ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2020) Volume 31, Issue 5
Elution of residual monomers from dental composite resins
Ezgi Sonkaya1, Seyhmus Bakır2, Elif Pınar Bakır3*
1Department of Restorative Dentistry, Cukurova University, Adana, Turkey
2Department of Restorative Dentistry, Dicle University, Diyarbakir, Turkey
- Corresponding Author:
- Elif Pinar Bakir
Department of Restorative Dentistry
Dicle University
Diyarbakir
Turkey
Accepted date: October 16, 2020
The biocompatibility of composite resins decreases with the increase of released monomer since they are cytotoxic on pulp or living tissues. Commercialy used composites should be choosen according to their biocompatibility to not jeopardize the patient’s health. Analyze of the composite biocompatibility and determination of the monomers elution of four different composite resin materials with nano filler content is completed in the study to investigate if there is difference and the most possible healty way of usage. The amount of Bis-GMA, UDMA and TEGDMA released from four different composite resins measured on three different day using the HPLC. Four groups (n=10, diameter: 5 mm, thickness: 2 mm) of each material were prepared. Samples were placed in 75% ethanol-water. On days 1, 15 and 30, 1 mL samples were taken for measurement. A total of 120 samples’ findings were analysed statistically. Bis-GMA and UDMA were released from all the materials used in the study. There were significant differences in the total monomer release of all composite resins in terms of time (P=0.001). Tetric Evoceram is the most residual monomer-releasing composite in experiment groups. The maximum amount of monomer release for all three monomers was on the 15th day. In order to reduce concerns about toxicity, taking measures to protect the pulp for example using cavity liners and bases specially in case of having less than 0.5 mm thickness of dentin. There is a need for these results to be supported by further clinical studies.
Keywords
Nanofiller Composite Resin, Residual Monomer, High Performance Liquid Chromato Figurey, Biocompatibility, Cytotoxicity.
Introduction
The use of long-life and biocompatible materials in restorative dentistry is important. The characteristics and biocompatibility of composite resins are determined by the monomers in their contents. The most commonly used monomers to form a polymer matrix are Bis- GMA (bisphenol A diglycidyl methacrylate), UDMA (urethane dimethacrylate), TEGDMA (triethylene glycol dimethacrylate) HEMA (hydroxyethyl metacrylate) and Bis-EMA (bisphenol A diglycidyl methacrylate ethoxylated) [1]. The mechanical properties of the polymer depend on the degree of conversion of the monomers and other components of the composite (e.g. The filler and initiator systems) [2]. The degree of conversion in composite resins is 40%-75%. Therefore, it is difficult to provide complete polymerization in each region of the resin at the same rate [3-5]. As the monomer conversion rate falls, it leads to increase of water absorption of the material, decrease of wear resistance and color stability, inadequate binding and unwanted tissue reactions [6,7].
The conversion rate of the Bis-GMA monomer with high molecular weight and viscosity is lower than that of other monomers. Reducing the Bis-GMA ratio increases polymerization shrinkage. Some of the dimethacrylates are densely bound to the cross linkage while others are loosely bound. Thus, an unreacted amount of monomers remains between the polymer chains [3].
UDMA monomer with similar weight to Bis-GMA but lower viscosity provides color stability in composite resins and increases adhesion. This monomer, which exhibits less polymerization shrinkage than Bis-GMA, has a high biocompatibility potential [8].
To reduce the viscosity of composite resins, TEGDMA is added as the diluent and the high reactivity, low viscosity and molecular weight of TEGDMA increases the degree of polymer conversion and shrinkage. The lipophilic nature of TEGDMA enables it to easily penetrate the cytoplasm and membrane lipid compartments of mammalian cells [9].
Residual monomers are separated from the mass by breaking the polymer chains with intraoral fluids which is a powerful organic solvent [4]. These monomers released from dental composites after interacting with intraoral fluids, enters the body circulatory system in three ways: the first, through the uptake of the secreted monomers from the gastrointestinal tract, the second through the absorption from the dentinal tubules [10,11] and the third through the uptake of volatile components in the lungs [12,13]. While residual monomers leached from the composite materials cause minor effects in the oral cavity over time, they may diffuse through dentin tubules into the pulp and affect pulpal cells [14]. These may cause allergic, cytotoxic or mutagenic reactions on pulp or live tissues. The catabolic effects of monomers have raised concerns about the reliability of composite resins [15].
In recent years, the focus of research on composite resins has been the problems of biocompatibility due to residual monomers. These studies have evaluated in particular when and what amount of monomers was released [16]. The differences in the amount of monomer released from the resin materials can be explained by the structural properties of the monomer [17,18]. There are different methods to test the presence of residual monomer [19]. HPLC is the most appropriate method for samples with high molecular weight and which are potentially perishable when heated [20].
Studies have shown that the amount of residual monomer release is changed when different polymerization times, light sources or aging periods are used. The degree of polymerization is known to be affected by filler particle type and content [21]. The main aim of this investigation is to assess the doses and risks associated with residual monomer released from four nanoparticle composite resin material at different time periods using the HPLC device. The null hypothesis that will be tested is that there is no significant difference between residual monomer amounts released from the same type but different brands composites.
Materials and Methods
The study used four different composite resin materials with nano filler (Grandio, FiltekTM Ultimate Universal Restorative, Clearfil Majesty Posterior and Tetric EvoCeram) of A2 color and activated by visible light (Table 1).
| Material | Type | Composition | Lot no | |
|---|---|---|---|---|
| Matrix | Filler | |||
| Grandio (VOCO Cuxhaven, Germany) |
Nano-hybrid composite |
Bis-GMA %10-12, UDMA %12-14, TEGDMA | Mixture of different dimethacrylates, silicate fillers, initiators, pigments, amines, additives. The inorganic filler ratio in the methacrylate matrix was 87% by weight (71.4% by volume). | 1719521 |
| Filtek Ultimate Universal Restorative(3MTM ESPETM St. Paul, USA) | Nanofilled composite | Bis-GMA %1-10, UDMA, TEGDMA %<1, PEGDMA %<5, BIS-EMA | The filler contains free-standing 20nm silica particles, 4-11nm zirconium particles and clusters together. The inorganic filler ratio in the methacrylate matrix was 78.5% (63.3% by volume). | N889185 |
| Clearfil Majesty Posterior (Kuraray Dental Inc, Japan) |
Nanofilled composite | Bis-GMA %<3, UDMA, TEGDMA %<3 | Hydrophobic aromatic dimethacrylate, silanated glass ceramics, surface treated alumina micro filler, silanated silica filler, dl-Camphorquinone, accelerators, pigments, others. The inorganic filler ratio in the methacrylate matrix was 92% by weight (82% by volume). | 3V0042 |
| Tetric EvoCeram(Ivoclar Vivadent, Principalit of Liechtenstein) | Nano-hybrid composite |
Bis-GMA %3-<10, UDMA %3-<10, Ytterbium trifluoride %3-<10, Ethoxylated Bis-EMA %3-<10 | Barium glass filler as filler, ytterbiumtrifluoride and mixed oxide 48.5% by weight, prepolymers 34.0% by weight, additives 0.4%, catalyst and stabilizing agents 0.3%, color pigments <0.1%. The inorganic filler ratio in the methacrylate matrix was 83.2% by weight | U23115 |
PEGDMA: Poly(ethylene glycol) dimethacrylate
Table 1: Chemical structures of composite resins and properties of standard monomers.
Bis-GMA, UDMA and TEGDMA (Sigma Aldrich, St Louis, MO, USA) were used as standard materials for the identification of the monomer peaks in the chromatograms. 99% ethanol, 99.8% acetonitrile, deionized water, micropipettes, 1.5 mL amber colored glass vials, amber colored and 20 m glass bottles with screw cap were also used.
For each resin-based dental material, 10 disc-shaped specimens (5 mm diameter and 2 mm thickness) were prepared using teflon rubber molds (n=40). BlueLex GT- 1200 (Monitex Industrial Co Ltd, Taiwan) was used for the polymerization of the samples. It was polymerized for 20 seconds according to the manufacturer’s instructions. The distance between the light source and sample were standardized using a 1 cm glass plate. Four different study groups were formed, including 10 samples of each material. Sof-Lex polishing discs (3 M TM ESPE TM, St Paul, USA) were used to remove the oxygen inhibition layer that could be formed on the surface of the material. Precision scales (Ohaus PA 224, USA) were used to weigh the completed polymerized discs.
Each of the 40 sample discs were placed in a 20 mL amber glass vials with a vacuum lid, containing 75% ethanol- water solution (Merck). All the vials were kept in the oven at 37°C for 30 days and were analyzed on day 1, 15 and 30. For the measurements, 1 mL samples were taken from these solutions into Eppendorf’s tubes, then transferred to 1.5 mL light impermeable ammonite glass vials. Thus, a total of 120 samples were transferred to the HPLC by sampling the samples from four different composite resin solutions at three different time points.
Six different solutions (0.1, 1, 10, 100, 500, 1000 ppm) from each monomer were prepared and injected into the HPLC system to calibrate the device. The Agilent 1260 Infinity II Quaternary LC (Agilent Technologies, USA) HPLC instrument was used. Eluates of the specimens were analyzed using HPLC, equipped with a Diode Array Detector (DAD) (204 nm) (Agilent Technologies, USA), and a symmetry silica column C18 (5 μm, 4.6 mm i.d./250 mm length) (Agilent ZORBAX Eclipse XDB columns-C18, Agilent Technologies, USA).
The eluent was a solution of acetonitrile/water (ACN Merck) 80/20 wt % at a flow rate of 1 mL/min. Identification and quantitative analysis of components were performed by comparison of the elution time and the integration of absorption peak area of the eluates with those of the authentic sample. The amount of monomer matching to the peak areas in chromatograms was calculated in ppm to acquire data for statistical analysis.
Statictical analysis
Data obtained in the study were analysed statistically using SPSS V21.0 software. The results were stated as mean ± standard deviation (SD) values. The Shapiro-Wilk test was used for assessment of normal distribution. The One Way Anova test was used on the basis of the residual amount of monomer release between different brands in each different measurement period. The Tukey HSD test was used to determine from which brand and which monomer the difference originated. The Bonferroni test was used for binary comparisons in repeated measurements. Total released monomer over 30 days is calculated linear point to point fitting method of the curve. A value of P<0.05 was considered statistically significant.
Results
Under the experimental conditions used in this study, the retention time of HPLC peaks of the standard solution of Bis-GMA, UDMA, and TEGDMA was found to be 5.3, 4.9, and 4.4 min, respectively.
Bis-GMA and UDMA were detected in all four different composite resin materials used in the study. Only TEGDMA release was not observed from the Tetric EvoCeram (TEC) material. Significant differences were observed in the total release time of monomers on different days. The results indicate that the highest values of Bis-GMA and TEGDMA monomers released from Grandio and Clearfil were reached on day 15. UDMA monomer reached the highest release value on day 1. All monomers released from Filtek and TEC reached the highest value on day 15. This result is significant at the P=0.001 level (Tables 2-4).
| Grandio (Mean ± SD) (ppm) |
FiltekTM Ultimate Universal (Mean ± SD) (ppm) |
Clearfil Majesty Posterior (Mean ± SD) (ppm) |
Tetric Evo Ceram (Mean ± SD) (ppm) |
P | ||
|---|---|---|---|---|---|---|
| Bis-GMA | 1. Day | 1.86 ± 0.19A | 0.91 ± 0.38A | 1.52 ± 1.12A | 4.70 ± 0.86A | 0.001* |
| 15. Day | 2.63 ± 0.28B | 1.46 ± 0.50B | 2.30 ± 1.78A | 9.42 ± 2.94B | 0.001* | |
| 30. Day | 1.38 ± 0.23C | 1.04 ± 0.34A | 1.49 ± 1.09A | 7.09 ± 2.81A | 0.001* | |
Note: *Expresses statistically significant difference
Different uppercase letters within the same column indicate a significant difference. This result is significant at the P=0.001 level.
Table 2: Average concentrations (ppm) of Bis-GMA eluted from composite resins in different time periods.
| Grandio (Mean ± SD) (ppm) |
FiltekTM Ultimate Universal (Mean ± SD) (ppm) |
Clearfil Majesty Posterior (Mean ± SD) (ppm) |
Tetric Evo Ceram (Mean ± SD) (ppm) |
P | ||
|---|---|---|---|---|---|---|
| UDMA | 1. Day | 0.32 ± 0.06A | 1.78 ± 0.60A | 0.15 ± 0.10A | 6.26 ± 1.14A | 0.001* |
| 15. Day | 0.28 ± 0.8B | 2.71 - 0.85B | 0.06 ± 0.18A | 11.27 ± 3.58B | 0.001* | |
| 30. Day | 0.16 ± 0.05C | 1.91 ± 0.57A | 0.04 ± 0.11B | 7.79 ± 3.13AC | 0.001* | |
Note: *Expresses statistically significant difference
Different uppercase letters within the same column indicate a significant difference. This result is significant at the P=0.001 level.
Table 3: Average concentrations (ppm) of UDMA eluted from composite resins in different time periods.
| Grandio (Mean ± SD) (ppm) |
FiltekTM Ultimate Universal (Mean ± SD) (ppm) |
Clearfil Majesty Posterior (Mean ± SD) (ppm) |
Tetric Evo Ceram (Mean ± SD) (ppm) |
p | ||
|---|---|---|---|---|---|---|
| TEGDMA | 1. Day | 0.55 ± 0.04A | 0.01 ± .009A | 0.87 ± 0.71A | 0 | 0.001* |
| 15. Day | 0.94 ± 0.08 B | 0.08 ± 0.04B | 1.41 ± 1.13B | 0 | 0.001* | |
| 30. Day | 0.14 ± 0.03C | 0.05 ± 0.01B | 0.81 ± 0.61A | 0 | 0.001* | |
Note: *Expresses statistically significant difference
Different uppercase letters within the same column indicate a significant difference. This result is significant at the P=0.001 level.
Table 4: Average concentrations (ppm) of TEGDMA eluted from composite resins in different time periods.
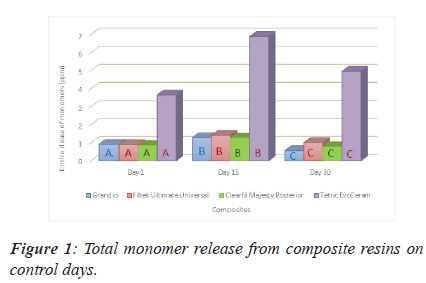
From the Figure 1 above we can see that TEC reported significantly more than the other three composite considering the monomer release on any of the control days. For any of the monomers on any control days, there is statistically difference between composites. The highest released total monomer amount were reached on day 15 for any composites (Figure 1).
Note: *There is a statistically significant difference between values of A,B,C among their colour group at the P=0.001 level.
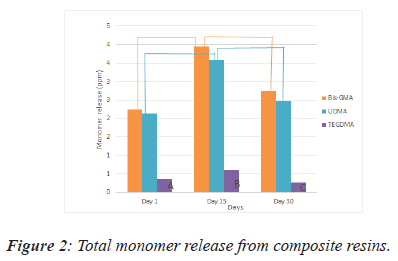
In Figure 2 there is a clear trend of increasing monomer release for on the 15th day. The amount of monomer release detected on the 30th day were significantly lower in comparison with the amount of monomer release on the 15th day in all groups. From this data, we can see that TEGDMA releases in the lowest value of monomer (Figure 2).
There is statistically significant differences between values of A,B,C and linear lines’ endpoints at the P=0.001 level.
There were no significant differences between Bis-GMA release from Clearfil on day 1 and 30. The difference of released Bis-GMA values from all other materials between 1st, 15th and 30th days was significant.
No significant difference was found between the 1st and 30th day of UDMA released from Filtek and TEC and between the 15th and 30th day values of UDMA released from Clearfil. In all other measurements, the difference of the released monomer values between 1st, 15th and 30th days was significant.
Only trace amounts of TEGDMA were detected in TEC. No significant difference was found between the 15th and 30th day of TEGDMA release from Filtek and on the 1st and 30th day of TEGDMA release from Clearfil. In all other measurements, the difference of the released TEGDMA monomer values between 1st, 15th and 30th days was significant.
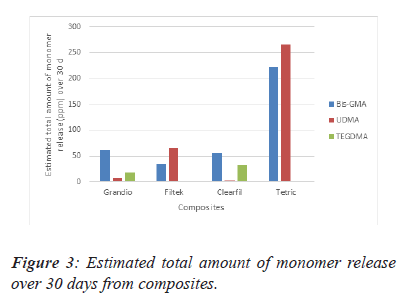
The differences between the composites are highlighted in Figure 3. From this data, we can see that the highest value of estimated total amount of monomer release is in TEC over 30 days. The highest TEGDMA release material was found to be Clearfil Majesty (Figure 3).
Note: *There is a statistically significant difference between all values. This result is significant at the P=0.05 level.
Discussion
Polymerization of dimethacrylate monomers in resin composites that forms a cross-linked polymer brings stability to composite. However, not all of these monomers are fully converted into a polymer and combined with fillers and additives. Thus, not only these unbound substances, but also uncured monomers release the significant amount of residual monomers [22]. This release starts with intraoral degradation processes that firstly cause mechanical [23], hydrolytic and enzymatic degradation, chain breaking and results with the production of the polymeric degradation products formation. Later release continues by aging of composite materials due to the interplay of water absorption, mechanical swelling and chemical/enzymatic degradation that can lead to porosity [24] and finally lead to gradual increase of released non- polymerized monomers initially trapped in the polymer network [23]. Besides all that, factors such as the type and monomer content of the material, the molecular size and chemical composition of the monomer, the degree of polymerization and the oxygen inhibition layer, the thickness of the sample and the chemical structure of the organic solvent are important in residual monomer release [25]. Investigating the validity of this thesis about the content of the material effect, we validated this thesis and observed that TEC released the highest amount of Bis- GMA and UDMA. During the Scientific Documentation review of the material, it is seen that it doesn’t contain TEGDMA and uses pre-polymerization method (Scientific Documentation, Tetric EvoCeram, Ivoclar Vivadent). This method aiming to improve the mechanical properties of the material produces high monomer release. This view is also supported by Kopperud (2013) who writes that, the heterogeneous distribution and agglomeration of the particles/pre-polymerized fillers in the TEC affects the scattering and penetration depth of the polymerizing light in the material and results in the hardness reduction of the material and residual monomer increase [26]. Our study correlates with this result. The null hypothesis of this study was rejected. Significant differences were seen between composites in terms of monomer releases.
Guler’ [27], Sideridou and Achilias’ [9], Polydorou’ et al. [28] and Polydorou’ et al [29] research findings supported the fact that Bis-GMA has the highest release rate among residual monomers when they investigate the monomer release in different dental materials. Moldovan et al. [30] also obtained similar findings where they compared the commercially used composites with the one they produced experimentally. The results of the current study, consistent with previous findings in literature, showed that the most released monomer was Bis-GMA and the least released was TEGDMA. Lowest release rate of TEGDMA, which is a reactive diluent typically used in dental materials [31], can be explain with its high conversion degree and lower molecular weight (Bis-GMA molecular weight: 512 g/ mol, UDMA :470 g/mol, TEGDMA:286 g/mol) [9] that leads to high reactivity by letting high polymerization performance in the composite.
The rate of changing monomers to polymer is called the degree of conversion and the maximum conversion degree is reached by the end of 24 hours which can vary from 50% to 70% [32-34]. Prior studies that have noted although monomer release can last for months, the maximum release occurs within the first days after polymerization [9,29]. In the literature, there is a contradicting view about the time needed for the complete elution of the extractable amount of unreacted monomers. Some studies have suggested that the elution is completed within 7 days with a peak at the end of first day, while others have found that it lasts for a long time, for example, 30 days [9]. Our study findings were consisted with the second group of studies. The reason of our findings can be rooted from using 75% ethanol/water organic solvent for degradation. Also Hürmüzlü’s work support this group of studies when he measured the amount of residual monomers of 3 bulkfil and one nanohybrid composite on day 1 and day 30 and observed higher release on the 30th day [35]. We have found that monomer release lasts 30 days like Hürmüzlü however the highest releasing rate is reached on the 15th day on contrary his findings. The reason is because that no intermediate extraction taken in his experiment.
Using oral environment in vivo experiments has an important effect on degradation of resin based composites but in vivo experiments are not always applicable, ethical and long period of time demanding. Thus, in vitro aging experiments are acceptable instead of in vivo experiments. There are many mediums for mimicking oral medium but the most often chosen is ethanol-water solution. 75% ethanol-water solution is chosen as the elution medium since it mimics and speeds up the expected process of daily degradation occurring orally by the food consumption and saliva [36]. Therefore, FDA recommends its usage in vitro experiments and many studies uses it like we did in our study [3,6,9,15,37]. However, ethanol/ water solution creates not clinically possible destruction on the composites in short amount of time which can be related to monomer release rate. This destruction that can occur in a month in ethanol solution corresponds to the several months or years of destruction that can occur in oral environment [36].
Bis-GMA and Bis-EMA which are BPA-based (bisphenol A), TEGDMA and UDMA which are non-BPA based are the monomers most commonly found in a dental composite resin matrix [38,39]. Both of these groups can act as endocrine disruptors by mimicking and disturb the hormone receptors. This leads to low fertility on any gender and problem with gene expression [40]. Besides the endocrine disrupting, non-based BPA monomers can be cytotoxic for the local application area. This can cause pulp deformation, gingival margin retraction, secondary caries, failure of the restoration and local allergic reactions [41]. Bis-GMA over 0.001 mM and UDMA over 0,05 mM for gingival and pulp fibroblasts [42] and TEGDMA over 1.2 ± 0.9 mM for gingival and TEGDMA over 2.6 ± 1.1 mM for pulp fibroblasts can lead to cytotoxicity depending on the time and the concentration [41]. In our study when we are questioning these residual monomers toxicity on human dental treatments, we had to consider the factors such as the surface area and storage time of our discs and relation between oral and chemical elution medium. Van Landuyt measured and determined the average tooth and fillings surface areas in her meta-analysis such as an occlusal restoration’s surface area is 19 mm2 [14]. According to our basic intuition we would expect that a linear correlation between the surface area and released monomers however as Pelka’s study indicated that there is no linear correlation but only high correlation [43]. Thus we can still consider an average restoration’s and our discs’ surface areas relation to estimating the release monomer amount. Molecular weight of the monomers and their release amount in our study gave us the mM unit released amounts. Even when we consider the surface areas’ ratio in our evaluation of released monomer in mM unit, we obtain higher amount of released monomer than toxic limits of monomers. However according to in vivo Michelsen study, there was no released Bis-GMA, UDMA and TEGDMA in human saliva taken 10 min, 24 h, and 7 days after filling treatment [44]. So our results can be explained with ethanol-water solution’s accelerating impact on the monomer release from restoration. Our testing medium provides an important clue about performance of composite but not about time-monomer release correlation. Thus, our experiment conditions don’t provide the necessary information to decide on the toxicity on the human pulp and gingival fibroblasts. Therefore, we need new studies which can help us to understand better the correlation between in vivo and in vitro conditions about the residual monomers.
Conclusion
Residual monomers are released from all composite brands we used even if on 30th day. In order to reduce concerns about toxicity, taking measures to protect the pulp for example using cavity liners and bases especially in case of having less than 0.5 mm thickness of dentin. Another precaution is making polymerization according to manufacturer’s instructions against inadequate polymerization and using composite resins with increased filler content, which may contribute to the biocompatibility of composite resins.
Acknowledgment
The study was supported by the coordinator of scientific research projects at Dicle University, Turkey (Project No: DIS.17.029).
I cannot express enough thanks to my sister for her continued support in every step of my article and encouragement: Zeynep Sonkaya (Master of Science Student M.Sc, Faculty of Informatics, Department of Informatics, Technical University of Munich, Munich, Germany). I offer my sincere appreciation for the learning opportunities provided by her. My completion of this project could not have been accomplished without the support of her.
References
- Schulz SD, Laquai T, Kummerer K, Bolek R, Mersch-Sundermann V, Polydorou O. Elution of monomers from provisional composite materials. Int J Poly Sci 2015; 2: 1-7.
- Yang J, Shen J, Wu X, He F, Xie H, Chen C. Effects of nano-zirconia fillers conditioned with phosphate ester monomers on the conversion and mechanical properties of Bis-GMA-and UDMA-based resin composites. J Dent 2020; 103306.
- Alshali RZ, Salim NA, Sung R, Satterthwaite JD, Silikas N. Analysis of long-term monomer elution from bulk-fill and conventional resin-composites using high performance liquid chromatography. Dent Mater 2015; 31: 1587-1598.
- Łagocka R, Mazurek-Mochol M, Jakubowska K, Bendyk-Szeffer M, Chlubek D, Buczkowska-Radlińska J. Analysis of Base Monomer Elution from 3 Flowable Bulk-Fill Composite Resins Using High Performance Liquid Chromatography (HPLC). Med Sci Monit 2018; 24: 4679-4690.
- Gladwin M, Bagby M. Clinical aspects of dental materials: theory, practice, and cases. 2013.
- Lempel E, Czibulya Z, Kovacs B, Szalma J, Toth A, Kunsagi-Mate S. Degree of conversion and BisGMA, TEGDMA, UDMA elution from flowable bulk fill composites. Int J Mol Sci 2016; 17: 732.
- Cadenaro M, Breschi L, Antoniolli F, Navarra CO, Mazzoni A, Tay FR, Pashley DH. Degree of conversion of resin blends in relation to ethanol content and hydrophilicity. Dent Mater 2008; 24: 1194-1200.
- Khatri CA, Stansbury JW, Schultheisz CR, Antonucci JM Synthesis, characterization and evaluation of urethane derivatives of Bis-GMA. Dental Materials 2003; 19(7): 584-588.
- Sideridou ID, Achilias DS. Elution study of unreacted Bis-GMA, TEGDMA, UDMA, and Bis-EMA from light-cured dental resins and resin composites using HPLC. J Biomed Mater Res B Appl Biomater 2005; 4: 617-626.
- Reichl FX, Seiss M, Kleinsasser N, Kehe K, Kunzelmann KH, Thomas P. Distribution and excretion of BisGMA in guinea pigs. J Dent Res 2008; 87: 378-380.
- Gerzina TM, Hume WR. Diffusion of monomers from bonding resin–resin composite combinations through dentine In-vitro. J Dent 1996; 24: 125-128.
- Rogalewicz R, Voelkel A, Kownacki I. Application of HS-SPME in the determination of potentially toxic organic compounds emitted from resin-based dental materials. J Environ Monit 2006; 8: 377-383.
- Marquardt W, Seiss M, Hickel R, Reichl FX. Volatile methacrylates in dental practices. J Adhes Dent 2009; 11: 101-107.
- Van Landuyt KL, Nawrot T, Geebelen B, De Munck J, Snauwaert J, Yoshihara K, Van Meerbeek B. How much do resin-based dental materials release? A meta-analytical approach. Dent Mater 2011; 27: 723-747.
- Pongprueksa P, De Munck J, Duca RC, Poels K, Covaci A, Hoet P. Monomer elution in relation to degree of conversion for different types of composite. J Dent 2015; 43: 1448-1455.
- Cramer NB, Stansbury JW, Bowman CN. Recent advances and developments in composite dental restorative materials. J Dent Res 2011; 90: 402-416.
- Al-Hiyasat AS, Darmani H, Milhem MM. Cytotoxicity evaluation of dental resin composites and their flowable derivatives. Clin Oral Investig 2005; 9: 21-25.
- Tabatabaei MH, Sadrai S, Bassir SH, Veisy N, Dehghan S. Effect of food stimulated liquids and thermocycling on the monomer elution from a nanofilled composite. Open Dent J 2013; 7: 62-67.
- Viljanen EK, Langer S, Skrifvars M, Vallittu PK. Analysis of residual monomers in dendritic methacrylate copolymers and composites by HPLC and headspace-GC/MS. Dent mater 2006; 22: 845-851.
- Moharamzadeh K, Van Noort R, Brook IM, Scutt AM. HPLC analysis of components released from dental composites with different resin compositions using different extraction media. J Mater Sci Mater Med 2007; 18: 133-137.
- Altunsoy M, Botsalı MS, Tosun G, Yaşar A. Effect of different exposure times on the amount of residual monomer released from adhesive systems. Acta Odontol Turc 2013; 30: 6-12.
- Durner J, Spahl W, Zaspel J, Schweikl H, Hickel R, Reichl FX. The toxicokinetics and distribution of 2-hydroxyethyl methacrylate in mice. Dent Mater 2010; 26: 91.
- Koin PJ, Kilislioglu A, Zhou M, Drummond JL, Hanley L. Analysis of the degradation of a model dental composite. J Dent Res 2008; 87: 661-665.
- Shajii L, Santerre JP. Effect of filler content on the profile of released biodegradation products in micro-filled bis-GMA/TEGDMA dental composite resins. Biomater 1999; 20: 1897-1908.
- Mun HJ, Im BS, Lee YG, Kim CW. Determination of Residual Monomers in Dental Pit and Fissure Sealants. Bull Korean Chem Soc 2000; 21: 1115-1118.
- Kopperud HM, Johnsen GF, Lamolle S, Kleven IS, Wellendorf H, Haugen HJ. Effect of short LED lamp exposure on wear resistance, residual monomer and degree of conversion for Filtek Z250 and Tetric EvoCeram composites. Dent Mater 2013; 29: 824-834.
- Guler C, Gorgen VA, Keskin G, Demir P. The Evaluation of Effect of Light Curing Type and Resin Shade on Microhardness of a Composite Resin and Two Different Compomer Restorative Materials. Turkiye Klinikleri J Pediatr Dent-Special Topics 2015; 1: 24-28.
- Polydorou O, Konig A, Hellwig E, Kummerer K. Long‐term release of monomers from modern dental‐composite materials. Eur J Oral Sci 2009; 117: 68-75.
- Polydorou O, Trittler R, Hellwig E, Kummerer K. Elution of monomers from two conventional dental composite materials. Dent Mater 2007; 23: 1535-1541.
- Moldovan M, Balazsi R, Soanca A, Roman A, Sarosi C, Prodan D, Cristescu I. Evaluation of the degree of conversion, residual monomers and mechanical properties of some light-cured dental resin composites. Mater 2019; 12: 2109.
- Barszczewska-Rybarek IM, Chroszcz MW, Chladek G. Novel Urethane-Dimethacrylate Monomers and Compositions for Use as Matrices in Dental Restorative Materials. Int J Mol Sci 2020; 21: 2644.
- Park SH. Comparison of degree of conversion for light-cured and additionally heat- cured composites. J Prosthet Dent 1996; 76: 613-618.
- Halvorson RH, Erickson RL, Davidson CL. Energy dependent polymerization of resin-based composite. Dent Mater 2002; 18: 463-469.
- Neves AD, Discacciati JA, Orefice RL, Yoshida MI. Influence of the power density on the kinetics of photopolymerization and properties of dental composites. J Biomed Mater Res B: Appl Biomater 2005; 72: 393-400.
- Hurmuzlu F, Kilic V. Analysis of Monomer Elution from Bulk-fill and Nanocomposites Cured with Different Light Curing Units Using High Performance Liquid Chromatography. J Photopolym Sci Tec 2020; 33: 27-36.
- Sideridou ID, Karabela MM, Bikiaris DN. Aging studies of light cured dimethacrylate-based dental resins and a resin composite in water or ethanol/water. Dent mater 2007; 23: 1142-1149.
- Cebe MA, Cebe F, Cengiz MF. Elution of monomer from different bulk fill dental composite resins. Dent Mater. 2015; 31: 141-149.
- Lofroth M, Ghasemimehr M, Falk A, Vult von Steyern P. Bisphenol A in dental materials – existence, leakage and biological effects. Heliyon 2019; 5: e01711.
- Diamantopoulo E, Samanidou V. Bioanalysis as a powerful tool in Dentistry: the case of short-term and long-term release of Monomers from dental Composites. J Appl Bioanal 2020; 6: 64-80.
- Fenichel P, Chevalier N, Brucker-Davis F. Bisphenol A: An endocrine and metabolic disruptor. Ann Endocrinol 2013; 74: 211-220.
- Goldberg M. In vitro and in vivo studies on the toxicity of dental resin components: A review. Clin Oral Investig 2008; 12: 1-8.
- Bakopoulou A, Papadopoulos T, Garefis P. Molecular toxicology of substances released from resin-based dental restorative materials. Int J Mol Sci 2009; 10: 3861-3899.
- Pelka M, Distler W, Petschelt A. Elution parameters and HPLC-detection of single components from resin composite. Clin Oral Investig 1999; 3: 194-200.
- Michelsen VB, Kopperud HB, Lygre GB, Bjorkman L, Jensen E, Kleven IS, Lygre H. Detection and quantification of monomers in unstimulated whole saliva after treatment with resin‐based composite fillings in vivo. Eur J Oral Sci 2012; 120: 89-95.