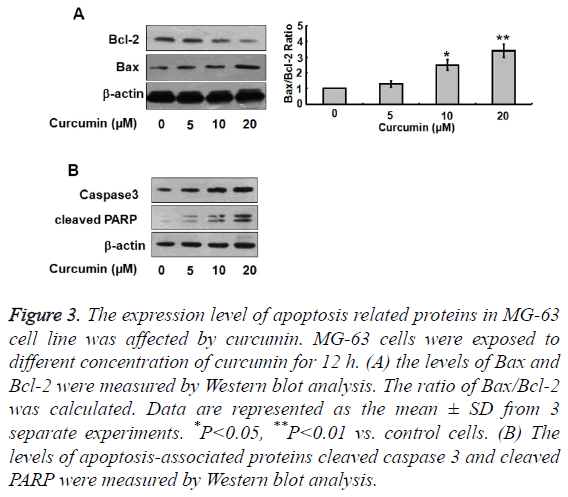
To further investigate the effect of curcumin on apoptosis in osteosarcoma cells, we used western blotting to detect Bcl-2, Bax, caspase-3, and cleaved PARP protein expression levels after treatment of cells with different concentrations of curcumin. Bcl-2 expression levels decreased significantly with increasing curcumin concentration, while Bax, caspase-3, and cleaved PARP expression levels increased with increasing curcumin concentration (Figure 3). The proportion of Bax/ Bcl-2 therefore increased with increasing curcumin concentration.
Figure 3: The expression level of apoptosis related proteins in MG-63 cell line was affected by curcumin. MG-63 cells were exposed to different concentration of curcumin for 12 h. (A) the levels of Bax and Bcl-2 were measured by Western blot analysis. The ratio of Bax/Bcl-2 was calculated. Data are represented as the mean ± SD from 3 separate experiments. *P<0.05, **P<0.01 vs. control cells. (B) The levels of apoptosis-associated proteins cleaved caspase 3 and cleaved PARP were measured by Western blot analysis.
Effect of curcumin on MG-63 and U2OS cell invasion ability
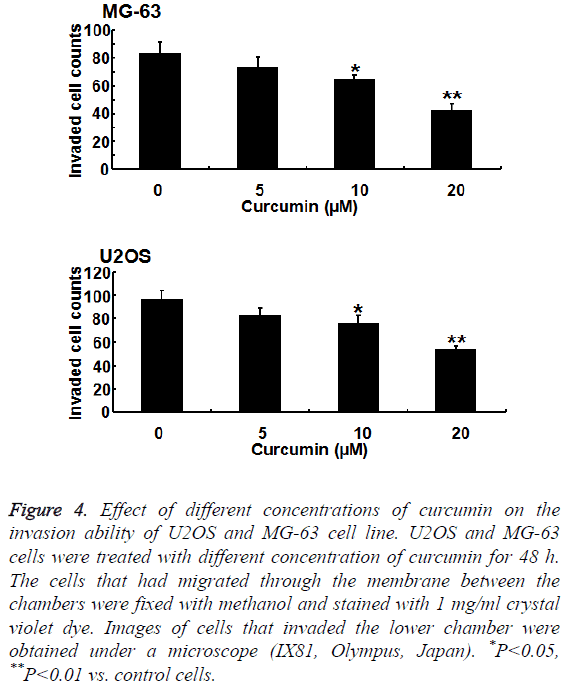
To further verify the effect of curcumin on osteosarcoma cell invasion ability, we studied the effect of curcumin on MG-63 and U2OS cells using a Transwell tumor invasion assay. The invasion ability of MG-63 and U2OS cells decreased significantly with increasing curcumin concentration. The inhibition of MG-63 cell line by curcumin was more significant than that of U2OS cell line (P<0.01) (Figure 4).
Figure 4: Effect of different concentrations of curcumin on the invasion ability of U2OS and MG-63 cell line. U2OS and MG-63 cells were treated with different concentration of curcumin for 48 h. The cells that had migrated through the membrane between the chambers were fixed with methanol and stained with 1 mg/ml crystal violet dye. Images of cells that invaded the lower chamber were obtained under a microscope (IX81, Olympus, Japan). *P<0.05, **P<0.01 vs. control cells.
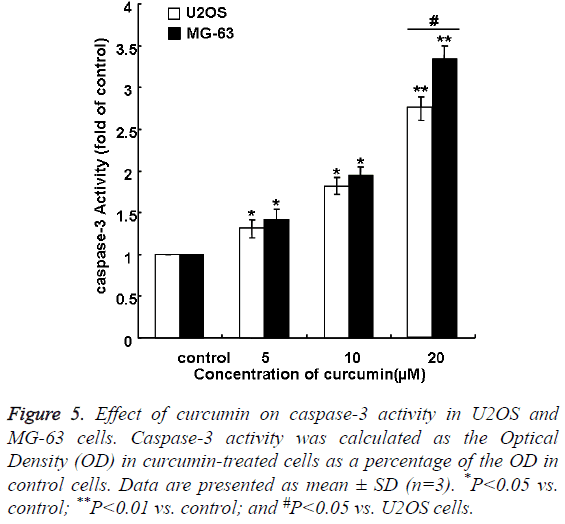
Analysis of caspase-3 activation in MG-63 and U2OS cells
Curcumin induced caspase-3 activation in a concentrationdependent manner in MG-63 and U2OS cells (Figure 5). The effect of curcumin in MG-63 group was larger than that in U2OS group. Caspase-3 activation increased to 3.34 fold of control levels on treatment with 20 μM curcumin in MG-63 group, compared with 2.75 fold of control levels on treatment with 20 μM curcumin in U2OS group (P<0.01).
Figure 5: Effect of curcumin on caspase-3 activity in U2OS and MG-63 cells. Caspase-3 activity was calculated as the Optical Density (OD) in curcumin-treated cells as a percentage of the OD in control cells. Data are presented as mean ± SD (n=3). *P<0.05 vs. control; **P<0.01 vs. control; and #P<0.05 vs. U2OS cells.
Discussion
Higher concentrations of curcumin showed higher rates of inhibition on proliferation of human osteosarcoma MG-63 and U2OS cell lines. Cell apoptosis levels and expression of apoptosis-related protein increased, and the invasion ability of the cell lines gradually decreased.
MG-63 cell is a human osteosarcoma cell line with a P53 gene mutation and is quite different from the U2OS cell line. The P53 gene is a tumor suppressor gene that can prevent cells from entering the S phase from the G1 phase, and can induce apoptosis of the cells. When the P53 gene has a mutation, the cell can lose the ability to monitor cell growth, differentiation and apoptosis, and can no longer repair damaged DNA. As a result, cancer is able to progress [7,8]. Because they have different genes, the signal transduction pathways of the MG-63 cell line and U2OS cell lines are different, which leads to different anti-apoptosis abilities of the cells. The degree of malignancy of the MG-63 cell line is significantly higher than that of the U2OS cell line. There are great differences in cell size, morphology, proliferation and migration ability of the two cell lines, which results in different types of osteosarcoma. MG-63 is the most commonly reported cell line. Because of the great differences in the biological behavior of different osteosarcoma cell lines, it is difficult to obtain the convincing results if only MG-63 cell line is studied. Therefore, we choose to use both the MG-63 and U2OS cell lines in our experiment to study the different reactions to curcumin.
According to our results, the MG-63 cell line is more sensitive to curcumin than the U2OS cell line is. This suggests that the P53 gene plays an important role in curcumin sensitivity, as the P53 gene is the main difference between the two cell lines. Our experiment shows the cell signal transduction pathway mediated by P53 is very important in the effect of curcumin on osteosarcoma, however, this is not the only signal transduction pathway. Curcumin inhibits osteosarcoma through multiple signaling pathways.
From 1974 to 1994, many chemotherapy drugs have been used to treat tumors. During this time, the survival rates of cancer patients have significantly improved and the local recurrence rate and metastasis rate has been reduced. However, the survival rate of osteosarcoma patients has not improved greatly in the last 20 years. According to a report by the US National Cancer Institute, the survival rate of osteosarcoma patients less than 45 years old was about 65% from 1975 to 2000. In osteosarcoma patients without metastasis, the 5-year survival rate increased to 70%, with surgical therapy combined with active adjuvant chemotherapy [9]. However, the 5-year survival rate was low for those with poor sensitivity to chemotherapy drugs [10]. Therefore, it is necessary to find a new, safer and more specific chemotherapy drug.
Curcumin has an anti-cancer effect on a variety of cancers. Curcumin can affect gene expression, transcription, proliferation and synthesis of the extracellular matrix, affecting a variety of molecular and cellular pathways [11,12]. Furthermore, curcumin can improve expression of tumor suppressor genes in tumor cell lines, including in osteosarcoma [13-15].
Bcl-2 family plays an important role in regulating cell apoptosis, mainly in promoting cell apoptosis and inhibiting cell apoptosis. Bcl-2 and Bax are the representative proteins for inhibiting cell apoptosis and promoting cell apoptosis, respectively. In osteosarcoma cell lines, Bcl-2 expression increased, and Bax expression decreased. Increasing Bcl-2 expression or decreasing Bax expression can inhibit tumor cell apoptosis; otherwise, they can promote cell apoptosis. If the Bcl-2/Bax ratio increases, it can obviously inhibit the occurrence and development of tumor [16].
Western Blot method showed that with the increase of curcumin concentration, Bax expression obviously increased, but Bcl-2 expression gradually decreased, and Bax/Bcl-2 ratio increased with the rise of curcumin concentration. So when the curcumin concentration gradually increased from 5 μmol/L to 20 μmol/L, the inhibition on osteosarcoma cells gradually improved.
PARP is a DNA repair enzyme. Caspase is closely related to the eukaryotic cells, and involved in the regulation of cell apoptosis; while cleaved PARP is the common cleaved substrate of various caspases and is an important apoptosis marker. In many cell lines, cleaved PARP can be used as a tool for the detection of apoptosis [17].
Caspase 3 is the effect enzyme in caspase family that implements cell apoptosis. Activated Caspase3 can result in cell apoptosis by multiple signaling pathways. Caspase 3 expression level can indicate cell apoptosis level [18].
Western Blot method showed that with increase of curcumin concentration, expression of cleaved PARP and Caspase3 obviously increased. So, it was suggested that tumor cell apoptosis level increased with the rise of curcumin concentration. Caspase-3 activity was significantly promoted by curcumin, which was consistent with the changes in caspase-3 protein levels.
Our experiment showed that the proliferation of osteosarcoma cell lines is significantly inhibited by curcumin, while the apoptosis is promoted, although the differences between the MG-63 and U2OS cell lines cannot be ignored. The inhibition effect of curcumin on osteosarcoma is in a concentration- and time-dependent manner. The differences are statistically significant. The Transwell tumor invasion assay showed that the invasion ability of osteosarcoma cell lines can also be inhibited by curcumin.
Previous studies have shown the effect of curcumin on osteosarcoma cells. However, our study is the first to find out differences in curcumin-induced cytotoxicity between p53 positive and negative osteosarcoma cells and the first to discuss the cause of the differences. We believe our studies benefit future studies and are of value for scientific community. More studies are needed to show the differences between several cell lines of osteosarcoma [19-21].
Curcumin is a widely used drug, and has a high metabolic rate. Curcumin is also insoluble in water, but soluble in organic solvents. When pH is greater than 7.2, curcumin is degraded. In human experiments, a very low concentration of curcumin was detected in human plasma after oral administration [22]. Even after oral absorption, curcumin can be degraded into glucuronide and sulfate after metabolism in the liver [23]. Therefore, further studies should be taken to find appropriate carriers of curcumin that are soluble in water and avoid degradation so that curcumin is released into the target region [21].
Acknowledgements
This study was supported by a grant from the Youth Foundation of the Second Hospital of Shandong University (No.Y2013010054).
References
- McTiernan A, Jinks RC, Sydes MR, Uscinska B, Hook JM. Presence of chemotherapy-induced toxicity predicts improved survival in patients with localised extremity osteosarcoma treated with doxorubicin and cisplatin: a report from the European Osteosarcoma Intergroup. Eur J Cancer 2012; 48: 703-712.
- Guo J, Li W, Shi H, Xie X, Li L. Synergistic effects of curcumin with emodin against the proliferation and invasion of breast cancer cells through upregulation of miR-34a. Mol Cell Biochem 2013; 382: 103-111.
- Zhang X, Chen Q, Wang Y, Peng W, Cai H. Effects of curcumin on ion channels and transporters. Front Physiol 2014; 5: 94.
- Xu X, Qin J, Liu W. Curcumin inhibits the invasion of thyroid cancer cells via down-regulation of PI3K/Akt signaling pathway. Gene 2014; 546: 226-232.
- Lim TG, Lee SY, Huang Z, Lim DY, Chen H. Curcumin suppresses proliferation of colon cancer cells by targeting CDK2. Cancer Prev Res (Phila) 2014; 7: 466-474.
- Guo Y, Shu L, Zhang C, Su ZY, Kong AN. Curcumin inhibits anchorage-independent growth of HT29 human colon cancer cells by targeting epigenetic restoration of the tumor suppressor gene DLEC1. Biochem Pharmacol 2015; 94: 69-78.
- Tullo A, DErchia AM, Sbisà E. Methods for screening tumors for p53 status and therapeutic exploitation. Expert Rev Mol Diagn 2003; 3: 289-301.
- Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature 2000; 408: 307-310.
- Endo-Munoz L, Cumming A, Sommerville S, Dickinson I, Saunders NA. Osteosarcoma is characterised by reduced expression of markers of osteoclastogenesis and antigen presentation compared with normal bone. Br J Cancer 2010; 103: 73-81.
- Moriceau G, Roelofs AJ, Brion R, Redini F, Ebetion FH. Synergistic inhibitory effect of apomine and lovastatin on osteosarcoma cell growth. Cancer 2012; 118: 750-760.
- Strimpakos AS, Sharma RA. Curcumin: preventive and therapeutic properties in laboratory studies and clinical trials. Antioxid Redox Signal 2008; 10: 511-545.
- Wilken R, Veena MS, Wang MB, Srivatsan ES. Curcumin: A review of anti-cancer properties and therapeutic activity in head and neck squamous cell carcinoma. Mol Cancer 2011; 10: 12.
- Choudhuri T, Pal S, Das T, Sa G. Curcumin selectively induces apoptosis in deregulated cyclin D1-expressed cells at G2 phase of cell cycle in a p53-dependent manner. J Biol Chem 2005; 280: 20059-20068.
- Liu E, Wu J, Cao W, Zhang J, Liu W. Curcumin induces G2/M cell cycle arrest in a p53-dependent manner and upregulates ING4 expression in human glioma. J Neurooncol 2007; 85: 263-270.
- Collins HM, Abdelghany MK, Messmer M, Yue B, Deeves SE. Differential effects of garcinol and curcumin on histone and p53 modifications in tumour cells. BMC Cancer 2013; 13: 37.
- Hu W, Xiao Z. Formononetin Induces Apoptosis of Human Osteosarcoma Cell Line U2OS by Regulating the Expression of Bcl-2, Bax and MiR-375 in vitro and in vivo. Cell Physiol Biochem 2015; 37: 933-939.
- Bressenot A, Marchal S, Bezdetnaya L, Garrier J, Guillemin F. Assessment of apoptosis by immunohistochemistry to active caspase-3, active caspase-7, or cleaved PARP in monolayer cells and spheroid and subcutaneous xenografts of human carcinoma. J Histochem Cytochem 2009; 57: 289-300.
- Ni B, Bai FF, Wei Y, Liu MJ, Feng ZX, Xiong QY. Apoptosis induced by lipid-associated membrane proteins from Mycoplasma hyopneumoniae in a porcine lung epithelial cell line with the involvement of caspase 3 and the MAPK pathway. Genet Mol Res 2015; 14: 11429-11443.
- Moran JM, Rodriguez-Velasco FJ, Roncero-Martin R, Vera V, Pedrera-Zamorano JD. Cytotoxic effects of curcumin in osteosarcoma cells. Int J Nanomedicine 2014; 9: 5273-5275.
- Dhule SS, Penfornis P, He J, Harris MR, Terry T, John V. The combined effect of encapsulating curcumin and c6 ceramide in liposomal nanoparticles against osteosarcoma. Mol Pharm 2014; 11: 417-427.
- Run C, Linlin S, Thomas JW. Selective inhibition of MG-63 osteosarcoma cell proliferation induced by curcumin-loaded self-assembled arginine-rich-RGD nanospheres. Int J Nanomedicine 2015; 10: 3351-3365.
- Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm 2007; 4: 807-818.
- Kumar A, Ahuja A, Ali J, Baboota S. Conundrum and therapeutic potential of curcumin in drug delivery. Crit Rev Ther Drug Carrier Syst 2010; 27: 279-312.