ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2016) Volume 27, Issue 4
Evaluation of the Bilichek transcutaneous bilirubinometer in the Chinese new-borns
Department of Neonatology, Children’s Hospital, School of Medicine, Zhejiang University, Hangzhou, PR China
- *Corresponding Author:
- Canyang Zhan
Department of Neonatology
School of Medicine Zhejiang
University PR
China
Accepted date: April 26, 2016
Objective: To evaluate the relation between transcutaneous bilirubin (TcB) by BiliChek and total serum bilirubin (TSB) in the Chinese neonates compared with another transcutaneous Bilirubinometer (VanHou).
Methods: TcB measurements were performed on the forehead by Bilichek and VanHou. Total serum bilirubin (TSB) was determined within 5 minutes after TcB measurement. The correlation and agreement between the two devices and TSB were assessed.
Results: A total of 172 babies were recruited. The mean TSB (± SD) was 11.1 ± 4.6 mg/dl, and the mean TcB (± SD) was respectively 13.4 ± 3.5 mg/dl (VanHou), 12.3 ± 4.5 mg/dl (Bilichek). When the TSB was lower than 9 mg/dl, the difference of VanHou TcB and TSB was much higher than that of Bilichek (3.51 vs. 1.28 mg/dl, p<0.01). When the TSB was 9 ~ 12 mg/dl, the bias of TcB and TSB was 2.31 mg/dl (VanHou) vs. 1.39 mg/dl (Bilichek) (p<0.01). The correlation between the TcB (Bilichek) and TSB was a little higher than the VanHou (0.94 vs. 0.92). Bilichek had a greater area under the ROC curve than VanHou when a threshold value of TSB was chosen at 9, 12 or 15 mg/dl. The Bland-Altman analysis demonstrated that TcB tended to overestimate the TSB, and compared with VanHou, Bilichek had a better agreement with the TSB.
Conclusions: Bilichek has a good correlation with TSB. Furthermore, when TSB was lower than 12 mg/dl, Bilichek is superior to the VanHou obviously.
Keywords
Bilichek, Total serum bilirubin, Transcutaneous bilirubin, Neonatal jaundice.
Abbreviations
TcB: Transcutaneous Bilirubin; TSB: Total Serum Bilirubin; AAP: American Academy Of Paediatrics; ROC: Receiver Operating Curve.
Introduction
Jaundice is one of the most common symptoms during the neonatal period globally, which is reported to be more prevalent in Asian babies [1]. Usually, visual inspection, transcutaneous bilirubin (TcB) meter and the total serum bilirubin (TSB) are used to monitor jaundice in neonates. The determination of TSB levels is an invasive, costly and timeconsuming work. Moreover, repeated blood samplings may lead to significant blood loss, especially for the preterm infants. On the contrary, the TcB measurement is non-invasive, easy and rapid. It is reported that TcB reduced the need for blood sampling in neonates with visible jaundice [2]. TcB could also decrease the readmission rate for hyperbilirubinemia [3,4]. In previous studies, TcB has been shown a good relationship with serum bilirubin level in various ethnic neonates [5-7]. Bilichek, as a new second generation transcutaneous bilirubin meter, can distinguish the light absorption of bilirubin from haemoglobin and melanin by means of spectral subtraction theory. Hemmati et al. showed a strong correlation between Bilichek and TSB in the South of Iran (r=0.969, r2=0.94), independently of sex, gestational age, postnatal age, and birth weight [8]. They also found that this device tended to underestimate the level of bilirubin when TSB>15 mg/dl. Raimondi et al. concluded that Bilicheck, compared with BiliMed, was a reliable screening tool for hyperbilirubinemia in the multiracial neonatal population [9]. The aim of our study was to determine the accuracy and agreement of Bilichek with serum bilirubin concentration in Chinese new-born infants.
Materials and Methods
Patient selection
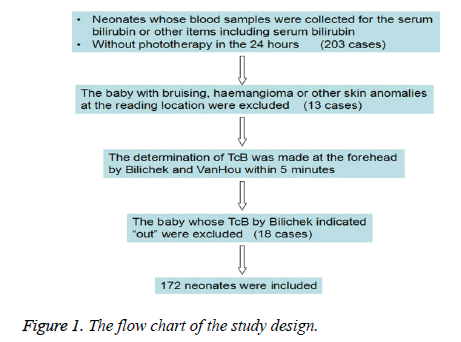
The study was conducted in the neonatal department at the Children's Hospital Zhejiang University School of Medicine. The study was approved by the local institutional review board. The inclusion criterion was the baby with the serum sample for the serum bilirubin or other items including serum bilirubin and no phototherapy in the 24 hours. Two hundred and three babies were consecutively enrolled from June to August 2014. The exclusion criteria were bruising, haemangioma or other skin anomalies at the reading location (13 cases) and indicating “out” by Bilichek (18 cases). Finally, 172 neonates were included (Figure 1).
Measurement of TcB and TSB
The determination of TcB was made at the forehead with the two instruments within 5 minutes, according to the manufacturer instructions. Bilichek (Children’s Medical Ventures, LLC, Monroeville, PA, USA) was calibrated before each measurement using a BiliCal™, and each measurement was performed with two readings in different points of neonatal forehead. VanHou (Vanhou Medical Equipment Ltd, Wuhan, China) was used on the forehead performing two readings too. Each device was used by one neonatologist unaware of the other one’s results and the TSB level. The blood sample for measurement was obtained by radial artery puncture, within 5 minutes after TcB measurement. The TSB was assayed with direct spectrophotometer after centrifugation.
Statistical analysis
The mean of the transcutaneous bilirubin was compared with the serum bilirubin concentration. The relationship between transcutaneous and serum bilirubin values was determined using linear regression analysis. The strength of this relationship was quantified by the Pearson correlation coefficients. The agreement between TcB and TSB was assessed by the method of Bland-Altman. Furthermore, receiver operating curve (ROC) was used to assess the predictive ability of the two studied devices. Excel (Microsoft Office Professional 2003) and SPSS Statistics 17.0 were used for statistical calculations and graphs.
Results
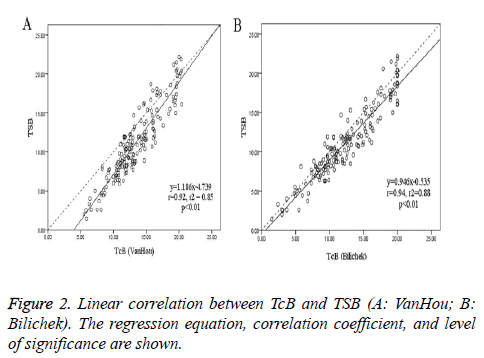
A total of 172 new born babies (134 terms and 38 preterm) were enrolled in the study. The patient characteristics were shown in Table 1. The mean TSB was 11.1 ± 4.6 mg/dl, and the mean TcB (± SD) was respectively 13.4 ± 3.5 mg/dl (VanHou), 12.3 ± 4.5 mg/dl (Bilichek). When the TSB was lower than 9 mg/dl, the difference of VanHou TcB and TSB was much higher than that of Bilichek (3.51 vs. 1.28 mg/dl, p<0.01). When the TSB was 9 ~ 12 mg/dl, the bias of TcB and TSB was 2.31 mg/dl (VanHou) vs. 1.39 mg/dl (Bilichek) (p<0.01). Figure 2 showed the relationship between TcB and TSB: y (TSB) = 1.186x (VanHou) - 4.739, r2=0.85, p<0.01; y (TSB) = 0.946x (Bilichek) - 0.535, r2=0.88, p<0.01. And the Pearson’s coefficient for BiliChek (0.94) was a little higher than VanHou (0.92).
| Variables | Mean ± SD | Median | Range |
|---|---|---|---|
| Gestational age (weeks) | 37.7 ± 2.3 | 38 | 31-42 |
| Birth weight (kg) | 3.0 ± 0.6 | 3 | 1.6-4.5 |
| Postnatal age (d) | 9.5 ± 6.9 | 8 | 0.1-32 |
| TcB- VanHou (mg/dl) | 13.4 ± 3.5 | 12.9 | 5.5-20.4 |
| TcB- Bilichek (mg/dl) | 12.3 ± 4.5 | 12.0 | 1.5-20 |
| TSB (mg/dl) | 11.1 ± 4.6 | 10.7 | 1.4-22.2 |
| TcB: Transcutaneous Bilirubin; TSB: Total Serum Bilirubin | |||
Table 1: Characteristics of the study population
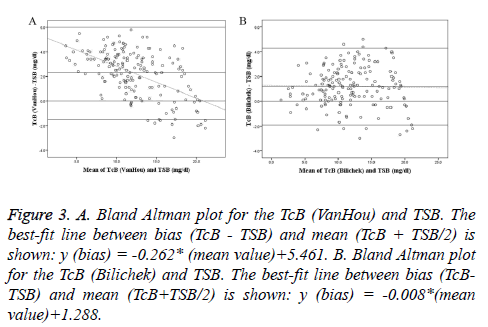
Bland-Altman plots of TcB vs. TSB were shown with regression line in Figure 3. At low bilirubin concentrations, TcB (VanHou) was higher than TSB; whereas at higher bilirubin concentrations, it tended to be lower than TSB. But the difference of the TcB (Bilichek) and TSB was relatively constant over the range of TSB. Mean of the differences and 95% agreement limits of the Bland-Altman bias plot were shown in Table 3. The mean differences between TcB and TSB (VanHou 2.3 mg/dl; BiliChek 1.2 mg/dl) could be considered clinically small. However, 95% agreement limits of the Bland- Altman bias plot showed a relatively large range, especially for the VanHou (-1.5 ~ 6.0 mg/dl).
Figure 3: A. Bland Altman plot for the TcB (VanHou) and TSB. The best-fit line between bias (TcB - TSB) and mean (TcB + TSB/2) is shown: y (bias) = -0.262* (mean value)+5.461. B. Bland Altman plot for the TcB (Bilichek) and TSB. The best-fit line between bias (TcBTSB) and mean (TcB+TSB/2) is shown: y (bias) = -0.008*(mean value)+1.288.
| SB (mg/dl) | Bias of TcB and TSB (mg/dl) | P value | |
|---|---|---|---|
| VanHou | Bilichek | ||
| ≤ 9 | 3.51 | 1.28* | <0.01 |
| 9 ~ 12 | 2.31 | 1.39* | <0.01 |
| 12 ~ 15 | 1.91 | 1.71 | 0.57 |
| >15 | 0.11 | 0.49 | 0.11 |
| TcB: Transcutaneous Bilirubin; TSB: Total Serum Bilirubin *: P<0.01, By T-Test. |
|||
Table 2: The difference of TcB and TSB.
| Bias of TcB and TSB (mg/dl) | Mean | Lower bound | Upper bound |
| VanHou | 2.3 | -1.5 | 6.0 |
| Bilichek | 1.2 | -1.9 | 4.3 |
| TcB: Transcutaneous Bilirubin; TSB: Total Serum Bilirubin | |||
Table 3: Mean of the Differences and 95% Agreement Limits of Bland- Altman Bias Plot.
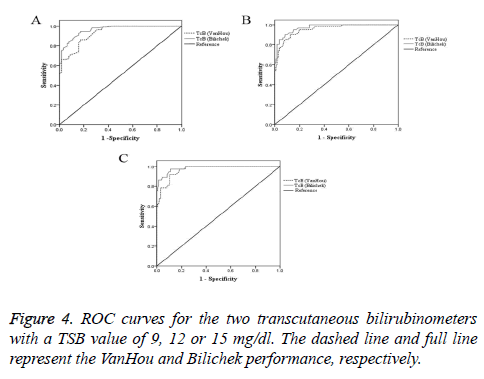
Figure 4 reported ROC curves for the two transcutaneous bilirubin meters with the TSB value of 9, 12 or 15 mg/dl. The areas under the curve and the sensitivity, specificity of the TcB at various clinically relevant cut-off points were showed in the Table 4. At cut-off points of TSB (9, 12 or 15 mg/dl), Bilichek performed better than VanHou.
| TSB level (mg/dl) | Area | Cut-off TcB (mg/dl) | Sensitivity (%) | Specificity (%) | |
|---|---|---|---|---|---|
| 9 | VanHou | 0.931 | 12.05 | 86.9 | 83.1 |
| Bilichek | 0.963 | 10.05 | 94.4 | 82.8 | |
| 12 | VanHou | 0.955 | 13.85 | 90.2 | 87.4 |
| Bilichek | 0.973 | 13.25 | 90.2 | 92.0 | |
| 15 | VanHou | 0.966 | 15.15 | 91.9 | 89.6 |
| Bilichek | 0.984 | 15.05 | 97.3 | 88.9 | |
| TcB: Transcutaneous Bilirubin; TSB: Total Serum Bilirubin | |||||
Table 4: Sensitivity, specificity, and area of the chosen cut off TcB.
Discussion
There were a large number of studies about the evaluation of Bilichek on the hyperbilirubinemia. Bilichek was regarded as a sensitive screening method for hyperbilirubinemia in term or preterm new born [10-12]. However, there were few studies about Bilichek in Chinese population, especially for large sample study. In our study, we provided the comparison of Bilichek and VanHou (one kind of transcutaneous bilirubin meters used in China commonly) in the Chinese new-borns.
The relation between TcB and TSB
The accuracy of TcB has been assessed in neonatal jaundice from various ethnic backgrounds. Most of the studies found that the Bilichek had a strong correlation with TSB (r=0.91), multiracial population, by Bhutani et al. [13]; r=0.969, Iranian, by Hemmati et al. [8]; r=0.83, multi-ethnic population, by Campbell et al. [14]; r=0.86, multiracial population, by Raimondi et al. [9]; r=0.85, Turkish, by Kaynak-Turkmen et al. [15]; r=0.95, Thai, by Janjindamai et al. [16]. However, Neocleous et al. reported that TcB (Bilichek) and TSB values did not correlate well and TcB values often proved imprecise in predicting actual TSB levels (r=0.439, Greek, 2014) [17].
In our study, the correlation of TcB derived from BiliChek and VanHou with serum bilirubin were 0.94 and 0.92 respectively. This result was better than another Chinese study of Bilichek by Ho EY et al., which reported a coefficient of 0.757 for forehead measurements, and 0.794 for sternum [18]. Moreover, the mean differences between TcB and TSB were VanHou 2.3 mg/dl, BiliChek 1.2 mg/dl respectively. So it is considered that both of Bilichek and VanHou are reliable screening tools for hyperbilirubinemia in Chinese population.
Looking at the Table 2 and Bland Altman plots, most of the TcB from both of two devices was higher than TSB. It was contrary to the results by the study of Conceicao et al. [19]. They found the mean TcB was lower than the TSB (8.6 vs. 10.2, p<0.05). In our study, the difference between TcB and TSB became smaller while the TSB was higher. Especially, when TSB>15 mg/dl, the TcB had a tendency to underestimation the bilirubin level. Hemmati et al. also found that TcB tended to underestimate the level of bilirubin when TSB>15 mg/dl [3]. Therefore, we should take the blood sample for TSB when the jaundice is severe.
The Comparison of Bilichek and Vanhou
In our study, we showed that both of Bilichek and VanHou had a good relation with TSB in Chinese new-borns. However, there appeared to be some deficiencies as well. Look at the Tables 1 and 2, the mean TcB concentration was 13.4 mg/dl (VanHou), 12.3 mg/dl (Bilichek) while mean TSB bilirubin level was 11.1 mg/dl. TcB (Bilichek) was much closer to the TSB than TcB (VanHou). Especially, when TSB was lower than 12 mg/dl, the bias of TcB and TSB from the VanHou was much higher than Bilichek, which might result more unnecessary phototherapy. Likewise, the figures of the Bland- Altman Blot also shown that the VanHou, compared to the Bilichek, tended to be more overestimated when the lower serum bilirubin level. The data from ROC showed a similar result. When the TSB value was set at 9 mg/dl, use of a cut-off point of 12.05 mg/dl (VanHou) and 10.05 mg/dl (Bilichek), the sensitivities and specificities were 86.9% and 83.1% for VanHou, 94.4% and 82.8% for Bilichek. The sensitivity of VanHou was much lower than Bilichek. When the TSB value was set at 12 mg/dl, use of a cut-off point of 13.85 mg/dl (VanHou) and 13.25 mg/dl (Bilichek), the sensitivities and specificities were 90.2% and 87.4% for VanHou, 90.2% and 92% for Bilichek. Bilichek had a more satisfactory specificity than VanHou.
The transcutaneous Bilirubinometer should be sensitive to prevent the kernicterus. Furthermore, the device must also have a desirable level of specificity because over diagnosis leads to unnecessary admissions and unexpected complications. The conventional phototherapy was suggested when the bilirubin level got to 9 mg/dl for the neonates in the first 24 hours after birth, and 12 mg/dl for the neonates in the first 48 hours after birth, according to the guidelines for the management of hyperbilirubinaemia in new-borns by American Academy of Paediatrics (AAP) Subcommittee on Hyperbilirubinemia [20]. A small difference between TcB and TSB might change the medical plan. As a result, we suggested that, in Chinese baby, Bilichek was more accurate than VanHou because of fewer false positive, especially when the TSB was lower than 12 mg/dl. However, the Bilichek was calibrated with BiliCal before every measurement and took the average of 5 readings. As a result, the Bilichek was more time consuming and much more expensive than the VanHou.
Limitation of the study
In this study, the sample size of the severe jaundice was small. It is reported that the good correlation between TcB and TSB might be related with the large number of relatively low bilirubin level [17,21]. Furthermore, the sample size of the preterm was small. We have found that when TSB ≤ 12 mg/dl, the bias of TcB and TSB from the VanHou was much higher than Bilichek. This range of bilirubin level has great clinical importance in the preterm. So, we should carry out another study in preterm in the future.
Conclusion
The findings of the present study indicate that the Bilichek has a good correlation with TSB. Furthermore, when the bilirubin levels ≤ 12 mg/dl Bilichek is superior to the VanHou obviously. Finally, for the neonates with TSB>15 mg/dl, both of the two devices tend to underestimate the level of bilirubin.
Acknowledgements
This study was supported by Grants from National Natural Science Foundation of China (81401235).
References
- Ho NK. Neonatal Jaundice In Asia. Baillieres Clin Haematol 1992; 5: 131-142.
- Mishra S, Chawla D, Agarwal R, Deorari AK, Paul VK, BhutaniVK. Transcutaneous Bilirubinometry Reduces The Need For Blood Sampling In Neonates With Visible Jaundice. Acta Paediatr 2009; 98: 1916-1919.
- Peterson JR, Okorodudu AO, Mohammad AA, Fernando A, Shattuck KE. Association Of Transcutaneous Bilirubin Testing In Hospital With Decreased Readmission Rate For Hyperbilirubinemia. Clin Chem 2005; 51: 540-544.
- Stevenson DK, Wong RJ, Vreman HJ. Reduction In Hospital Readmission Rates For Hyperbilirubinemia Is Associated With Use Of Transcutaneous Bilirubin Measurements. Clin Chem 2005; 5: 481-482.
- El-Beshbishi SN, Shattuck KE, Mohammad AA, Petersen JR. Hyperbilirubinemia and Transcutaneous Bilirubinometry. Clin Chem 2009; 55: 1280-1287.
- Ho HT, Ng TK, Tsui KC, Lo YC. Evaluation Of New Transcutaneous Bilirubinometer In Chinese New-borns. Arch Dis Child Foetal Neonatal Ed 2006; 91: F434-438.
- Mahajan G, Kaushal RK, Sankhyan N, Sharma RL, Nakra M. Transcutaneous Bilirubinometer In Assessment of Neonatal Jaundice In Northern India. Indian Pediatr 2005; 42: 41-45.
- Hemmati F, Kiyani Rad NA. The Value of Bilicheck® As A Screening Tool For Neonatal Jaundice In The South Of Iran. Iran J Med Sci 2013; 38: 122-128.
- Raimondi F, Lama S, Landolfo F, Sellitto M, Borrelli AC, Maffucci R, Milite P, Capasso L. Measuring Transcutaneous Bilirubin: A Comparative Analysis Of Three Devices On A Multiracial Population. BMC Pediatr 2012; 12: 70.
- Badiee Z, Mohammadizadeh M, Shamee M. Diagnostic Usefulness Of Transcutaneous Bilirubinometry In Very Preterm Newborns. Int J Prev Med 2012; 3: 262–265.
- Mishra S, Chawla D, Agarwal R, Deorari AK, Paul VK. Transcutaneous Bilirubin Levels In Healthy Term And Late Preterm Indian Neonates. Indian J Pediatr 2010; 77: 45-50.
- De Luca D, Zecca E, De Turris P, Barbato G, Marras M, Romagnoli C. Using Bilicheck For Preterm Neonates In A Sub-Intensive Unit: Diagnostic Usefulness And Suitability. Early Hum Dev 2007; 83: 313-317.
- Bhutani VK, Gourley GR, Adler S, Kreamer B, Dalin C, Johnson LH. Noninvasive Measurement Of Total Serum Bilirubin In A Multiracial Predischarge Newborn Population to Assess The Risk Of Severe Hyperbilirubinemia. Pediatrics 2000; 106: E17.
- Campbell DM, Danayan KC, McGovern V, Cheema S, Stade B, Sgro M. Transcutaneous Bilirubin Measurement At The Time Of Hospital Discharge In A Multiethnic Newborn Population. Paediatr Child Health. 2011; 16: 141-145.
- Kaynak-Turkmen M, Aydogdu SA, Gokbulut C, Yenisey C, Soz O, Cetinkaya-Cakmak B. Transcutaneous Measurement Of Bilirubin In Turkish New-borns: Comparison With Total Serum Bilirubin. Turk J Pediatr 2011; 53: 67-74.
- Janjindamai W, Tansantiwong T. Accuracy Of Transcutaneous Bilirubinometer Estimates Using Bilicheck In Thai Neonates. J Med Assoc Thai 2005; 88: 187-190.
- Neocleous C, Adramerina A, Limnaios S, Symeonidis S, Spanou C, Malakozi M, Mpampalis E. A Comparison Between Transcutaneous And Total Serum Bilirubin In Healthy-Term Greek Neonates With Clinical Jaundice. Prague Med Rep 2014; 115: 33-42.
- Ho EY, Lee SY, Chow CB, Chung JW. Bilicheck Transcutaneous Bilirubinometer: A Screening Tool For Neonatal Jaundice In The Chinese Population. Hong Kong Med J 2006; 12: 99-102.
- Conceicao CM, Dornaus MF, Portella MA, Deutsch AD, Rebello CM. Influence of assessment site in measuring transcutaneous bilirubin. Einstein (Sao Paulo) 2014; 12: 11-15.
- American Academy of Paediatrics Subcommittee on Hyperbilirubinemia. Management Of Hyperbilirubinemia In The New Born Infant 35 Or More Weeks Of Gestation. Paediatrics 2004; 114: 297-316.
- Engle WD, Jackson GL, Sendelbach D, Manning D, Frawley WH. Assessment Of A Transcutaneous Device In The Evaluation Of Neonatal Hyperbilirubinemia In A Primarily Hispanic Population. Paediatrics 2002; 110: 61-67.