ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 2
Evaluation of non-invasive diagnostic methods as indicators of fibrosis in patients with nonalcoholic fatty liver disease
1Division of Gastroenterology, Cerrahpasa Faculty of Medicine, Istanbul University, Turkey
2Division of Pathology, Cerrahpasa Faculty of Medicine, Istanbul University, Turkey
3Division of Nephrology, Haydarpasa Numune Training and Research Hospital, Turkey
- *Corresponding Author:
- Mustafa Canbakan
Haydarpasa Numune Education and Research Hospital
Istanbul, Turkey
Accepted on May 25, 2016
Aims: Non-alcoholic fatty liver disease (NAFLD) is an increasingly recognized cause of chronic-liverdisease which may be diagnosed incidentally in asymptomatic patients. Non-alcoholic steatohepatitis (NASH) may cause cirrhosis and hepatocellular carcinoma. To date, no established non-invasive test exists to accurately predict fibrosis in NASH. Although liver biopsy is the gold standard for the diagnosis of NASH, it occasionally has some serious complications. We aimed to compare the diagnostic accuracy of some widely used non-invasive fibrosis scoring systems.
Methods: We retrospectively assessed the files of 40 subjects with biopsy proven diagnosis of NAFLD. The subjects were grouped as mild and advanced fibrosis. Demographic, medical historical, and laboratory data were recorded. FIB4 index, APRI-, BARD-, and BAAT-scores were calculated. The agreement between biopsy findings and non-invasive scoring systems was assessed with Kappa statistics.
Results: 17 of the patients (58% males, mean age 58.8 years) had advanced fibrosis while 23 of them had mild fibrosis (52% males, mean age 54.5 years). Patients with advanced fibrosis had significantly higher AST and fasting glucose levels and waist circumferences (p<0.05 for each). None of the studies noninvasive scoring systems had a strong or independent correlation with biopsy findings. BAAT and BARD scores were relatively sensitive (88.9% for each) but non-specific (12.9 and 35.5, respectively), APRI score was highly specific (95.7%) but non-sensitive (35%), and FIB4 score was moderately sensitive (65%) and specific (69.6%) to detect advanced fibrosis.
Conclusions: In this study, none of the non-invasive tests used were independent factors for advanced fibrosis. These scores were more likely to exclude rather than detect advanced fibrosis. When the increase in NAFLD prevalence is considered, it is obvious that studies performed with more reliable noninvasive tests which would be used to detect or exclude advanced fibrosis are needed.
Keywords
Non-alcoholic fatty liver disease, Steatohepatitis, Fibrosis.
Introduction
Non-alcoholic fatty liver disease (NAFLD) is characterized with hepatocellular accumulation of triglycerides in patients with alcohol abuse less than 30 g/day. NAFLD involves two different clinical conditions including non-alcoholic fatty liver (simple steatosis) and steatohepatitis (NASH). NAFLD is associated with metabolic syndrome, obesity, diabetes and hyperlipidemia; non-alcoholic fatty liver disease is observed in 80% of the patients with metabolic syndrome. While simple steatosis is observed in approximately 30% of the general population, steatohepatitis is observed with a lower rate (5%) [1,2]. Progression to cirrhosis is observed in 20-25% of the patients with steatohepatitis and a risk for hepatocellular carcinoma exists in 5% [3]. NAFLD is generally asymptomatic and is found during routine screening with increased serum transaminase levels and/or increased liver echogenity in USG. The golden standard is liver biopsy in the diagnosis and staging of NAFLD and in the differentiation of simple steatosis and steatohepatitis. The negative aspects of liver biopsy can be listed as follows: it is an invasive procedure, sampling may be insufficient, sampling may be performed in an inappropriate site and the result may be assessed erroneously [4,5]. Therefore, many non-invasive methods have been developed in staging of NAFLD [6]. These tests include routine laboratory parameters, specific direct fibrosis markers and methods including elastography [7,8]. The BAAT score (body mass index, ALT, triglyceride), FIB4 index (age, ALT, AST, platelet count), BARD score (body mass index, ALT/AST, presence of DM), APRI score (AST, PLT), Fibrotest, FibroScan and European liver fibrosis score (ELF) are examples of the methods. In this study, it was aimed to evaluate the non-invasive methods as indicators of fibrosis in patients who were found to have NAFLD and to compare the diagnostic values of these methods as indicators of fibrosis.
Patients and Methods
Patients aged between 18 and 65 years who presented to our Gastroenterology-Hepatology outpatient’s clinic between 2001 and 2013 with different complaints and had hepatosteatosis on abdominal ultrasonography (USG) and underwent liver biopsy were included in the study and their files were retrospectively examined. Our exclusion criteria were as follows: history of alcohol consumption (>30 g/day); HBsAg and/or anti-HCV positivity; autoimmune hepatitis, Wilson’s disease, hemochromatosis, or other chronic liver diseases; and use of steatogenic drugs (corticosteroid, methotrexate etc). 40 patients were included in the study. The following study parameters and liver biopsy results were recorded. AST, ALT, GGT values, fasting insulin, fasting glucose, lipid profile (HDL, LDL, triglyceride and total cholesterol) and TSH values, leukocyte count, platelet count, hemoglobin level, age, gender, height, weight, waist circumference, drugs used (antihypertensive, antidiabetic etc) were recorded as the study parameters. Insulin resistance (IR) was calculated using homeostasis model assessment (HOMA) method [(fasting plasma glucose mg/dl/18) × (fasting insulin level mIU/ml/22.5)]. A HOMA-IR of ≥ 2.5 was considered to indicate IR (35). The body mass index (BMI) were assessed as follows: (lean: <18.5 kg/m2, normal: 18.5-24.9 kg/m2, overweight: 25-29.9 kg/m2, obese: 30-39.9 kg/m2, morbid obese: >40 kg/m2). The patients were divided into two groups according to the status of fibrosis as group 1 (no fibrosis or mild fibrosis; F0-F1) and group 2 (moderate or advanced fibrosis; F2-F4). The diagnosis of metabolic syndrome was made in the patients who had at least 3 of 5 criteria according to ATP III criteria [9]. The diagnosis of diabetes mellitus (DM) was made according to American Diabetes Association (ADA) criteria or when the subjects were under anti-diabetic treatment [10]. A diagnosis of hypertension was made in the patients who used at least one antihypertensive drug and whose blood pressure value measured in the follow-up was ≥ 140/90mm/Hg according to the ESH/ESC guidelines [11].
The same pathologist (NK), who was blinded to the clinical and biochemical data, reviewed all liver biopsy specimens. As previously reported by Brunt et al. NASH was defined by the presence of hepatic steatosis, cytologic ballooning, scattered, mainly acinar or portal inflammation, with or without Mallory bodies and/or fibrosis [12]. The stage of fibrosis was scored based on a 5point scale (0: no fibrosis; 1: perisinusoidal or portal fibrosis; 2: perisinusoidal and portal/ periportal fibrosis; 3: septal or bridging fibrosis; and 4: cirrhosis). The non-invasive methods for assessment of fibrosis we used in the study are shown in Table 1. The results we obtained in this retrospective study were used by calculation using these methods. The concordance between the scores obtained with these calculations and the biopsy results were assessed and compared with each other.
| APRI Score | (AST/upper limit AST†/PLT‡) × 100 |
| FIB4 Index | (Age × AST)/(PLT X √ALT |
| BARD Score | BMI ≥ 28 -1 point |
| AST/ALT† ≥ 0.8 -2 point | |
| Diagnosis of diabetes -1 point | |
| BAAT Score | BMI ≥ 28 -1 point |
| Age ≥ 50 -1 point | |
| ALT ≥ 2 × ULN -1 point | |
| TG ≥ 150 mg/dl -1 point | |
| †AST - ALT Upper limit: 30IU/L for females and 35IU/L for males, ‡PLT: Platelet Count; ULN: Upper Limit of Normal; TG: Triglycerides; BMI: Body Mass Index | |
Table 1. Non-invasive Fibrosis assessment methods.
Statistical analysis
Pearson or Spearman analyses were used to examine the correlation of the factors with each other. The Student’s t test was used to compare the groups. Receiver operating curve (ROC) analysis was used to determine the diagnostic cut-off values to distinguish between no/mild and moderate/severe fibrosis. The ROC curve analysis results were provided with sensitivity, specificity, area under the curve (AUC), and confidence intervals. Multivariate logistic regression analyses were performed for each non-invasive fibrosis score separately to determine independent factors associated with fibrosis according to biopsy. P value<0.05 was considered statistically significant. All statistical analyses were performed using SPSS® statistical software program (Ver.15.0, Chicago, Illinois, USA).
Results
Twenty two of the patients were male and 18 were female. The mean age was 56.3±9.11 years. No difference was found in the degree of fibrosis in the male and female patient’s groups (p=0.67).
Twenty-three NAFLD patients (M/F: 12/11; mean age ± SD: 54.48 ± 7.69) had mild fibrosis, and 17 patients (M/F: 7/ 10; mean age ± SD: 58.76 ± 10.47) had advanced fibrosis. Patients with advanced fibrosis had higher waist circumferences and AST and fasting glucose levels than those with mild fibrosis (p=0.044, 0.045, and 0.045, respectively). Other study variables were similar between the groups. Their main data are shown in Table 2. 11 of the 17 patients with advanced fibrosis had type 2 DM, 12 of them had metabolic syndrome, 11 of them had obesity, and insulin resistance was found in 3 of 6 non-diabetic patients. The post-hoc power analysis indicated that the power of this study was 15.1%.
| Group 1* (n=23) | Group 2* (n=17) | p | |
|---|---|---|---|
| Age (year) | 54.48 ± 7.69 | 58.76 ± 10.47 | 0.14 |
| Sex (male/female) | 12/11 (52% /48%) | 10 /7 (58% /42%) | 0.5 |
| Diabetes | 10 (43%) | 11 (64%) | 0.18 |
| Metabolic Syndrome | 13 (56%) | 12 (70%) | 0.5 |
| Obesity | 9 (39%) | 11 (65%) | 0.1 |
| Insulin resistance† | 9/13 (69%) | 3/6 (50%) | 0.4 |
| Body mass index (kg/m2) | 29.7 ± 2.57 | 31.12 ± 5.19 | 0.3 |
| Waist circumference (cm) | 104.52 ± 8.52 | 111.18 ± 11.65 | 0.044 |
| AST (U/L) | 25.26 ± 9.11 | 43.65 ± 41.29 | 0.045 |
| ALT (U/L) | 37.52 ± 25.57 | 53.12 ± 48.02 | 0.3 |
| GGT (U/L) | 45.13 ± 37.01 | 74.41 ± 40.19 | 0.1 |
| HDL cholesterol (mg/dl) | 48.09 ± 14.21 | 47.47 ± 14.56 | 0.9 |
| Triglycerides (mg/dl) | 169.26 ± 90.69 | 141.65 ± 78.21 | 0.3 |
| Fasting Glucose (mg/dl) | 95.57 ± 35.53 | 118.29 ± 32.53 | 0.045 |
| Fasting insulin (mIU/l) † | 15.45 ± 6.05 | 15.01 ± 4.38 | 0.14 |
| Homa-IR † | 3.16 ± 1.37 | 3.14 ± 1.46 | 0.6 |
| Platelet Count µ/L | 246478 ± 71743 | 233411 ± 72228 | 0.7 |
| *Group 1 denotes patients with mild fibrosis while group 2 denotes patients with advanced fibrosis. †Only non-diabetic patients were compared (diabetic patients were not included in this comparison). Significant p values are written with bold text. |
|||
Table 2. Demographic and biochemical characteristics of patients with mild and advanced fibrosis.
Evaluation of the non-invasive methods
Fibrosis assessments with non-invasive methods are given in Table 3. In kappa analysis, APRI and FIB4 scores had fair and statistically significant agreement with histologic fibrosis stage (kappa=0.3 and p=0.011; kappa=0.34 and p=0.03, respectively). BAAT or BARD scores did not show statistically significant agreement with histologic fibrosis stage.
| BAAT | Group 1 (n=23) | Group 2 (n=17) | |
| Mean score | 2.35 ± 1.12 | 2.29 ± 1.16 | Kappa: 0.09 P :0.8 |
| 0-1 | 5 (21%) | 4 (24%) | |
| 2-4 | 18 (79%) | 13 (76%) | |
| BARD | Group 1 (n=23) | Group 2 (n=17) | |
| Mean score | 2.09 ± 1.34 | 2.65 ± 1.11 | Kappa:0.13 P:0.16 |
| 0-1 | 9 (39%) | 3(18%) | |
| 2-4 | 14 (61%) | 14(82%) | |
| APRI | Group 1 (n=23) | Group 2 (n=17) | |
| <0.61 | 22 (%) | 11 (65%) | Kappa:0.33 P:0.011 |
| ≥ 0.61 | 1 (%) | 6 (35%) | |
| FIB4 Index | Group 1 (n=23) | Group 2 (n=17) | |
| <1.08 | 16 (70%) | 6 (35%) | Kappa:0.34 P :0.03 |
| >1.08 | 7 (30%) | 11 (65%) |
Table 3. Non-invasive scores and assessment of fibrosis.
The sensitivity, specificity, positive predictive, and negative predictive values of non-invasive methods to indicate advanced fibrosis are listed in Table 4. Using ROC curve analysis the cut-off value for APRI score was found to be 0.61 and 1.08 for FIB4 index. ROC curve analysis revealed that the BAAT and BARD scores were relatively sensitive (88.9% and 88.9%, respectively) but non-specific (12.9% and 35.5%, respectively) to detect advanced fibrosis. The APRI score was highly specific (95.7%) but non-sensitive (35%) while a FIB4 score>1.08 was moderately sensitive (65%) and specific (69.6%) to detect advanced fibrosis.
| BAAT ≥ 2 | BARD ≥ 2 | APRI ≥ 0.61 | FIB4>1.08 | |
|---|---|---|---|---|
| Sensitivity | 88.9 | 88.9 | 35 | 65 |
| Specificity | 12.9 | 35.5 | 95.7 | 69.6 |
| Positive predictive value | 22.9 | 28.6 | 85.7 | 61.1 |
| Negative predictive value | 80 | 91.7 | 66.7 | 72.7 |
Table 4. ROC curve analysis results for noninvasive methods to detect moderate-advanced fibrosis.
Multivariate logistic regression analysis showed that increased BAAT, BARD, FIB4 and APRI scores were risk factors for fibrosis but without statistical significance (OR: 1.12, CI: 0.36-3.49; p=0.83; OR: 1.13, CI: 0.55-2.29; p=0.73; OR: 1.82, CI: 0.36-9.17; p=0.46;and OR: 1.89, CI: 0.07-5.78; p=0.70) (Table 5). Multivariate linear regression analysis showed that none of the non-invasive markers was independently correlated with degree of fibrosis (p=0.72, 0.42, 0.25, and 0.33, respectively) (Table 6).
| OR | Confidence interval | P | |
|---|---|---|---|
| BAAT | 1.12 | 0.36-3.49 | 0.83 |
| BARD | 1.13 | 0.55-2.29 | 0.73 |
| FIB4 | 1.82 | 0.36-9.17 | 0.46 |
| APRI | 1.89 | 0.07-50.78 | 0.70 |
Table 5. Fibrosis score- advanced fibrosis - Logistic regression analysis.
| b | p | |
|---|---|---|
| BAAT | -0.084 | 0.72 |
| BARD | -0.139 | 0.42 |
| FIB4 | 0.303 | 0.25 |
| APRI | -1.140 | 0.33 |
Table 6. Fibrosis score-fibrosis degree - linear regression analysis.
Discussion
In this study, we non-invasively evaluated the study subjects in terms of fibrosis scores and compared these results with biopsy results. While some of non-invasive methods were relatively sensitive but non-specific, some others were relatively specific but non-sensitive. Furthermore, no independent correlation was found between advanced fibrosis on biopsy and the non-invasive fibrosis markers used widely in adult patients who were found to have hepatosteatosis on ultrasonography and underwent biopsy.
Although modern imaging methods are used recently for the diagnosis of NAFLD, the gold standard diagnostic method is still liver biopsy. Although development of fibrosis is not common in these patients, it is very important to diagnose fibrosis with liver biopsy at an early time, because complications including cirrhosis and development of HCC cause to great mortality and morbidity. When the prevalence of NAFLD is considered, performing biopsy in all patients will increase both the cost and the risk of development of biopsy-related complications, though to a small extent. The BAAT score was designed as a method based on scoring. In a study performed by Ratzui et al. 93 patients with NAFLD were evaluated. Advanced fibrosis was not found in any of 30 patients who had a BAAT score of 0-1. The negative predictive value in terms of group 2 (moderate-advanced fibrosis) was found to be 100% (sensitivity 100%, specificity 47%). In 4 patients, only the BAAT score was calculated and moderate-advanced fibrosis was found in all of them; the specificity and positive predictive value were found to be 100%, while the sensitivity was found to be only 14% [13]. In the study of Ratziu et al. a significant portion of the patients included in the study were composed of the patients who had dyslipidemia and who were investigated because of increased hepatic function test results. Since the demographic properties of these patients were not equally distributed and the patients who had moderate-advanced fibrosis had statistically higher BMI and triglyceride level, the BAAT score was calculated to be high in these patients. Thus, the majority of the patients with a low BAAT score were composed of the patients who had mild fibrosis or who had no fibrosis. In our study, the BAAT score was found to be weak in terms of detecting or excluding moderate-advanced fibrosis.
The BARD score is another method based on scoring. In a study performed by McPherson et al. 145 patients were evaluated. Mild fibrosis (F0-1) was found in 99 of these patients and moderate-advanced fibrosis (F2-4) was found in 46 of these patients. In the F0-1 group, the median BARD sore was calculated to be 2 (0-4) and in the other group, the median BARD score was calculated to be 3 [1,2,14,15]. As a result of this study, the sensitivity was found to be 89%, the specificity was found to be 44%, the positive predictive value was found to be 42% and the negative predictive value was found to be 95% in terms of determining advanced fibrosis when the BARD score was considered ≥ 2-4 [16]. In a study conducted in Poland, 104 patients were evaluated and the sensitivity was found to be 86%, the specificity was found to be 72%, the positive predictive value was found to be 35% and the negative predictive value was found to be 97% in terms of detecting advanced fibrosis when the BARD score was considered ≥ 2-4 [17]. In our study, similar results were obtained in terms of sensitivity and positive predictive value, high negative predictive value whereas lower values were obtained in terms of specificity. Conclusively, while the BARD score was weak in terms of detecting advanced fibrosis, it was relatively reliable to exclude advanced fibrosis in our study.
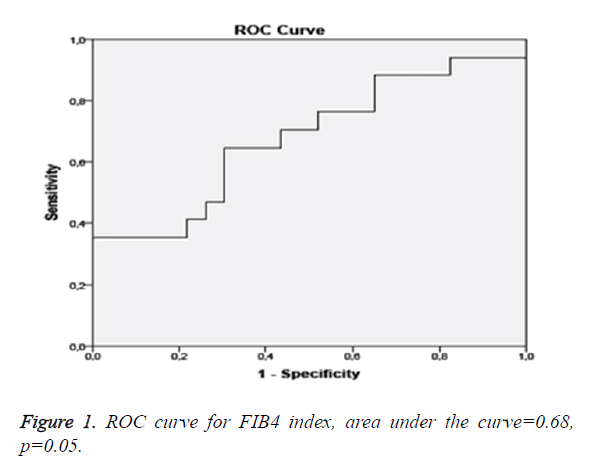
The FIB4 index is a method based on calculation in contrast to the other two scores. In a study conducted by Shah et al. 541 patients were evaluated and advanced fibrosis was found in 126 patients. In this study, the upper cut-off value for exclusion of advanced fibrosis in ROC curve analysis was found to be 1.31. It was observed that the FIB4 index was below the cut-off value in 327 patients. While mild fibrosis (F0-F1) was present in 294 of 327 patients, advanced fibrosis was found in 33 patients. When the FIB4 index was below the cut-off value, the negative predictive value of the FIB4 index was reported to be 90% in terms of excluding advanced fibrosis (sensitivity 74%, specificity 71%, and positive predictive value 43%). Again in this study, the lower cut-off value which could predict advanced fibrosis optimally was calculated to be 2.67. Advanced fibrosis was found in 41 of 51 patients who were above this value. When the FIB4 index was above this cut-off value, the sensitivity was found to be 33%, the specificity was found to be 98%, the positive predictive value was found to be 80% and the negative predictive value was found to be 83% [18]. In another study conducted in Japan, 576 NAFLD patients were evaluated. It was observed that the upper cut-off value to exclude advanced fibrosis with ROC curve analysis was 1.45 (Figure 1). In patients whose FIB4 index was below this value, the negative predictive value in terms of excluding advanced fibrosis was found to be 98% (sensitivity 90%, specificity 64%, and positive predictive value 24%). In the study, the lower cut-off value which would predict advanced fibrosis with ROC curve analysis in the best was found to be 3.25. It was observed that the sensitivity was 48%, the specificity was 95%, the positive predictive value was 53% and the negative predictive value was 98% in terms of detecting advanced fibrosis [19]. In our study, the optimal FIB4 cut-off value to exclude advanced fibrosis with ROC curve analysis was found to be 1.08. The positive predictive value in terms of detecting advanced fibrosis was found to be 61.1% (sensitivity 65%, specificity 69.6% and negative predictive value 72.7%). Our results were (similar) in accordance with the previous studies. Conclusively, FIB4 was relatively more reliable in excluding and detecting advanced fibrosis.
The APRI score is another method based on calculation. Studies conducted with this score generally compare this score with the other noninvasive methods. In a study performed by Shin et al. 134 patients with chronic hepatic disease were evaluated. Chronic viral hepatitis was found in 111 cases and NAFLD was found in 23 cases. The best cut-off value for diagnosis with ROC curve analysis was found to be 0.5. The sensitivity of the score in terms of detecting advanced fibrosis was found to be 93%, the specificity was found to be 48%, the positive predictive value was found to be 75% and the negative predictive value was found to be 80%. When the cut-off value was considered 1.5, the sensitivity was found to be 58%, the specificity was found to be 88% and the positive predictive value was found to be 89% [20]. In the study conducted by Krueger et al. 111 patients who were diagnosed with NAFLD by biopsy were evaluated. The APRI score ROC value was found to be 0.8 and at a cut-off value of 0.95, the sensitivity in terms of detecting advanced fibrosis was found to be 75%, the specificity was found to be 86%, the positive predictive value was found to be 54% and the negative predictive value was found to be 93% [21]. In the study conducted by Sumida et al. which compared the non-invasive methods, the sensitivity in terms of detecting advanced fibrosis was found to be 67%, the specificity was found to be 81%, the positive predictive value was found to be 31% and the negative predictive value was found to be 95% at an APRI score cut-off value of ≥ 1 [19]. Another study by Barone et al. [22] investigated the association of endothelial dysfunction with liver fibrosis in patients with HCV infection, and they found a significant correlation between fibro scan and APRI scores in this study. In our study, the APRI cut-off value to exclude advanced fibrosis with ROC curve analysis was found to be 0.54. The APRI scores of 8 patients were found to be above the cut-off value. Advanced fibrosis was found in 6 of these 8 patients. With these results the sensitivity was found to be 44.4%, the specificity was found to be 87.1%, the positive predictive value was found to be 50% and the negative predictive value was found to be 84.4%. Thus in this study APRI score was weak in terms of sensitivity but had high specificity. The fact that patients with chronic hepatitis were included and advanced fibrosis and increased AST values were present in most of these patients affected calculation of the score in the first study. According to these results, the APRI score can be used to exclude advanced fibrosis rather than detecting its presence similar to the other non-invasive methods.
The small number of patients and the retrospective nature are limitations of our study. Thus we cannot reach definite cause-effect relationship with this data. The limited sample size might have led to underestimation of some significant associations. However, the fact that the demographic and biochemical variables of the groups with mild and advanced fibrosis were not statistically different enhances the reliability of our findings.
In conclusion, an ideal non-invasive test should be considerably sensitive and specific to evaluate hepatic fibrosis, should be used in all chronic hepatic diseases and not bring additional financial cost to patients. Unfortunately, there is no non-invasive test meeting these criteria at the present time and further investigations are needed. In our study, none of the non-invasive tests used were independent factors for advanced fibrosis. These scores were more likely to exclude rather than detect advanced fibrosis. When the increase in NAFLD prevalence is considered, it is obvious that studies performed with more reliable noninvasive tests which would be used to detect or exclude advanced fibrosis should be conducted.
References
- Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology 2004; 40: 1387-1395.
- Wanless IR, Lentz JS. Fatty liver hepatitis (steatohepatitis) and obesity: an autopsy study with analysis of risk factors. Hepatology 1990; 1106-1110.
- Parekh S, Anania FA. Abnormal lipid and glucose metabolism in obesity: implications for nonalcoholic fatty liver disease. Gastroenterology 2007; 132: 2191-2207.
- Bedossa P, Dargere D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology 2003; 38: 1449-57.
- Ratziu V, Charlotte F, Heurtier A, Gombert S, Giral P, Bruckert E. Sampling Variability of Liver Biopsy in Nonalcoholic Fatty Liver Disease. Gastroenterology 2005; 128: 1898-1906.
- Pinzani M, Vizzutti F, Arena U, Marra F. Technology Insight: noninvasive assessment of liver fibrosis by biochemical scores and elastography. Nat Clin Pract Gastroenterol Hepatol 2008; 5: 95-106.
- Ratziu V, Massard J, Charlotte F, Messous D, Imbert-Bismut F, Bonyhay L. Diagnostic value of biochemical markers (FibroTest-FibroSURE) for the prediction of liver fibrosis in patients with non-alcoholic fatty liver disease. BMC Gastroenterol 2006; 6:6.
- Guha IN, Parkes J, Roderick P, Chattopadhyay D, Cross R, Harris S. Noninvasive markers of fibrosis in nonalcoholic fatty liver disease: Validating the European Liver Fibrosis Panel and exploring simple markers. Hepatology 2008; 47: 455-460.
- Pasternak R. Adult Treatment Panel II versus Adult Treatment Panel III: what has changed and why? Am J Cardiol 2002; 89: 3c-7c.
- Clinical Pratice Recommendation 2013 by the American Diabetes Association 2013.
- Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 2013; 31: 1281-357.
- Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol 1999; 94: 2467-2474.
- Ratziu V, Giral P, Charlotte F, Bruckert E, Thibault V, Theodorou I. Liver fibrosis in overweight patients. Gastroenterology 2000; 118: 1117-1123.
- Brunt EM. Nonalcoholic steatohepatitis: definition and pathology. Semin Liver Dis 2001; 21: 3-16.
- Greenberg NJ, Burakoff R. Current Diagnosis & Treatment Gastroenterology. Hepatology &Endoscopy 2012.
- McPherson S, Stewart SF, Henderson E, Burt AD, Day CP. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut 2010; 59: 1265-1269.
- Raszeja-Wyszomirska J, Szymanik B, Lawniczak M, Kajor M, Chwist A, Milkiewicz P. Validation of the BARD scoring system in Polish patients with nonalcoholic fatty liver disease (NAFLD). BMC Gastroenterol 2010; 10: 67.
- Shah AG, Lydecker A, Murray K, Tetri BN, Contos MJ, Sanyal AJ. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2009; 7: 1104-1112.
- Sumida Y, Yoneda M, Hyogo H, Itoh Y, Ono M, Fujii H. Validation of the FIB4 index in a Japanese nonalcoholic fatty liver disease population. BMC Gastroenterol 2012; 12: 2.
- Shin WG, Park SH, Jun SY, Jung JO, Moon JH, Kim JP. Simple tests to predict hepatic fibrosis in nonalcoholic chronic liver diseases. Gut Liver. 2007; 1: 145-50.
- Kruger FC, Daniels CR, Kidd M, Swart G, Brundyn K, van Rensburg C. APRI: a simple bedside marker for advanced fibrosis that can avoid liver biopsy in patients with NAFLD/NASH. S Afr Med J. 2011; 101: 477-480.
- Barone M, Viggiani MT, Amoruso A, Schiraldi S, Zito A, Devito F. Endothelial Dysfunction Correlates with Liver Fibrosis in Chronic HCV Infection. Gastroenterology Research and Practice 2015.